2. 同济大学附属同济医院骨科, 上海 200065
2. Department of Orthopaedics, Tongji Hospital, Tongji University, Shanghai 200065, China
神经病理性疼痛(neuropathic pain, NPP)是由躯体感觉神经系统损伤或疾病引起的疼痛,是腰椎间盘突出症、腰椎间孔狭窄的常见并发症,临床主要表现为自发痛、痛觉过敏和痛觉异常[1-5]。重复经颅磁刺激(repetitive transcranial magnetic stimulation, rTMS)是一种无痛、无创、安全性高的治疗方法,近年来被广泛应用于神经科学领域。多项动物实验及临床研究发现,高频rTMS对各种原因导致的NPP(如带状疱疹后疼痛、三叉神经痛、脊髓损伤后患肢痛等)有较好的镇痛效果[6-9],但其如何发挥镇痛作用目前尚无定论。研究显示,神经胶质细胞如卫星胶质细胞和星形胶质细胞的激活及突触可塑性增强在NPP的发病中发挥了重要作用[10-12]。本研究通过建立慢性背根神经节受压(chronic compression of dorsal root ganglion, CCD)致NPP大鼠模型,探讨rTMS对NPP的镇痛效果及对卫星胶质细胞、星形胶质细胞活化、突触可塑性的影响。
1 材料和方法 1.1 实验动物清洁级健康10周龄雄性SD大鼠50只,体重为220~250 g,购自上海杰思捷实验动物有限公司[实验动物生产许可证号:SCXK(沪)2018-0004],自由饮食。
1.2 主要仪器与试剂MagPro X100重复经颅磁刺激仪(丹麦Tonica公司),von Frey纤维丝(美国Stolting公司),兔GAPDH抗体(英国Abcam公司),兔胶质纤维酸性蛋白(glial fibrillary acidic protein, GFAP)抗体(武汉三鹰生物技术有限公司),小鼠突触后致密区95(postsynaptic density-95, PSD-95)抗体(英国Abcam公司),山羊抗兔二抗、山羊抗小鼠二抗(上海碧云天生物技术有限公司),快速封闭液(上海碧云天生物技术有限公司),HRP化学发光试剂盒(美国Millipore公司),IL-18、TNF-α ELISA检测试剂盒(上海西唐生物科技有限公司)。
1.3 实验分组与处理大鼠随机分为3组:假手术+假刺激组、CCD致NPP模型+假刺激组(NPP+假刺激组)、NPP+rTMS组,剔除术前先天存在条件偏爱和痛觉过敏及术后造模失败的大鼠后,每组成功纳入12只。NPP+rTMS组大鼠刺激部位为大脑皮质M1区,上午接受假刺激,下午接受真刺激;假手术+假刺激组和NPP+假刺激组大鼠上午、下午均接受假刺激。
1.4 CCD致NPP大鼠模型建立CCD致NPP大鼠模型的建立采用既往报道的方法[13],即将免受磁场干扰的“ L”型聚乳酸棒置于大鼠左侧L4/5椎间孔。假手术组大鼠仅暴露左侧L4/5椎间孔,不插入“ L”型聚乳酸棒,其他操作与模型组相同。各组大鼠术后均腹腔注射20万U青霉素预防感染,每天1次,连续3 d。
1.5 机械痛阈测量在25 ℃、安静、明亮的环境中进行机械痛阈测试。将大鼠置于底部为金属网格(网孔大小为0.5 cm×0.5 cm)的透明丙烯酸塑料盒内,适应15~30 min,采用“ up-down ”法[14]测试机械痛阈,von Frey纤维丝从0.6 g递增至26.0 g[15]。用von Frey纤维丝尖端垂直刺激大鼠足底第3、4趾间皮肤,大鼠抬足或舔舐被刺激侧记为阳性反应,否则为阴性反应。在出现首次阳性反应后再用“ up-down ”法测试3次或26.0 g刺激时仍为阴性则停止测试。术后第1天,疼痛行为不明显的大鼠将被剔除(6 g无阳性反应)。
1.6 条件性位置偏爱(conditioned place preference, CPP)实验 1.6.1 基础值测定造模前1 d,将大鼠置于有CPP系统的中间室,抽去隔板,让大鼠可在A、B箱和中间室自由探索,A、B箱的唯一不同点为底板纹路不同,其余条件均相同。让大鼠自由活动15 min,剔除存在偏爱(在某一箱中逗留时间>720 s或<180 s)的大鼠。
1.6.2 rTMS干预及训练期在造模后第8~18天,假手术+假刺激组、NPP+假刺激组大鼠上午接受假刺激,与A箱配对半小时;4 h后再次接受假刺激,与B箱配对半小时。NPP+rTMS组大鼠上午接受假刺激,与A箱配对半小时;4 h后接受高频rTMS,与B箱配对半小时。
