2. 内蒙古医科大学附属医院外科实验室, 呼和浩特 010050;
3. 中国医科大学基础医学院免疫学教研室, 沈阳 110021
2. Laboratory of Surgery, Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010050, Inner Mongolia Autonomous Region, China;
3. Department of Immunology, College of Basic Medical Sciences, China Medical University, Shenyang 110021, Liaoning, China
疟疾是一种严重危害人类健康的虫媒传染病,WHO疟疾报告指出,2017年全球仍有43.5万人死于疟疾[1]。在疟疾的高密度流行区,疟原虫对现有抗疟药物、按蚊对杀虫剂均逐渐产生了抗药性[2-3],耐药疟原虫和抗杀虫剂按蚊的增加与流行增大了疟疾的防控难度[4]。我国自启动《中国消除疟疾行动计划(2010-2020)》以来,疟疾发病率得到了有效控制[5]。2018年,我国全国范围内虽无本地感染疟疾病例,但仍有2 671例境外输入病例[6]。因此,研发安全、有效的疫苗对全球疟疾的防控尤为关键。疟疾疫苗主要分为红前期疫苗、红内期疫苗和传播阻断疫苗(transmission-blocking vaccine, TBV)三大类。红前期疫苗与红内期疫苗可分别降低疟疾的感染率及临床发病率,但两者的候选抗原均暴露于人体的免疫系统,存在广泛的基因多态性[7],有一定的缺陷;TBV针对疟原虫的有性发育阶段发挥作用,可阻止疟原虫在蚊体内的后续发育,彻底切断疟原虫的传播[8-9],被认为是加速疟疾控制和支持最终根除疟疾的有效策略[10]。但TBV的研发进展依然迟缓,迄今为止,已发现的候选抗原非常有限,且现有的TBV候选抗原诱导的特异性抗体尚不能完全阻断疟疾的传播[11]。因此,筛选并增加新型疟原虫有性阶段候选抗原,明确其分子结构、表达阶段、生物学功能及其免疫学特性,将为TBV的研发提供必要的理论依据。
静息巯基氧化酶(quiescin sulfhydryl oxidase, QSOX)是促进生物体内蛋白质二硫键正确形成、促进蛋白质发挥生理活性的关键酶[12-13],其功能是催化二硫键的从头形成并产生过氧化氢。典型的QSOX蛋白由硫氧化还原蛋白1(thioredoxin 1, Trx1)、硫氧化还原蛋白2(thioredoxin 2, Trx2)、ψErv(pseudo-essential for respiration and viability)与Erv/ALR(essential for respiration and viability/augmenter of liver regeneration)结构域组成[14],原核生物(如锥虫)、绿色藻类和高等植物的QSOX部分或完全失去了Trx2结构域[15-16]。近年来,QSOX因在肿瘤组织中过表达并与胰腺癌[17]、心力衰竭[18]、乳腺癌[19]和前列腺肿瘤[20]等疾病的发生密切相关而备受关注。氧化还原相关蛋白的折叠不仅对原生动物的生长调控与生命周期等发挥着至关重要的作用[21],还对宿主与寄生虫间的相互作用[22]、寄生虫所致的病理进程[15]等存在影响。然而,目前关于QSOX在疟原虫属中的表达阶段及其发挥的相关生物学功能、QSOX是否可以成为疟疾防控的新靶位等信息尚不清楚。
本研究应用生物信息学方法选取伯氏疟原虫QSOX(Plasmodium berghei QSOX, PbQSOX),采用基因打靶技术获取了PbQSOX-HA标签型疟原虫,并通过蛋白质印迹法与间接免疫荧光实验(indirect immunofluorescence assay, IFA)观察PbQSOX的主要表达阶段及表达特点,为研究抗疟原虫QSOX抗体的传播阻断潜能及TBV候选抗原的筛选提供新的实验依据。
