2. 宁夏医科大学基础医学院, 银川 750000;
3. 海军军医大学(第二军医大学)第三附属医院泌尿外科, 上海 201805;
4. 海军军医大学(第二军医大学)附属公利医院耳鼻咽喉科, 上海 200135
2. College of Basic Medicine Sciences, Ningxia Medical University, Yinchuan 750000, Ningxia Hui Autonomous Region, China;
3. Department of Urology, The Third Affiliated Hospital of Naval Medical University (Second Military Medical University), Shanghai 201805, China;
4. Department of Otolaryngology, Gongli Hospital, Naval Medical University (Second Military Medical University), Shanghai 200135, China
胰岛素抵抗是2型糖尿病最主要的发病机制[1]。格列酮类药物是临床治疗2型糖尿病的常用胰岛素增敏药物,其主要通过激活过氧化物酶体增殖物激活受体γ(peroxisome proliferators-activated receptor γ,PPARγ)调控与胰岛素效应有关的多种基因的转录,增加胰岛素抵抗敏感性[2-7]。但部分2型糖尿病患者对此类药物敏感性低,治疗效果不佳[2, 8]。如能在用药早期评估患者对该药物是否敏感,对指导临床精准用药具有重要价值。为深入研究PPARγ的下游分子通路,本研究以高脂诱导的2型糖尿病模型大鼠为研究对象,观察血清miRNA-223水平与格列酮药物的疗效相关性;以HepG2细胞为研究对象,观察PPARγ是否通过调控miRNA-223改善高脂诱导的胰岛素抵抗细胞葡萄糖吸收的降低。
1 材料和方法 1.1 试剂与材料人肝癌HepG2细胞购自中国科学院上海生命科学研究院细胞生物学研究所细胞库,均按细胞培养说明用含10% FBS、1% 双抗(100 U/mL青霉素和100 μg/mL链霉素)的MEM培养基,于37 ℃、5% CO2、饱和湿度的细胞培养箱中培养。软脂酸(货号P0500-10G,纯度≥99%)、雷公藤红素(货号C0869,纯度≥98%)、DMSO购自美国Sigma公司;PPARγ、葡萄糖转运蛋白(glucose transporter,GLUT)-1、GLUT-2、GLUT-4、胰岛素受体底物(insulin receptor substrate,IRS)-1、IRS-2、β-肌动蛋白抗体均购自美国Cell Signaling Technology公司;siRNA-PPARγ基因沉默转染序列用siRNA-Mate转染,siRNA转染序列及siRNA-Mate均购于上海吉玛制药技术有限公司;ReverTra Ace qPCR反转录试剂盒购自日本ToYoBo公司;蛋白质印迹检测试剂盒购自上海碧云天生物技术有限公司。
1.2 2型糖尿病大鼠模型建立15只Wistar雄性大鼠购自浙江维通利华实验动物技术有限公司[动物生产许可证号:SCXK(浙)2019-0001]。大鼠以高脂饲料喂养6~8周诱发胰岛素抵抗,然后用亚致病剂量(25 mg/kg)链脲佐菌素(streptozotocin,STZ)静脉注射诱发高血糖症。判断标准:空腹血糖≥10 mmol/L,胰岛素敏感性降低,符合此标准的大鼠确定为2型糖尿病大鼠。对成功建模的15只2型糖尿病大鼠使用吡格列酮(每天10 mg/kg)灌胃治疗7 d,在治疗前与治疗7 d后分别采集静脉血检测空腹血糖、血清miRNA-233表达水平及炎症因子表达水平。
1.3 细胞转染和处理将HepG2细胞铺于6孔板中,24 h后根据试剂说明书操作,加入基因转染序列和Lipofectamine 3000转染试剂,转染48 h后加PBS清洗,抽提蛋白或RNA。HepG2细胞生长至对数生长期,以含有浓度为250 μmol/L软脂酸或浓度为1 μmol/L吡格列酮(PPARγ激活剂)的细胞培养液培养24 h,收集细胞进行后续实验。
1.4 血糖检测使用葡萄糖氧化酶-过氧化物酶法检测大鼠空腹血浆葡萄糖水平。