1.6.3 逗留时间测定在造模后第19天,3组大鼠均不给予真或假rTMS刺激,抽去隔板,让大鼠在A、B箱中自由活动15 min,分别记录大鼠在A、B箱中的逗留时间。假设rTMS可以缓解疼痛,那么在真刺激后被放入B箱配对的大鼠会对B箱产生偏爱,而假刺激后放入A箱配对的大鼠无疼痛缓解,则大鼠不会对A箱产生偏爱。连续训练11 d后,大鼠会形成条件反射。因此当大鼠被放在A、B箱中自由活动时,会选择停留在B箱。反之,若rTMS无镇痛作用,则大鼠不会对B箱产生偏爱,那么大鼠在A、B箱中的停留时间将会比较平均。
1.7 rTMS干预方法CCD致NPP大鼠模型建立后第8天,大鼠被固定在自制固定器(已申请发明专利,专利申请号:202010093329.2)上,使用直径为9 cm的圆形线圈刺激大鼠大脑皮质M1区,刺激频率为20 Hz,刺激强度为35%的机器最大输出强度(最大磁场强度为4.2 T),每串80个脉冲,共1 600个脉冲,每串间隔10 s。假刺激组将线圈与大鼠颅骨垂直,仅让大鼠接受机器发出的声音刺激。造模后第8天开始干预,连续干预11 d。
1.8 蛋白质印迹法检测大鼠背根神经节(dorsal root ganglion, DRG)和脊髓背角中GFAP、PSD-95蛋白的表达在造模后第20天,腹腔注射3%戊巴比妥钠深度麻醉大鼠,眼眶采血后冰上快速取出大鼠左侧L4、L5节段DRG和脊髓背角组织。组织经冰上匀浆裂解、BCA法蛋白定量,金属浴5 min使蛋白变性,80 V恒压电泳,200 mA转膜120 min,加入一抗GFAP抗体(稀释比例1∶1 000)、PSD-95抗体(稀释比例1∶1 000)4 ℃孵育过夜,加入二抗(稀释比例1∶1 000)室温孵育2 h,ECL显色。以GAPDH为内参照,计算GFAP和PSD-95的相对表达量,利用ImageJ软件进行灰度值分析。
1.9 ELISA检测大鼠血清促炎因子IL-18、TNF-α的表达在造模后第20天,各组大鼠用3%戊巴比妥钠腹腔注射深度麻醉后,眼眶采血,4 ℃ 1 503×g离心10 min后收集血清。具体操作按试剂盒说明书进行。
1.10 统计学处理应用SPSS 20.0软件进行统计学分析。数据以x±s表示;各组大鼠的机械痛阈值采用双因素方差分析,自发痛、蛋白质印迹法检测结果及ELISA结果采用单因素方差分析;组间两两比较时,若方差齐选择Tukey检验,若方差不齐选择Dunnett T3检验。检验水准(α)为0.05。
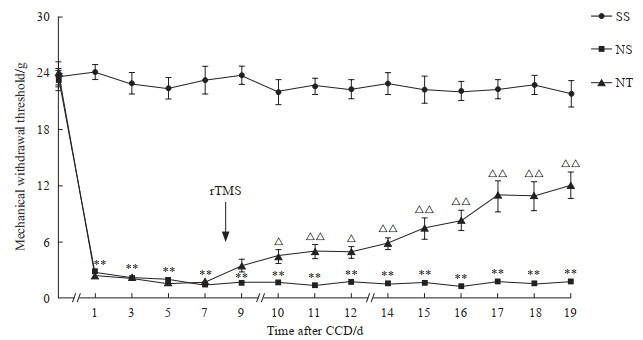
2 结果 2.1 rTMS干预缓解了大鼠CCD造模后的机械痛阈和自发痛NPP+假刺激组大鼠DRG持续受压后机械痛阈值迅速且持续降低,从CCD造模后第1天开始与假手术+假刺激组相比差异均有统计学意义(P均<0.01)。在rTMS干预后第1天,NPP+rTMS组大鼠机械痛阈值略高于NPP+假刺激组,但差异无统计学意义(P>0.05);而从rTMS干预后第2天开始NPP+rTMS组机械痛阈值逐渐上升,与NPP+假刺激组比较差异均有统计学意义(P均<0.05)。见图 1。
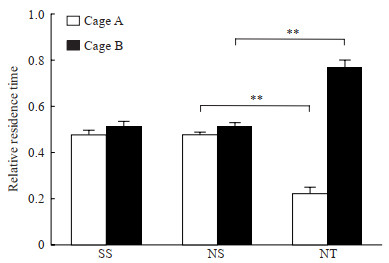
CPP实验结果显示,在经rTMS干预后,NPP+rTMS组大鼠对具备rTMS干预条件的B箱产生了偏爱,在B箱中逗留时间长于NPP+假刺激组(P<0.01),而假手术+假刺激组、NPP+假刺激组大鼠在A、B两箱中的逗留时间差异均无统计意义(P均>0.05)。见图 2。
|
图 1 rTMS干预后大鼠机械痛阈值变化 Fig 1 Changes of mechanical withdrawal threshold of rats after rTMS intervention SS group: Sham operation+sham stimulation group; NS group: NPP+sham stimulation group; NT group: NPP+rTMS group. **P < 0.01 vs SS group; △P < 0.05, △△P < 0.01 vs NS group. n=12, x±s. rTMS: Repetitive transcranial magnetic stimulation; NPP: Neuropathic pain; CCD: Chronic compression of dorsal root ganglion. |
|