1 材料和方法 1.1 质粒、疟原虫株及实验动物PbQSOX-HA标签型打靶质粒由英国PlasmoGEM机构提供,伯氏疟原虫(ANKA株)由中国医科大学免疫学教研室惠赠,6~8周龄雌性BALB/c小鼠(体重20~25 g)购自北京维通利华实验动物技术有限公司[实验动物生产许可证号:SCXK(京)2016-0006]。本研究的动物实验经内蒙古医科大学医学与实验动物伦理委员会审批(YKD2017243),小鼠的饲养和免疫遵照内蒙古医科大学动物实验相关规范进行。
1.2 主要试剂高保真PCR酶(PCR-Plus-Neo)购自日本ToYoBo公司;限制性内切酶NotⅠ、DNA分子量标准、蛋白质分子量标准、DNA提取与质粒提取试剂盒、外周血PCR试剂盒均购自日本TaKaRa公司;疟原虫电转液试剂盒NucleofectorⅡ购自瑞士Lonza公司;小鼠抗HA标签单克隆抗体、HRP标记的羊抗鼠IgG与封片剂ProLong® Gold Antifade Mountant购自美国Invitrogen公司;鼠抗伯式疟原虫动合子表面抗原(Plasmodium berghei ookinete surface antigen, Pbs21)单克隆抗体由日本自治医科大学Hiroyuki Matsuoka教授赠送。其余化学试剂均为国产分析纯,购自北京鼎国昌盛生物技术有限责任公司。
1.3 PbQSOX蛋白信号肽、跨膜区及结构域预测运用信号肽分析系统SignalP-5.0 Server(http://www.cbs.dtu.dk/services/SignalP/)分析PbQSOX蛋白的信号肽,运用跨膜区预测软件TMHMM Server 2.0(http://www.cbs.dtu.dk/services/TMHMM/)分析PbQSOX蛋白的跨膜区,应用SMART(http://smart.embl-heidelberg.de/)与BLAST(http://blast.ncbi.nlm.nih.gov/Blast.cgi)在线工具对PbQSOX蛋白的结构域进行预测。
1.4 PbQSOX-HA标签型质粒的获得PbQSOX-HA标签型打靶质粒由英国PlasmoGEM机构提供(ID:PbGEM-325531),质粒的设计原理及构建流程等详细信息见PlasmoGEM官方网站(http://plasmogem.sanger.ac.uk/)。
1.5 PbQSOX-HA标签型打靶质粒的线性化及电转染PbQSOX-HA标签型打靶质粒保存于TSAG宿主菌中,将菌株扩增培养后提取PbQSOX-HA标签质粒,质粒经限制性内切酶NotⅠ在37 ℃下酶切6 h,使其线性化。将线性化的质粒用DNA纯化试剂盒进行纯化。取1×107个培养并纯化的成熟裂殖体至1.5 mL离心管内,低速离心弃上清。将10 μg线性化的PbQSOX-HA标签质粒加入到100 μL疟原虫电转液中,与裂殖体混匀后转至电转杯内,并使用Lonza Nucleofector的U-033程序进行电转染。电转染后将混合液中加入50 μL RPMI 1640培养基,液体混匀后经尾静脉注射至BALB/c小鼠体内。
1.6 PbQSOX-HA标签型疟原虫的筛选、克隆及PCR鉴定小鼠感染经电转染的疟原虫24 h后,给予乙胺嘧啶饮用水(0.07 mg/mL),第6天开始取尾静脉血,通过Giemsa染色观察是否有疟原虫出现,对疟原虫血症达到0.5%的电转染小鼠外周血用PCR方法进行基因型鉴定。利用Primer Premier 5.0软件设计特异性鉴定引物,引物P1(5'-TGAAATGCTAAATGATGTTCCA-3')和P2(5'-AGCCTTTTCGCTGCGGCCAT-3')扩增出的是野生型PbQSOX基因,而引物P1和P3(5'-CATACTAGCCATTTTATGTA-3')扩增出的是发生同源重组的序列。