1.5 miRNA-223水平检测使用总RNA提取试剂盒(上海飞捷生物技术有限公司、上海硕盟生物科技有限公司)提取血清和细胞总RNA。使用ReverTra Ace qPCR反转录试剂盒进行反转录反应合成cDNA。按照SYBR® Premix Ex TaqTM试剂盒(上海吉玛生物有限公司)使用说明进行qPCR检测。miRNA-223引物由上海吉玛制药技术有限公司合成。qPCR检测及数据采集均使用ABI PRISM 7500HT序列检测系统(美国Applied Biosystems公司),GAPDH和U6作为内参。
1.6 炎症因子水平检测采用美国R & D公司ELISA试剂盒,利用双抗体夹心法测定炎症因子IL-1β、IL-6、IL-8和TNF-α水平。
1.7 蛋白质印迹实验取对数生长期细胞,以每孔1×106个细胞的密度接种于6孔板中,加入MEM完全培养基3 mL。提取蛋白质,用BCA法测定蛋白质浓度。配制10%分离胶电泳,转膜后加入一抗(1∶500稀释),4℃过夜,用TBST清洗3次后,加入二抗(1∶2 000稀释)室温孵育2 h,TBST清洗3次后用凝胶图像处理系统分析目的条带灰度值并计算相对表达量。
1.8 细胞葡萄糖吸收实验清洗细胞,并加入含有1 mL葡萄糖的无血清、无酚红DMEM培养基。再培养12 h后,收集培养基,根据试剂说明书使用葡萄糖检测试剂盒(南京建成生物工程研究所),用葡萄糖氧化酶法测定细胞外葡萄糖。将培养起始点的葡萄糖水平设为1,计算培养后的葡萄糖水平。
1.9 统计学处理采用SPSS 22.0软件进行数据分析,符合正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验或配对t检验。检验水准(α)为0.05。
2 结果 2.1 吡格列酮治疗前后大鼠空腹血糖、血清miRNA-223水平、血清炎症因子的变化与治疗前相比,吡格列酮治疗7 d后大鼠空腹血糖下降[(5.64±0.71)mmol/L vs(7.60±0.70)mmol/L,P < 0.001],血清miRNA-223水平升高至治疗前的1.31±0.25倍(P < 0.001),血清炎症因子IL-1β、IL-6、IL-8、TNF-α相对表达量均较治疗前下降(分别为0.29±0.08 vs 0.42±0.09、3.91±1.23 vs 6.76±0.81、13.36±2.71 vs 20.23±2.07、0.67±0.36 vs 1.33±0.36),差异均有统计学意义(P均 < 0.001)。提示吡格列酮能降低2型糖尿病模型大鼠的空腹血糖及血清炎症因子IL-1β、IL-6、IL-8和TNF-α的含量,升高血清miRNA-223水平。
2.2 软脂酸诱导HepG2细胞对葡萄糖吸收能力、PPARγ表达及miRNA-223水平的影响用软脂酸(250 μmol/L)处理24 h后,软脂酸组HepG2细胞中PPARγ蛋白表达较空白对照组下降(49.00±0.29)%(图 1)。与空白对照组相比,软脂酸组血清miRNA-223水平是空白对照组的(0.51±0.29)倍,软脂酸组细胞外葡萄糖剩余量升高为空白对照组的(1.59±0.23)倍(P均 < 0.05)。
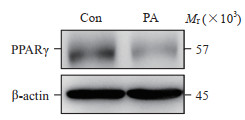
|
图 1 PA对HepG2细胞PPARγ蛋白表达的影响 Fig 1 Effect of PA on expression of PPARγ protein in HepG2 cells PPARγ: Peroxisome proliferators-activated receptors γ; PA: Palmitic acid; Con: Control. |
2.3 PPARγ蛋白在调控HepG2细胞葡萄糖吸收、miRNA-223水平及炎症因子中的作用
结果(图 2A~2D)显示,在软脂酸诱导HepG2细胞中上调PPARγ蛋白表达可以逆转软脂酸导致的miRNA-223水平下降,也可以改善软脂酸导致的细胞葡萄糖吸收减弱,对软脂酸活化的炎症因子也有抑制作用(P均 < 0.05)。用RNA干扰技术抑制PPARγ表达后,miRNA-223表达水平下降至转染对照组的(0.19±0.22)倍(P < 0.05),细胞外葡萄糖剩余量为转染对照组的(1.41±0.23)倍(P均 < 0.05),但是炎症因子水平与转染对照组相比差异无统计学意义(P均 > 005)(图 2E、2F)。

|
