2. 海军军医大学(第二军医大学)中医系, 上海 200433
2. Department of Traditional Chinese Medicine, Naval Medical University(Second Military Medical University), Shanghai 200433, China
女性绝经后由于体内雌激素水平急剧下降,导致动脉粥样硬化、冠心病等心血管疾病的发生风险升高,与同年龄男性相当[1-2]。体内外实验证实雌激素可通过减轻氧化应激改善心肌细胞功能异常[3-4]。有研究报道17β-雌二醇(17β-estradiol,E2)是一种强大的内源性抗氧化剂,能够减少肝脏和血液中的脂质过氧化[5]。E2缺乏导致活性氧产生增加、血管内皮功能障碍,而E2替代疗法可以减轻这些病理变化,这一结果表明雌激素急剧下降诱导的氧化应激在绝经后心血管功能异常中发挥着重要作用[6]。近期研究发现,二肽基肽酶3(dipeptidyl peptidase 3,DPP3)是一种锌依赖性水解酶,参与降解具有4~12个氨基酸残基的寡肽[7]。DPP3与多种病理生理过程有关,包括血压调节、疼痛信号传导和癌细胞对氧化应激的防御[8]。文献报道,卵巢切除(ovariectomy,OVX)小鼠的肝脏中DPP3表达减少,而E2替代治疗后DPP3表达上调[5],但受到E2调节的DPP3是否通过调节氧化应激参与绝经导致的心脏功能异常尚未明确。血红素加氧酶1(heme oxygenase 1,HO-1)是哺乳动物血红素降解的限速酶,作为一种有效的抗氧化剂,其表达的转录控制是通过抗氧化反应元件所介导[9]。研究证实DPP3结合Kelch样环氧氯丙胺相关蛋白1(Kelch-like ECH-associated protein 1,KEAP1)以取代核因子E2相关因子2(nuclear factor erythroid-2-related factor-2,NRF2),从而抑制NRF2泛素化并驱动NRF2与抗氧化反应元件结合,进而促进HO-1表达[10]。本研究通过明确DPP3和HO-1在OVX大鼠心脏中的表达变化,以及补充E2对OVX大鼠心脏中DPP3和HO-1表达、氧化应激和心脏功能的影响,旨在为雌激素改善OVX大鼠心血管功能提供新的理论依据。
1 材料和方法 1.1 OVX造模20只健康雌性SD大鼠(体重200~250 g)购自上海西普尔-必凯实验动物有限公司[动物生产许可证号:SCXK(沪)-2017-0012],饲养于12 h光照/12 h黑暗、室温25 ℃的清洁级动物房,自由进食和饮水。大鼠通过吸入体积分数为2%的异氟烷和氧气混合气体麻醉,取仰卧位固定,下腹部备皮、消毒,切开皮肤,切口长0.8~1 cm,钝性分离腹部正中肌肉和筋膜,进入腹腔,找到子宫,沿子宫“ Y ”形走行找到卵巢。钝性分离卵巢,OVX模型组(OVX组,n=10)大鼠在结扎卵巢周围血管后完整切除双侧卵巢;假手术对照组(Sham组,n=10)仅接受开腹分离卵巢但不切除卵巢,逐层缝合、关闭腹腔,缝合皮肤。术后肌内注射青霉素(20 000 U/kg)预防感染。术后大鼠正常饲养6周。
1.2 E2补充OVX组及Sham组大鼠各按体重大小依次编号后采用随机数表法分为2个亚组,每个亚组5只大鼠。OVX造模后2周,一个亚组的大鼠于颈部皮下注射溶于生理盐水的E2(购自美国Sigma公司,产品批号:Lot#SLB D2317V),根据课题组前期研究[11]确定E2剂量为每天30 μg/kg(E2组),另一个亚组于颈部皮下注射相应体积的生理盐水作为溶剂(vehicle,Veh)对照,每天注射1次,持续注射4周。4个亚组根据有无OVX和E2补充分别命名为Sham+Veh组、Sham+E2组、OVX+Veh组和OVX+E2组。