图 2 CPP实验检测各组大鼠在A、B两箱中的逗留时间 Fig 2 CPP test detecting stay in cage A or B of rats in each group SS group: Sham operation+sham stimulation group; NS group: NPP+sham stimulation group; NT group: NPP+rTMS group. **P < 0.01. n=12, x±s. CPP: Conditioned place preference; NPP: Neuropathic pain; rTMS: Repetitive transcranial magnetic stimulation. |
|
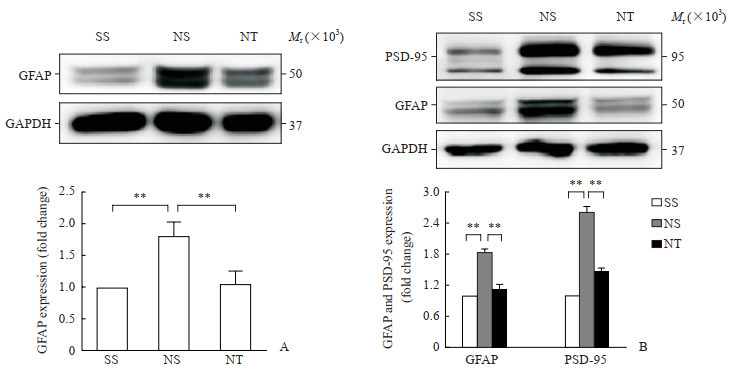
图 3 蛋白质印迹法检测各组大鼠DRG和脊髓背角中GFAP及PSD-95蛋白表达 Fig 3 Protein expression of GFAP and PSD-95 in DRG and spinal cord dorsal horn of rats in each group detected by Western blotting A: Expression of GFAP protein in DRG; B: Expression of GFAP and PSD-95 proteins in spinal cord dorsal horn. SS group: Sham operation+sham stimulation group; NS group: NPP+sham stimulation group; NT group: NPP+rTMS group. **P < 0.01. n=12, x±s. DRG: Dorsal root ganglion; GFAP: Glial fibrillary acidic protein; PSD-95: Postsynaptic density-95; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; NPP: Neuropathic pain; rTMS: Repetitive transcranial magnetic stimulation. |
|
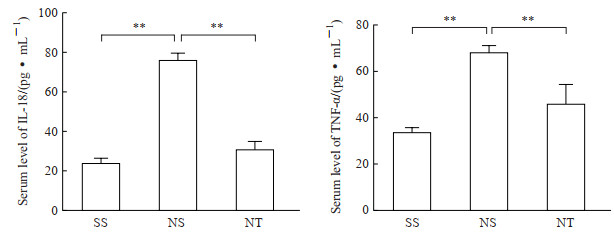
图 4 ELISA检测各组大鼠血清中IL-18、TNF-α表达 Fig 4 Serum levels of IL-18 and TNF-α of rats in each group detected by ELISA SS group: Sham operation+sham stimulation group; NS group: NPP+sham stimulation group; NT group: NPP+rTMS group. **P < 0.01. n=12, x±s. ELISA: Enzyme-linked immunosorbent assay; IL-18: Interleukin 18; TNF-α: Tumor necrosis factor α; NPP: Neuropathic pain; rTMS: Repetitive transcranial magnetic stimulation. |
2.2 rTMS干预抑制大鼠CCD造模后DRG及脊髓背角中GFAP和PSD-95表达
蛋白质印迹法检测结果显示,在大鼠DRG,NPP+假刺激组卫星胶质细胞活化标志物GFAP的蛋白表达水平高于假手术+假刺激组和NPP+rTMS组(P均<0.01);而在大鼠脊髓背角,NPP+假刺激组星形胶质细胞活化标志物GFAP和参与突触连接形成的PSD-95的蛋白表达水平均高于假手术+假刺激组和NPP+rTMS组(P均<0.01)。见图 3。
2.3 rTMS干预降低大鼠血清中促炎因子IL-18、TNF-α水平ELISA检测结果显示,NPP+假刺激组大鼠血清中促炎因子IL-18、TNF-α水平均高于假手术+假刺激组和NPP+rTMS组(P均<0.01)。见图 4。
3 讨论腰椎间盘突出症或腰椎间孔狭窄常压迫腰神经根导致长期的NPP,这是全球范围内引起人们工作能力丧失及活动受限的主要原因之一[16]。NPP机制复杂,常规的治疗方法(如药物)往往效果不佳。对大脑皮质M1区进行高频rTMS是欧洲神经病学学会推荐的治疗NPP的有效方法。国内外多项研究也证明了高频rTMS在NPP中的镇痛效果[6-9]。本研究结果同样显示,高频rTMS缓解了大鼠DRG持续受压引起的NPP。
大部分学者认为外周敏化和中枢敏化是NPP的重要表现,而卫星胶质细胞和星形胶质细胞活化、突触可塑性变化及炎症是外周敏化和中枢敏化的重要原因[17-19]。卫星胶质细胞与星形胶质细胞均为神经胶质细胞,前者位于外周神经系统DRG上,后者则广泛分布在中枢神经系统。但两者在功能上被认为无明显差异,且存在着共同的活化标志物GFAP。神经损伤时,GFAP表达增多,说明神经系统中卫星胶质细胞和星形胶质细胞呈活跃状态。研究表明,抑制卫星胶质细胞和星形胶质细胞活化可以有效缓解NPP[20-21]。