图 2 PPARγ在调控细胞葡萄糖吸收能力、miRNA-223及炎症因子水平中的作用 Fig 2 Role of PPARγ in regulating cellular glucose absorption and levels of miRNA-223 and inflammatory factors A, E: PPARγ protein expression detected by Western blotting; B: miRNA-223 expression detected by quantitative real-time polymerase chain reaction; C: Glucose absorption detected by glucose oxidase peroxidase; D, F: Inflammatory factors (IL-1β, IL-6, IL-8, and TNF-α) detected by enzyme-linked immunosorbent assay. *P < 0.05. n=3, x±s. PPARγ: Peroxisome proliferators-activated receptor γ; PA: Palmitic acid; Con: Control; IL: Interleukin; TNF-α: Tumor necrosis factor α. |
2.4 miRNA-223在调控葡萄糖吸收及相关蛋白中的作用
结果显示,过表达组miRNA-223水平为转染对照组的(3.82±0.13)倍(P < 0.05)。过表达miRNA-223可以改善软脂酸诱导的细胞葡萄糖吸收下降(P < 0.05)、逆转软脂酸诱导的炎症因子IL-1β、IL-6、IL-8、TNF-α水平增高(P < 0.05)。过表达miRNA-223后,与转染对照组相比,葡萄糖吸收调节相关蛋白GLUT-1表达上升(1.23±0.21)倍、GLUT-4上升(3.44±0.42)倍、IRS-1蛋白上升(2.00±0.13)倍。抑制miRNA-223水平后,转染对照组miRNA-223表达水平为抑制组的(5.27±0.15)倍(P < 0.05)。抑制miRNA-223组细胞外葡萄糖剩余量为转染对照组的(1.34±0.26)倍。与转染对照组相比,抑制miRNA-223组葡萄糖吸收调节相关蛋白GLUT-1下降(10.70±0.18)%、GLUT-4下降(71.67±0.21)%、IRS-1下降(59.00±0.17)%(P均 < 0.05),但4种炎症因子IL-1β、IL-6、IL-8、TNF-α水平无明显变化(P均 > 0.05)。过表达或抑制miRNA-223水平时PPARγ蛋白表达差异无统计学意义(P > 0.05)。见图 3。

|
图 3 miRNA-223在调控细胞葡萄糖吸收及相关蛋白中的作用 Fig 3 Role of miRNA-223 in regulating cellular glucose absorption and related proteins A: Glucose absorption detected by glucose oxidase peroxidase; B, D, E, G: PPARγ protein expression detected by Western blotting; C, F: Inflammatory factors (IL-1β, IL-6, IL-8, and TNF-α) detected by enzyme-linked immunosorbent assay. *P < 0.05. n=3, x±s. PPARγ: Peroxisome proliferators-activated receptor γ; PA: Palmitic acid; Con: Control; IL: Interleukin; TNF-α: Tumor necrosis factor α. |
3 讨论
胰岛素抵抗是2型糖尿病的主要发病机制,使用胰岛素增敏药物是治疗2型糖尿病的主要手段之一[9-10]。噻唑烷二酮(thiazolidinedione,TZD)类药物为胰岛素增敏剂,可以起到减轻胰岛素抵抗和保护胰岛β细胞的作用[2]。TZD类药物主要通过PPARγ起作用。TZD类药物单药控制血糖的时间较二甲双胍、格列本脲更持久,是临床改善胰岛素敏感性的常用药[7]。临床治疗时有15%~20%患者用TZD类药物后无法增加胰岛素敏感性,降糖效果不佳[2, 8, 11],目前仍不清楚PPARγ通过何种下游通路调节胰岛素敏感性。