1.3 心血管指标监测皮下注射E2治疗4周结束时称量大鼠体重,腹腔注射2%戊巴比妥钠0.03 mL/kg麻醉,将大鼠置于体温控制板上,维持体温在37 ℃左右,进行气管插管和右侧颈总动脉插入动脉导管,连接至PowerLab/8SP系统(美国ADInstruments公司)。首先,测量平均动脉压和心率,记录10 min后将动脉导管沿颈总动脉经主动脉进入左心室,测量左心室压力上升最大速率(maximum rate of rise of left ventricular pressure,dp/dtmax)和左心室压力下降最大速率(maximum rate of drop of left ventricular pressure,-dp/dtmax)。
1.4 标本留取测量完心血管指标的大鼠在处死前从股静脉取血,抗凝静置10 min后,8×g离心15 min,吸取血浆,转移至-80 ℃冰箱备用。处死大鼠取出心脏,排空血液,称量心脏质量后用液氮快速冷冻后,转移至-80 ℃冰箱备用。
1.5 心脏组织氧化应激测定将大鼠心脏从心尖处剪取约100 mg组织,液氮冷冻后研磨组织块,按每10 mg组织加入50 μL RIPA裂解液(南京碧云天生物科技有限公司,P0013B),提取组织样本的总蛋白,冰上静置10 min后离心,吸取上清,待用,通过BCA法测定各样本上清液中蛋白浓度。活性氧和丙二醛含量、过氧化氢酶(catalase,CAT)和超氧化物歧化酶(superoxide dismutase,SOD)是氧化应激的重要标志物,被广泛认为是氧化损伤的指标。活性氧和丙二醛含量分别采用动物组织活性氧光泽精化学发光法(lucigenin chemiluminescence,LCL)定量检测试剂盒(上海哈灵生物科技有限公司,货号HL10113.5)和丙二醛检测试剂盒(南京碧云天生物科技有限公司,货号S0131S)检测。氧化应激相关酶CAT和SOD活性分别采用CAT检测试剂盒(南京碧云天生物科技有限公司,货号S0051)和总SOD活性检测试剂盒(南京碧云天生物科技有限公司,货号S0101S)检测,具体检测步骤按照说明书进行。
1.6 DPP3和HO-1表达检测根据1.5节中蛋白提取方法获得心脏组织蛋白样本,按终浓度为5 μg/μL加入上样缓冲液,混匀后加热至100 ℃变性10 min,-20 ℃保存备用。对变性蛋白样本依次行SDS-PAGE、转膜、5%脱脂奶粉溶液封闭和洗膜。4℃过夜标记DPP3一抗和HO-1一抗(货号分别为ab133735和ab68477,均购自英国Abcam公司,工作浓度均为1∶1 000,以GAPDH作为内参照),洗膜3次。加入二抗,室温孵育1.5 h,洗膜。经化学发光法显示蛋白条带,并采用Gel-Pro Analyzer 1软件对蛋白条带灰度值进行分析。
1.7 统计学处理应用GraphPad Prism 6.0软件进行统计学分析。计量资料以x±s表示,组间比较采用双因素方差分析,多重比较采用Tukey检验。检验水准(α)为0.05。
2 结果 2.1 E2对OVX大鼠心血管功能的影响OVX+Veh组大鼠的体重、心脏质量和心脏质量与体重之比均高于Sham+Veh组(P均 < 0.05),而OVX+E2组大鼠的体重和心脏质量均较OVX+Veh组无明显降低,但心脏质量与体重之比下降(P < 0.05)。与Sham+Veh组比,OVX+Veh组大鼠心率、平均动脉压均升高(P均 < 0.05),dp/dtmax绝对值和-dp/dtmax绝对值均降低(P均 < 0.05);而接受E2补充治疗的OVX+E2组大鼠心率和平均动脉压均降低,dp/dtmax绝对值和-dp/dtmax绝对值均增加,与OVX+Veh组相比差异均有统计学意义(P均 < 0.05)。见表 1。
|
|
表 1 E2补充治疗后各组大鼠的心血管功能指标比较 Tab 1 Comparison of cardiovascular function indexes of rats in each group after E2 supplementary treatment |
2.2 E2对OVX大鼠心脏氧化应激的影响