PSD-95是位于中枢神经系统突触后膜的特殊结构,具有参与突触连接形成、维持突触可塑性、参与疼痛调控等生物学功能,在神经元发生、突触长时程增强中发挥重要作用。研究表明,应用PSD-95抑制剂[22]或阻断PSD-95的作用[23]均可延缓NPP进展。
IL-18及TNF-α均为致炎细胞因子,它们不仅可通过调控初级感觉神经元上多种离子通道的表达使神经元兴奋性持续增高,从而导致外周敏化,还可使脊髓背角突触传递效率持续增强而引起中枢敏化[24-25]。
本研究中,建立CCD致NPP大鼠模型后,大鼠DRG中GFAP表达增加,脊髓背角中GFAP和PSD-95蛋白表达均增加,血清中促炎因子IL-18、TNF-α水平增高。而高频rTMS不仅抑制了大鼠DRG及脊髓背角中GFAP的表达,还降低了脊髓背角中PSD-95蛋白的表达及血清中促炎因子IL-18、TNF-α的水平。这些结果表明,高频rTMS可能通过抑制DRG中卫星胶质细胞活化、脊髓背角中星形胶质细胞活化和突触连接形成及改善炎症微环境,有效地缓解外周敏化及中枢敏化,从而改善NPP症状。
| [1] |
SCHOLZ J, FINNERUP N B, ATTAL N, AZIZ Q, BARON R, BENNETT M I, et al. The IASP classification of chronic pain for ICD-11:chronic neuropathic pain[J]. Pain, 2019, 160: 53-59. DOI:10.1097/j.pain.0000000000001365 |
| [2] |
PATEL E A, PERLOFF M D. Radicular pain syndromes: cervical, lumbar, and spinal stenosis[J]. Semin Neurol, 2018, 38: 634-639. DOI:10.1055/s-0038-1673680 |
| [3] |
RAINVILLE J, LOPEZ E. Comparison of radicular symptoms caused by lumbar disc herniation and lumbar spinal stenosis in the elderly[J]. Spine (Phila Pa 1976), 2013, 38: 1282-1287. DOI:10.1097/BRS.0b013e31828f463e |
| [4] |
RICE A S C, SMITH B H, BLYTH F M. Pain and the global burden of disease[J]. Pain, 2016, 157: 791-796. DOI:10.1097/j.pain.0000000000000454 |
| [5] |
FINNERUP N B, ATTAL N, HAROUTOUNIAN S, MCNICOL E, BARON R, DWORKIN R H, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis[J]. Lancet Neurol, 2015, 14: 162-173. DOI:10.1016/S1474-4422(14)70251-0 |
| [6] |
O'CONNELL N E, MARSTON L, SPENCER S, DESOUZA L H, WAND B M. Non-invasive brain stimulation techniques for chronic pain[J/CD]. Cochrane Database Syst Rev, 2018, 4: CD008208.
|
| [7] |
KHEDR E M, KOTB H, KAMEL N F, AHMED M A, SADEK R, ROTHWELL J C. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain[J]. J Neurol Neurosurg Psychiatry, 2005, 76: 833-838. DOI:10.1136/jnnp.2004.055806 |
| [8] |