miRNA是一类短序列非编码单链RNA,通过与靶基因mRNA互补配对在转录后水平对基因表达进行负调控[12]。miRNA与代谢性疾病密切相关,在胰岛素分泌及血糖、血脂调控过程中起重要作用[13-14]。miRNA-223与代谢性疾病关系密切,在血糖、血脂尤其在胰岛素敏感性调控方面起重要作用[15]。本课题组前期研究发现,脂肪酸诱导的胰岛素抵抗细胞模型中miRNA-223水平下降,过表达miRNA-223可提高胰岛素敏感性,在细胞水平敲除miRNA-223能削弱药物治疗的降糖效果[14]。值得关注的是,糖尿病患者血清中miRNA-223水平低于正常人[16],而且在细胞水平上也证实miRNA-223是调控PPARγ诱导巨噬细胞极化的关键调控者[17]。本研究结果发现吡格列酮有效降低了2型糖尿病模型大鼠的血糖、升高血清miRNA-223水平,提示吡格列酮的降血糖作用可能与血清miRNA-223水平升高有关。
2型糖尿病是一种炎症性疾病,慢性炎症与2型糖尿病的胰岛素抵抗密切相关[18]。炎症因子如TNF和IL等在2型糖尿病的发病机制中起着重要作用[19]。IL-1β可通过激活转录因子NF-κB调节B细胞中抗凋亡和促凋亡基因的表达。IL-6可促进肝脏合成超敏CRP等急性时相蛋白,促进炎症和胰岛素抵抗的发生,是2型糖尿病的重要独立影响因素[20]。有研究显示,作为中性粒细胞中含量最为丰富的miRNA之一,miRNA-223可以直接抑制中性粒细胞中IL-6的表达,进而减轻酒精性脂肪肝导致的肝损伤[21]。本研究通过ELISA方法检测吡格列酮治疗前后2型糖尿病模型大鼠血清中炎症因子水平,结果发现吡格列酮可以降低血清炎症因子IL-1β、IL-6、IL-8和TNF-α的表达水平,提示在用吡格列酮治疗后血糖降至正常水平与血清炎症因子表达降低存在一定的关联。
有研究者发现软脂酸可以诱导胰岛素抵抗[22],近些年大量胰岛素抵抗体外实验选用软脂酸为诱导剂。miRNA-223在高糖饮食诱导血糖异常、高脂血症和胰岛素抵抗发病机制中有重要意义[23],对IRS及GLUT家族均有一定的调节作用[14, 24]。本研究发现软脂酸诱导HepG2细胞可降低细胞葡萄糖吸收、抑制PPARγ蛋白和miRNA-223表达,而活化PPARγ可改善上述现象。抑制PPARγ表达也可导致细胞葡萄糖吸收下降、miRNA-223水平降低,但对细胞炎症因子没有调节作用。本研究通过过表达或抑制miRNA-223表达,发现调节miRNA-223水平对细胞葡萄糖吸收及GLUT-4、IRS-1蛋白表达有调节作用,提示miRNA-223可能通过调节上述蛋白进而调节葡萄糖吸收,但在没有高脂刺激的条件下对炎症因子没有调节作用。结果提示PPARγ和miRNA-223可能仅抑制高脂刺激后的炎症因子水平,对正常条件下的炎症因子并无调节作用,PPARγ可能通过上调miRNA-223进而通过GLUT改善细胞葡萄糖吸收。
综上所述,PPARγ通过抑制炎症反应、调节miRNA-223及其下游GLUT-4、IRS-1等相关蛋白改善胰岛素抵抗。本研究为指导临床治疗2型糖尿病精准用药提供了一定的理论依据。
| [1] |
MIRHOSSEINI N, VATANPARAST H, MAZIDI M, KIMBALL S M. Vitamin D supplementation, glycemic control, and insulin resistance in prediabetics: a meta-analysis[J]. J Endocr Soc, 2018, 2: 687-709. DOI:10.1210/js.2017-00472 |
| [2] |
PAN D S, WANG W, LIU N S, YANG Q J, ZHANG K, ZHU J Z, et al. Chiglitazar preferentially regulates gene expression via configuration-restricted binding and phosphorylation inhibition of PPARγ[J/OL]. PPAR Res, 2017, 2017: 4313561. DOI: 10.1155/2017/4313561.