OVX+Veh组大鼠心脏组织中抗氧化酶CAT和SOD的活性较Sham+Veh组均降低(P均 < 0.05),而活性氧和丙二醛水平均增高(P均 < 0.05)。E2补充治疗4周后OVX+E2组大鼠的心脏组织中CAT和SOD的活性均较OVX+Veh组增高(P均 < 0.05),活性氧和丙二醛水平均较OVX+Veh组降低(P均 < 0.05)。见图 1。
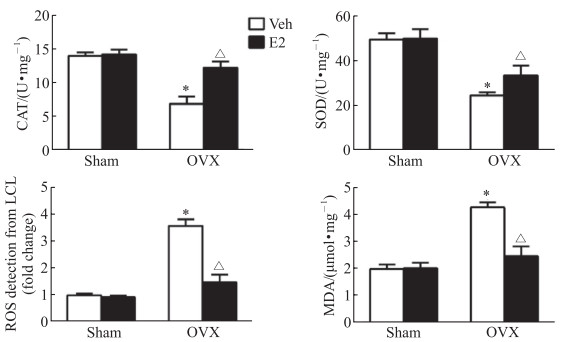
|
图 1 E2补充治疗对各组大鼠心脏组织氧化应激指标的影响 Fig 1 Effect of E2 supplement on oxidative stress indexes in cardiac tissues of rats *P < 0.05 vs Sham+Veh group; △P < 0.05 vs OVX+Veh group. n=5, x±s. E2: 17β-estradiol; Veh: Vehicle; OVX: Ovariectomy; CAT: Catalase; SOD: Superoxide dismutase; ROS: Reactive oxygen species; LCL: Lucigenin chemiluminescence; MDA: Malondialdehyde. |
2.3 E2对OVX大鼠心脏DPP3和HO-1蛋白表达的影响
与Sham+Veh组相比,OVX+Veh组大鼠心脏组织中DPP3和HO-1的蛋白表达水平均降低(P均 < 0.05);E2补充治疗4周后OVX+E2组大鼠心脏组织中的DPP3和HO-1蛋白表达水平均较OVX+Veh组升高(P均 < 0.05)。见图 2。
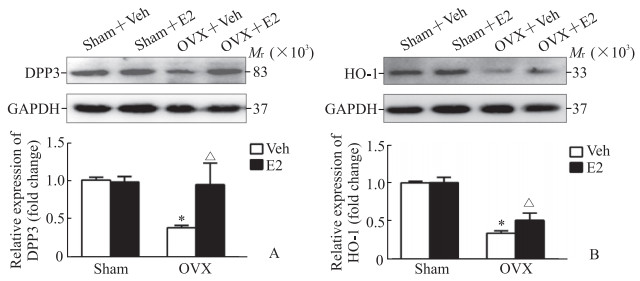
|
图 2 蛋白质印迹法检测各组大鼠心脏组织中DPP3(A)和HO-1(B)蛋白表达 Fig 2 Expression of DPP3 (A) and HO-1 (B) in cardiac tissues of rats in different groups detected by Western blotting *P < 0.05 vs Sham+Veh group; △P < 0.05 vs OVX+Veh group. n=5, x±s. DPP3: Dipeptidyl peptidase 3; HO-1: Heme oxygenase 1; Veh: Vehicle; E2: 17β-estradiol; OVX: Ovariectomy; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. |
3 讨论
随着社会生活节奏加快,女性面临的压力增加,导致卵巢功能早衰;此外,随着妇科肿瘤发病率的增加,接受卵巢切除手术的女性患者与日俱增,这些情况都可导致女性体内雌激素水平急剧下降,给心血管系统带来潜在风险,但是绝经所致的雌激素下降通过何种机制导致女性心脏功能异常尚未明确[12-13]。本研究通过OVX造成雌性大鼠手术绝经,实验结果显示OVX大鼠的心脏质量与体重之比增加,平均动脉压升高,心率加快,衡量左心室舒张功能的指标-dp/dtmax绝对值降低,而补充E2后上述指标的改变程度均有所改善,表明雌激素疗法对OVX大鼠心脏具有一定的保护作用。假手术组大鼠卵巢功能没有损伤,其在生理状态下仍然能够分泌足够的雌激素,因此,假手术组大鼠未观察到因雌激素急剧下降而引起的心脏功能改变,此外,假手术组大鼠外源性补充E2后对其心血管功能也未产生明显影响。