SUN X, LONG H, ZHAO C, DUAN Q, ZHU H, CHEN C, et al. Analgesia-enhancing effects of repetitive transcranial magnetic stimulation on neuropathic pain after spinal cord injury: an fNIRS study[J]. Restor Neurol Neurosci, 2019, 37: 497-507. |
| [9] |
QUESADA C, POMMIER B, FAUCHON C, BRADLEY C, CRÉAC'H C, MURAT M, et al. New procedure of high-frequency repetitive transcranial magnetic stimulation for central neuropathic pain: a placebo-controlled randomized crossover study[J]. Pain, 2020, 161: 718-728. DOI:10.1097/j.pain.0000000000001760 |
| [10] |
ISHIGURO H, KAITO T, HASHIMOTO K, KUSHIOKA J, OKADA R, TSUKAZAKI H, et al. Administration of ONO-2506 suppresses neuropathic pain after spinal cord injury by inhibition of astrocytic activation[J]. Spine J, 2019, 19: 1434-1442. DOI:10.1016/j.spinee.2019.04.006 |
| [11] |
LIM H, LEE H, NOH K, LEE S J. IKK/NF-κB-dependent satellite glia activation induces spinal cord microglia activation and neuropathic pain after nerve injury[J]. Pain, 2017, 158: 1666-1677. DOI:10.1097/j.pain.0000000000000959 |
| [12] |
D'MELLO R, MARCHAND F, PEZET S, MCMAHON S B, DICKENSON A H. Perturbing PSD-95 interactions with NR2B-subtype receptors attenuates spinal nociceptive plasticity and neuropathic pain[J]. Mol Ther, 2011, 19: 1780-1792. DOI:10.1038/mt.2011.42 |
| [13] |
SONG X J, VIZCARRA C, XU D S, RUPERT R L, WONG Z N. Hyperalgesia and neural excitability following injuries to central and peripheral branches of axons and somata of dorsal root ganglion neurons[J]. J Neurophysiol, 2003, 89: 2185-2193. DOI:10.1152/jn.00802.2002 |
| [14] |
CHAPLAN S R, BACH F W, POGREL J W, CHUNG J M, YAKSH T L. Quantitative assessment of tactile allodynia in the rat paw[J]. J Neurosci Methods, 1994, 53: 55-63. DOI:10.1016/0165-0270(94)90144-9 |
| [15] |
MIAO X R, FAN L C, WU S, MAO Q, LI Z, LUTZ B, et al. DNMT3a contributes to the development and maintenance of bone cancer pain by silencing Kv1.2 expression in spinal cord dorsal horn[J/OL]. Mol Pain, 2017, 13: 1744806917740681. DOI: 10.1177/1744806917740681.