|
| [3] |
LAM V Q, ZHENG J, GRIFFIN P R. Unique interactome network signatures for peroxisome proliferator-activated receptor gamma (PPARγ) modulation by functional selective ligands[J]. Mol Cell Proteomics, 2017, 16: 2098-2110. DOI:10.1074/mcp.RA117.000308 |
| [4] |
CHEN Y D, MA H M, ZHU D S, ZHAO G W, WANG L L, FU X J, et al. Discovery of novel insulin sensitizers: promising approaches and targets[J/OL]. PPAR Res, 2017, 2017: 8360919. DOI: 10.1155/2017/8360919.
|
| [5] |
VIGUEIRA P A, MCCOMMIS K S, HODGES W T, SCHWEITZER G G, COLE S L, OONTHONPAN L, et al. The beneficial metabolic effects of insulin sensitizers are not attenuated by mitochondrial pyruvate carrier 2 hypomorphism[J]. Exp Physiol, 2017, 102: 985-999. DOI:10.1113/EP086380 |
| [6] |
SHARMA S, TALIYAN R. Histone deacetylase inhibitors: future therapeutics for insulin resistance and type 2 diabetes[J]. Pharmacol Res, 2016, 113(Pt A)): 320-326. |
| [7] |
HAN L, SHEN W J, BITTNER S, KRAEMER F B, AZHAR S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part Ⅱ: PPAR-β/δ and PPAR-γ[J]. Future Cardiol, 2017, 13: 279-296. DOI:10.2217/fca-2017-0019 |
| [8] |
CAMPOS M L, CERQUEIRA L B, SILVA B C U, FRANCHIN T B, GALDINO-PITTA M R, PITTA I R, et al. New pioglitazone metabolites and absence of opened-ring metabolites in new N-substituted thiazolidinedione[J]. Drug Metab Dispos, 2018, 46: 879-887. DOI:10.1124/dmd.117.079012 |
| [9] |
WANG X, YANG X H, CAI Y H, WANG S M, WENG W Q. High prevalence of erectile dysfunction in diabetic men with depressive symptoms: a meta-analysis[J]. J Sex Med, 2018, 15: 935-941. DOI:10.1016/j.jsxm.2018.05.007 |
| [10] |
BAI L T, GAO J L, WEI F, ZHAO J, WANG D W, WEI J P. Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes[J/OL]. Front Pharmacol, 2018, 9: 423. DOI: 10.3389/fphar.2018.00423.