本实验进一步检测了心脏组织中氧化应激相关指标,实验结果显示OVX大鼠心脏组织中活性氧产生增加,细胞脂质氧化程度更明显,抗氧化相关酶SOD和CAT表达均下调,而补充E2可减轻OVX大鼠心脏组织中氧化应激且上调抗氧化相关酶SOD和CAT的表达,提示OVX大鼠心脏舒张功能改变与雌激素下降导致心脏氧化应激水平增加有关。有研究证实外源性补充雌激素可减少心脏组织中过氧化氢和超氧阴离子的产生,从而改善OVX大鼠血流动力学和心率变异性[14]。此外,mRen2. Lewis大鼠OVX后雌激素水平降低可导致心脏舒张功能障碍加重[15]。本研究结果与这些研究结果一致,表明绝经大鼠心脏功能异常可能与雌激素水平急剧下降诱导的氧化应激有关。
目前,已经明确绝经引起雌激素水平急剧下降诱导氧化应激,从而导致心脏功能异常[16];但是,绝经个体雌激素降低后诱导心脏氧化应激的机制并未明确。本研究结果表明,OVX大鼠心脏组织中DPP3和HO-1的蛋白表达水平均降低,而补充E2上调了OVX大鼠心脏中DPP3和HO-1的蛋白表达。HO-1是一种应激反应酶,被各种引起氧化应激的物质、缺氧、高氧、促炎细胞因子所诱导[17]。HO-1作为一种有效的抗氧化剂在体内发挥生物学效应[18],在动物模型中,KEAP1-NRF2-抗氧化反应元件通路已被证明可以通过上调HO-1的表达减轻氧化应激[19]。此外,有研究报道DPP3与NRF2竞争性结合细胞质中KEAP1上的相同位点,从而释放出细胞质中结合的NRF2,增加其核转位并与抗氧化反应元件结合,从而上调HO-1的表达[20]。本研究结果发现E2可诱导HO-1的蛋白质表达,这一结果与表达增加的DPP3蛋白与KEAP1结合从而释放NRF2,最终增加其反应基因(如HO-1)的转录一致。本研究明确了OVX及E2补充治疗后心脏组织中DPP3和HO-1蛋白表达水平的变化,并提出了E2通过上调DPP3表达,经KEAP1-NRF2-抗氧化反应元件通路介导,诱导HO-1蛋白表达,减轻氧化应激,从而改善OVX大鼠心脏功能的可能机制,为进一步明确雌激素改善OVX大鼠心血管功能的机制研究提供了理论依据。
尽管本研究明确了DPP3和HO-1在OVX大鼠心脏中的表达情况变化,以及补充E2对OVX大鼠心脏中DPP3和HO-1表达、氧化应激和心脏功能的影响,但仍存在不足之处:(1)雌激素主要通过与其受体结合而发挥生物学效应,而可与雌激素结合的受体主要有两大类[21],一类为细胞膜受体,如G蛋白偶联雌激素受体[22],主要通过非基因表达途径发挥心血管保护效应。研究报道E2可通过G蛋白偶联雌激素受体介导增强OVX大鼠心脏组织中线粒体的生物学功能,从而减轻氧化应激[23]。还有研究报道激活G蛋白偶联雌激素受体30通过抑制心脏诱导型一氧化氮合酶活性和NO产生,改善OVX的糖尿病雌性大鼠心肌纤维化程度[24]。另一类为细胞内受体,其又分为α-雌激素受体和β-雌激素受体,主要通过基因表达途径发挥心血管保护效应。研究证实E2或α-雌激素受体激动剂逆转了OVX大鼠心肌中活性氧和丙二醛水平的增加及CAT和SOD活性的降低,而β-雌激素受体或G蛋白偶联雌激素受体激动剂则不然[25]。但是本研究中E2调节心脏组织中DPP3和HO-1蛋白表达主要与α-雌激素受体还是β-雌激素受体有关,还是通过G蛋白偶联雌激素受体介导的非基因途径发挥效应需要进一步实验验证。(2)本研究主要针对雌激素调节心脏组织中DPP3蛋白表达对绝经后心血管功能的影响,并未明确E2是通过何种机制调节DPP3蛋白表达,还需要进一步实验进行探索。
研究表明运动训练可通过改善压力感受性反射延缓衰老和减轻OVX引起的心脏和肾脏氧化应激[26];游泳训练也可预防OVX自发性高血压大鼠的冠状动脉内皮功能障碍[27],这些研究结果表明非药物干预可以调节OVX大鼠心血管系统氧化应激。尽管补充E2可上调OVX大鼠心脏中DPP3蛋白表达,但全身给予E2后除了作用于心血管系统发挥保护性作用外,无法排除外源性E2对其他系统的不良效应。此外,目前绝经后女性雌激素替代治疗也存在诸多争议,研究表明绝经后女性补充雌激素可增加肿瘤、脑卒中和肺栓塞等不良事件的风险,并认为绝经后女性补充雌激素的整体疗效弊大于利[28-29]。另一方面,绝经后女性补充雌激素时所使用的剂量也一直难以确定[30]。在未来研究中可以重点关注通过运动训练等非药物干预是否可以上调OVX大鼠心脏组织中DPP3的蛋白表达,以期为绝经后女性心血管疾病防治提供新的靶点和策略。