引物由北京六合华大基因科技有限公司合成。PCR反应条件为:94 ℃ 5 min;94 ℃ 30 s,50 ℃ 30 s,68 ℃ 1 min,共30个循环;68 ℃ 10 min。PCR鉴定出重组正确的疟原虫基因型后,在小鼠的疟原虫血症未达3%时,取尾静脉血,用生理盐水将小鼠血液稀释至每100 μL含1~2个伯氏疟原虫感染红细胞(Plasmodium berghei-parasitized red blood cell, PbRBC)。将稀释的疟原虫血液经尾静脉注射至BALB/c小鼠(每只100 μL),至少注射10只小鼠进行克隆。小鼠给予乙胺嘧啶饮用水(0.07 mg/mL),当小鼠外周血中出现耐药型疟原虫株后,感染血液经上述PCR方法再次进行鉴定。将鉴定正确的疟原虫进行保存与传代。
1.7 PbQSOX-HA标签型疟原虫裂殖体、配子体及动合子的培养与纯化(1)裂殖体:雌性BALB/c小鼠经腹腔注射1.2 mg苯肼,3 d后腹腔注射1×107个PbRBC。待疟原虫血症达5%~10%,用乙醚麻醉小鼠后,经心脏采血并将血液置于裂殖体培养基中(按50 mL RPMI 1640培养基和0.5 mL血液的比例配制,培养基中含20% FBS和50 mg/L青霉素与链霉素),在5% CO2、5% O2、90% N2培养箱中36.5 ℃下培养12~16 h。当70%~80%的疟原虫发育到成熟的裂殖体时,培养物经55% Nycodenz密度梯度液离心分离,收集中间灰白层裂殖体,用PBS清洗2次,吉姆萨染色后于显微镜下观察裂殖体。
(2)配子体:用上述同样的方法感染小鼠,感染第4天,给小鼠饮用磺胺嘧啶饮用水(20 mg/L)2 d。用乙醚麻醉小鼠后,心脏采血并将血液置于37 ℃预热的PBS中防止配子体活化。血液经48% Nycodenz密度梯度液1 300×g离心30 min分离,收集中间灰白层配子体,用RPMI 1640培养基清洗2次,吉姆萨染色后于显微镜下观察配子体。
(3)动合子:用上述同样的方法感染小鼠,感染第3天,用乙醚麻醉小鼠后,心脏采血,将1 mL小鼠血液置于9 mL动合子培养液(RPMI 1640培养基,含50 mg/L青霉素、50 mg/L链霉素、100 mg/L新霉素、20% FBS、1 mg/L肝素钠,pH 8.3)中,19~20 ℃培养24 h。培养悬液经62% Nycodenz密度梯度液1 300×g离心30 min分离,收集中间灰白层动合子,用PBS清洗2次,吉姆萨染色后于显微镜下观察动合子。
1.8 蛋白质印迹法检测PbQSOX蛋白表达将纯化的裂殖体、配子体与动合子分别用0.15%的皂角苷-PBS裂解去除红细胞,经裂解液(1% Triton X-100,2% SDS,PBS溶解,含蛋白酶抑制剂)冰上裂解30 min提取疟原虫抗原。取等量的裂殖体、配子体与动合子疟原虫抗原进行10% SDS-PAGE,电泳完毕后转至NC膜。以5%脱脂奶粉溶液4 ℃封闭过夜。TBST漂洗3次,每次5 min。加入TBST稀释的小鼠抗HA标签抗体(稀释比例1∶2 000),室温孵育3 h。洗膜3次,每次5 min。加入TBST稀释的HRP标记的羊抗鼠IgG(稀释比例1∶5 000),室温摇动孵育1 h,TBST洗膜3次。用ECL发光法在荧光图像分析系统中检测结果。
1.9 IFA检测PbQSOX蛋白表达疟原虫经PBS洗1次后均匀涂布在玻片上,用4%多聚甲醛溶液室温固定20 min。用50 mmol/L甘氨酸/PBS漂洗后,加入(或不加)0.1% Triton X-100透膜10 min。用含5%脱脂奶粉的PBS 37 ℃封闭30 min。加入1∶500稀释的小鼠抗HA标签单克隆抗体,37 ℃孵育2 h。以鼠抗Pbs21单克隆抗体(稀释比例1∶500)作为阳性对照。PBS洗3次,加入FITC标记的羊抗鼠IgG(稀释比例1∶500),37 ℃孵育60 min。疟原虫细胞核经DAPI染色5 min,用抗淬灭封片剂封片,于荧光显微镜下观察结果。