|
| [16] |
LIDGREN L. The bone and joint decade 2000-2010[J]. Bull World Health Organ, 2003, 81: 629. |
| [17] |
FERRARI L F, LOTUFO C M, ARALDI D, RODRIGUES M A, MACEDO L P, FERREIRA S H, et al. Inflammatory sensitization of nociceptors depends on activation of NMDA receptors in DRG satellite cells[J]. Proc Natl Acad Sci USA, 2014, 111: 18363-18368. DOI:10.1073/pnas.1420601111 |
| [18] |
KAWASAKI Y, ZHANG L, CHENG J K, JI R R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord[J]. J Neurosci, 2008, 28: 5189-5194. DOI:10.1523/JNEUROSCI.3338-07.2008 |
| [19] |
TANG Y, LIU L, XU D, ZHANG W, ZHANG Y, ZHOU J, et al. Interaction between astrocytic colony stimulating factor and its receptor on microglia mediates central sensitization and behavioral hypersensitivity in chronic post ischemic pain model[J]. Brain Behav Immun, 2018, 68: 248-260. DOI:10.1016/j.bbi.2017.10.023 |
| [20] |
LEE J Y, CHOI H Y, JU B G, YUNE T Y. Estrogen alleviates neuropathic pain induced after spinal cord injury by inhibiting microglia and astrocyte activation[J]. Biochim Biophys Acta Mol Basis Dis, 2018, 1864: 2472-2480. DOI:10.1016/j.bbadis.2018.04.006 |
| [21] |
OLIVEIRA M E, SANTOS F M, BONIFÁCIO R P, FREITAS M F, MARTINS D O, CHACUR M. Low level laser therapy alters satellite glial cell expression and reverses nociceptive behavior in rats with neuropathic pain[J]. Photochem Photobiol Sci, 2017, 16: 547-554. DOI:10.1039/C6PP00360E |
| [22] |
ANDREASEN J T, NASSER A, CABALLERO-PUNTIVERIO M, SAHLHOLT M, BACH A, GYNTHER M, et al. Effects of the dimeric PSD-95 inhibitor UCCB01-144 in mouse models of pain, cognition and motor function[J]. Eur J Pharmacol, 2016, 780: 166-173. DOI:10.1016/j.ejphar.2016.03.045 |
| [23] |
XU F, ZHAO X, LIU L, SONG J, ZHU Y, CHU S, et al. Perturbing NR2B-PSD-95 interaction relieves neuropathic pain by inactivating CaMKⅡ-CREB signaling[J]. Neuroreport, 2017, 28: 856-863. DOI:10.1097/WNR.0000000000000849 |
| [24] |
李秋月, 许海玉, 杨洪军. 促炎因子TNF-α, IL-1β, IL-6在神经病理性疼痛中的研究进展[J]. 中国中药杂志, 2017, 42: 3709-3712. |
| [25] |
LIU S, LIU Y P, LV Y, YAO J L, YUE D M, ZHANG M Y, et al. IL-18 contributes to bone cancer pain by regulating glia cells and neuron interaction[J]. J Pain, 2018, 19: 186-195. |
 2021, Vol. 42
2021, Vol. 42