| [1] |
HICKEY M, MOSS K M, MISHRA G D, KREJANY E O, DOMCHEK S M, WARK J D, et al. What Happens After Menopause? (WHAM): a prospective controlled study of cardiovascular and metabolic risk 12 months after premenopausal risk-reducing bilateral salpingo-oophorectomy[J]. Gynecol Oncol, 2021, 162: 88-96. DOI:10.1016/j.ygyno.2021.04.038 |
| [2] |
BITTNER V. Menopause, age, and cardiovascular risk: a complex relationship[J]. J Am Coll Cardiol, 2009, 54: 2374-2375. DOI:10.1016/j.jacc.2009.10.008 |
| [3] |
PERSKY A M, GREEN P S, STUBLEY L, HOWELL C O, ZAULYANOV L, BRAZEAU G A, et al. Protective effect of estrogens against oxidative damage to heart and skeletal muscle in vivo and in vitro[J]. Proc Soc Exp Biol Med, 2000, 223: 59-66. DOI:10.1046/j.1525-1373.2000.22308.x |
| [4] |
ÖZDEMIR KUMRAL Z N, KOLGAZI M, ÜSTÜNOVA S, KASıMAY ÇAKıR Ö, ÇEVIK Ö D, ENER G, et al. Estrogen receptor agonists alleviate cardiac and renal oxidative injury in rats with renovascular hypertension[J]. Clin Exp Hypertens, 2016, 38: 500-509. DOI:10.3109/10641963.2015.1116550 |
| [5] |
MAAK ŠAFRANKO Ž, SOBOANEC S, ŠARI A, JAJANIN-JOZI N, KRSNIK Ž, ARALICA G, et al. The effect of 17β-estradiol on the expression of dipeptidyl peptidase Ⅲ and heme oxygenase 1 in liver of CBA/H mice[J]. J Endocrinol Investig, 2015, 38: 471-479. DOI:10.1007/s40618-014-0217-z |
| [6] |
RIBON-DEMARS A, PIALOUX V, BOREAU A, MARCOUILLER F, LARIVIÈRE R, BAIRAM A, et al. Protective roles of estradiol against vascular oxidative stress in ovariectomized female rats exposed to normoxia or intermittent hypoxia[J/OL]. Acta Physiol (Oxf), 2019, 225: e13159. DOI: 10.1111/apha.13159.