|
| [11] |
LEE B C, LEE J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance[J]. Biochim Biophys Acta, 2014, 1842: 446-462. DOI:10.1016/j.bbadis.2013.05.017 |
| [12] |
PETRIE J R, GUZIK T J, TOUYZ R M. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms[J]. Can J Cardiol, 2018, 34: 575-584. DOI:10.1016/j.cjca.2017.12.005 |
| [13] |
SINGHAL A, AGRAWAL A, LING J. Regulation of insulin resistance and type Ⅱ diabetes by hepatitis C virus infection: a driver function of circulating miRNAs[J]. J Cell Mol Med, 2018, 22: 2071-2085. DOI:10.1111/jcmm.13553 |
| [14] |
ZHANG X, XUE X C, WANG Y, CAO F F, YOU J, UZAN G, et al. Celastrol reverses palmitic acid-induced insulin resistance in HepG2 cells via restoring the miRNA-223 and GLUT4 pathway[J]. Can J Diabetes, 2019, 43: 165-172. DOI:10.1016/j.jcjd.2018.07.002 |
| [15] |
ESTEVES J V, ENGUITA F J, MACHADO U F. MicroRNAs-mediated regulation of skeletal muscle GLUT4 expression and translocation in insulin resistance[J/OL]. J Diabetes Res, 2017, 2017: 7267910. DOI: 10.1155/2017/7267910.
|
| [16] |
DEIULⅡS J A, SYED R, DUGGINENI D, RUTSKY J, RENGASAMY P, ZHANG J, et al. Visceral adipose microRNA 223 is upregulated in human and murine obesity and modulates the inflammatory phenotype of macrophages[J/OL]. PLoS One, 2016, 11: e0165962. DOI: 10.1371/journal.pone.0165962.
|
| [17] |
YING W, TSENG A, CHANG R C, MORIN A, BREHM T, TRIFF K, et al. MicroRNA-223 is a crucial mediator of PPARγ-regulated alternative macrophage activation[J]. J Clin Invest, 2015, 125: 4149-4159. DOI:10.1172/JCI81656 |
| [18] |
ZATALIA S R, SANUSI H. The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus[J]. Acta Med Indones, 2013, 45: 141-147. |
| [19] |
PIETROPAOLO M, BARINAS-MITCHELL E, KULLER L H. The heterogeneity of diabetes: unraveling a dispute: is systemic inflammation related to islet autoimmunity?[J]. Diabetes, 2007, 56: 1189-1197. DOI:10.2337/db06-0880 |
| [20] |
FAAM B, ZARKESH M, DANESHPOUR M S, AZIZI F, HEDAYATI M. The association between inflammatory markers and obesity-related factors in Tehranian adults: Tehran lipid and glucose study[J]. Iran J Basic Med Sci, 2014, 17: 577-582. |
| [21] |
LI M, HE Y, ZHOU Z, RAMIREZ T, GAO Y Q, GAO Y H, et al. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47phox-oxidative stress pathway in neutrophils[J]. Gut, 2017, 66: 705-715. DOI:10.1136/gutjnl-2016-311861 |
| [22] |
HUNNICUTT J W, HARDY R W, WILLIFORD J, MCDONALD J M. Saturated fatty acid-induced insulin resistance in rat adipocytes[J]. Diabetes, 1994, 43: 540-545. DOI:10.2337/diab.43.4.540 |
| [23] |
SUD N, ZHANG H Y, PAN K C, CHENG X, CUI J, SU Q Z. Aberrant expression of microRNA induced by high-fructose diet: implications in the pathogenesis of hyperlipidemia and hepatic insulin resistance[J]. J Nutr Biochem, 2017, 43: 125-131. DOI:10.1016/j.jnutbio.2017.02.003 |
| [24] |
MACARTNEY-COXSON D, DANIELSON K, CLAPHAM J, BENTON M C, JOHNSTON A, JONES A, et al. MicroRNA profiling in adipose before and after weight loss highlights the role of miRNA-223-3p and the NLRP3 inflammasome[J]. Obesity (Silver Spring), 2020, 28: 570-580. DOI:10.1002/oby.22722 |
 2022, Vol. 43
2022, Vol. 43