|
| [7] |
KOMENO M, PANG X L, SHIMIZU A, MOLLA M R, YASUDA-YAMAHARA M, KUME S, et al. Cardio- and reno-protective effects of dipeptidyl peptidase Ⅲ in diabetic mice[J/OL]. J Biol Chem, 2021, 296: 100761. DOI: 10.1016/j.jbc.2021.100761.
|
| [8] |
BLET A, DENIAU B, SANTOS K, VAN LIER D P T, AZIBANI F, WITTEBOLE X, et al. Monitoring circulating dipeptidyl peptidase 3(DPP3) predicts improvement of organ failure and survival in sepsis: a prospective observational multinational study[J/OL]. Crit Care, 2021, 25: 61. DOI: 10.1186/s13054-021-03471-2.
|
| [9] |
SUN W, YU J, KANG Q. Upregulation of heme oxygenase-1 by Brahma-related gene 1 through NRF2 signaling confers protective effect against high glucose-induced oxidative damage of retinal ganglion cells[J/OL]. Eur J Pharmacol, 2020, 875: 173038. DOI: 10.1016/j.ejphar.2020.173038.
|
| [10] |
LU K, ALCIVAR A L, MA J, FOO T K, ZYWEA S, MAHDI A, et al. NRF2 induction supporting breast cancer cell survival is enabled by oxidative stress-induced DPP3-KEAP1 interaction[J]. Cancer Res, 2017, 77: 2881-2892. DOI:10.1158/0008-5472.CAN-16-2204 |
| [11] |
HAO F, GU Y, TAN X, DENG Y, WU Z T, XU M J, et al. Estrogen replacement reduces oxidative stress in the rostral ventrolateral medulla of ovariectomized rats[J/OL]. Oxid Med Cell Longev, 2016, 2016: 2158971. DOI: 10.1155/2016/2158971.
|
| [12] |
HICKEY M, MISHRA G D. Timing and type of menopause and risk of cardiovascular disease[J]. Menopause, 2021, 28: 477-479. DOI:10.1097/GME.0000000000001747 |
| [13] |
WILD R A, HOVEY K M, ANDREWS C, ROBINSON J G, KAUNITZ A M, MANSON J E, et al. Cardiovascular disease (CVD) risk scores, age, or years since menopause to predict cardiovascular disease in the Women's Health Initiative[J]. Menopause, 2021, 28: 610-618. DOI:10.1097/GME.0000000000001753 |
| [14] |
CAMPOS C, CASALI K R, BARALDI D, CONZATTI A, ARAÚJO A S, KHAPER N, et al. Efficacy of a low dose of estrogen on antioxidant defenses and heart rate variability[J/OL]. Oxid Med Cell Longev, 2014, 2014: 218749. DOI: 10.1155/2014/218749.
|
| [15] |
JESSUP J A, ZHANG L, CHEN A F, PRESLEY T D, KIM-SHAPIRO D B, CHAPPELL M C, et al. Neuronal nitric oxide synthase inhibition improves diastolic function and reduces oxidative stress in ovariectomized mRen2.Lewis rats[J]. Menopause, 2011, 18: 698-708. DOI:10.1097/gme.0b013e31820390a2 |
| [16] |
ABBAS S Z, SANGAWAN V, DAS A, PANDEY A K. Assessment of cardiovascular risk in natural and surgical menopause[J]. Indian J Endocrinol Metab, 2018, 22: 223-228. DOI:10.4103/ijem.IJEM_620_17 |
| [17] |
FUNES S C, RIOS M, FERNÁNDEZ-FIERRO A, COVIÁN C, BUENO S M, RIEDEL C A, et al. Naturally derived heme-oxygenase 1 inducers and their therapeutic application to immune-mediated diseases[J/OL]. Front Immunol, 2020, 11: 1467. DOI: 10.3389/fimmu.2020.01467.
|
| [18] |
DORÉ S, TAKAHASHI M, FERRIS C D, ZAKHARY R, HESTER L D, GUASTELLA D, et al. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury[J]. PNAS, 1999, 96: 2445-2450. DOI:10.1073/pnas.96.5.2445 |
| [19] |
LEVONEN A L, HILL B G, KANSANEN E, ZHANG J, DARLEY-USMAR V M. Redox regulation of antioxidants, autophagy, and the response to stress: implications for electrophile therapeutics[J]. Free Radic Biol Med, 2014, 71: 196-207. DOI:10.1016/j.freeradbiomed.2014.03.025 |
| [20] |
MANOLAGAS S C. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis[J]. Endocr Rev, 2010, 31: 266-300. DOI:10.1210/er.2009-0024 |
| [21] |
TAHERI M, SHOOREI H, DINGER M E, GHAFOURI-FARD S. Perspectives on the role of non-coding RNAs in the regulation of expression and function of the estrogen receptor[J/OL]. Cancers, 2020, 12: 2162. DOI: 10.3390/cancers12082162.
|
| [22] |
ROUHIMOGHADAM M, LU A S, SALEM A K, FILARDO E J. Therapeutic perspectives on the modulation of G-protein coupled estrogen receptor, GPER, function[J/OL]. Front Endocrinol (Lausanne), 2020, 11: 591217. DOI: 10.3389/fendo.2020.591217.
|
| [23] |
SBERT-ROIG M, BAUZÁ-THORBRÜGGE M, GALMÉS-PASCUAL B M, CAPLLONCH-AMER G, GARCÍA-PALMER F J, LLADÓ I, et al. GPER mediates the effects of 17β-estradiol in cardiac mitochondrial biogenesis and function[J]. Mol Cell Endocrinol, 2016, 420: 116-124. DOI:10.1016/j.mce.2015.11.027 |
| [24] |
WANG X W, TAN Y Z, XU B, LU L H, ZHAO M G, MA J P, et al. GPR30 attenuates myocardial fibrosis in diabetic ovariectomized female rats: role of iNOS signaling[J]. DNA Cell Biol, 2018, 37: 821-830. DOI:10.1089/dna.2018.4208 |
| [25] |
STEAGALL R J, YAO F R, SHAIKH S R, ABDEL-RAHMAN A A. Estrogen receptor α activation enhances its cell surface localization and improves myocardial redox status in ovariectomized rats[J]. Life Sci, 2017, 182: 41-49. DOI:10.1016/j.lfs.2017.06.005 |
| [26] |
DA SILVA DIAS D, MORAES-SILVA I C, BERNARDES N, DE OLIVEIRA BRITO-MONZANI J, STOYELL-CONTI F F, MACHI J F, et al. Exercise training initiated at old stage of lifespan attenuates aging-and ovariectomy-induced cardiac and renal oxidative stress: role of baroreflex[J/OL]. Exp Gerontol, 2019, 124: 110635. DOI: 10.1016/j.exger.2019.110635.
|
| [27] |
CLAUDIO E R, ALMEIDA S A, MENGAL V, BRASIL G A, SANTUZZI C H, TIRADENTES R V, et al. Swimming training prevents coronary endothelial dysfunction in ovariectomized spontaneously hypertensive rats[J/OL]. Braz J Med Biol Res, 2017, 50: e5495. DOI: 10.1590/1414-431X20165495.
|
| [28] |
ROSSOUW J E, ANDERSON G L, PRENTICE R L, LACROIX A Z, KOOPERBERG C, STEFANICK M L, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial[J]. JAMA, 2002, 288: 321-333. DOI:10.1001/jama.288.3.321 |
| [29] |
KELLER K B, LEMBERG L. Estrogen plus progestin, benefits and risks: the " Women's Health Initiative" trials[J]. Am J Crit Care, 2005, 14: 157-160. DOI:10.4037/ajcc2005.14.2.157 |
| [30] |
PINKERTON J V, LIU J H, SANTORO N F, THURSTON R C, JOFFE H, FAUBION S S, et al. Workshop on normal reference ranges for estradiol in postmenopausal women: commentary from The North American Menopause Society on low-dose vaginal estrogen therapy labeling[J]. Menopause, 2020, 27: 611-613. DOI:10.1097/GME.0000000000001576 |
 2021, Vol. 42
2021, Vol. 42