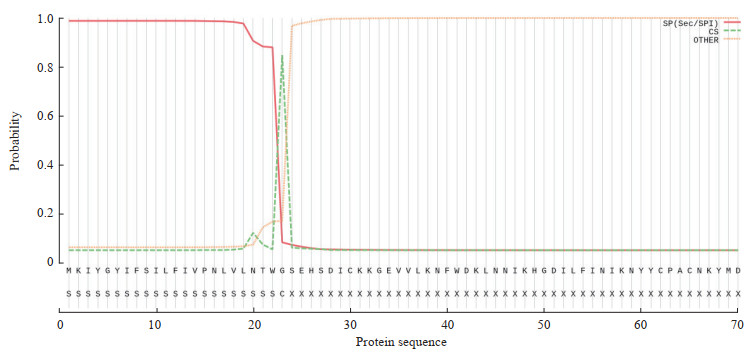
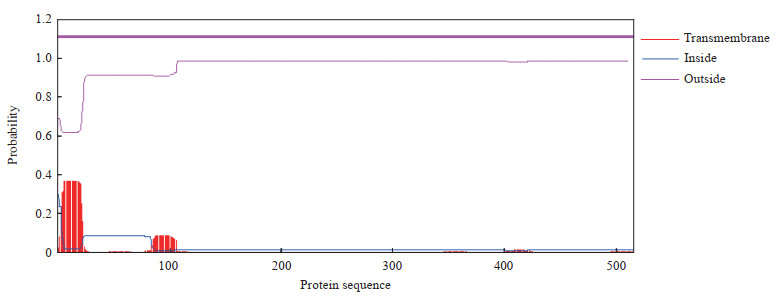
2 结果 2.1 PbQSOX蛋白信号肽、跨膜区及结构域预测PbQSOX在疟原虫数据库PlasmoDB中的登录号为PBANKA_145540,基因定位于伯氏疟原虫14号染色体2 148 296~2 149 846 bp之间,蛋白由516个氨基酸组成(分子量为61 537)。运用信号肽分析系统SignalP-5.0 Server对PbQSOX蛋白的信号肽进行分析,结果显示PbQSOX蛋白含有1个信号肽(图 1);运用跨膜区预测软件TMHMM Server 2.0进行分析,结果显示PbQSOX蛋白无跨膜区,蛋白均在细胞膜外表达(图 2);应用SMART与BLAST在线工具对PbQSOX蛋白的结构域进行预测,结果显示PbQSOX蛋白存在Trx1、ψErv与Erv/ALR结构域,并含3个关键的Cys-X-X-Cys活化基序(X代表任意氨基酸),分别为CPAC、CRNC与CNYC(图 3)。
|
图 1 SignalP-5.0 Server对PbQSOX蛋白的信号肽预测结果 Fig 1 Signal peptide of PbQSOX protein predicted by SignalP-5.0 Server PbQSOX: Plasmodium berghei quiescin sulfhydryl oxidase; SP (Sec/SPI): "Standard" secretory signal peptides transported by the Sec translocon and cleaved by signal peptidase Ⅰ; CS: Cleavage site; OTHER: The probability that the sequence does not have any kind of signal peptide. |
|
图 2 TMHMM Server 2.0对PbQSOX蛋白的跨膜区预测结果 Fig 2 Transmembrane domain of PbQSOX protein predicted by TMHMM Server 2.0 PbQSOX: Plasmodium berghei quiescin sulfhydryl oxidase. |

|
图 3 PbQSOX蛋白的结构域及活化基序分布模式图 Fig 3 Domain and activation motifs of PbQSOX protein The motifs were predicted by SMART and BLAST. Red, blue, green and yellow areas represent the signal peptide (Sig.), Trx1, ψErv and Erv/ALR structure domains, respectively. PbQSOX: Plasmodium berghei quiescin sulfhydryl oxidase; Trx1: Thioredoxin 1; ψErv: Pseudo-essential for respiration and viability; Erv/ALR: Essential for respiration and viability/augmenter of liver regeneration; aa: Amino acid; CPAC, CRNC and CNYC: Three Cys-X-X-Cys dithiol/disulfide motifs. |
2.2 PbQSOX-HA标签型疟原虫株的获取及基因型鉴定
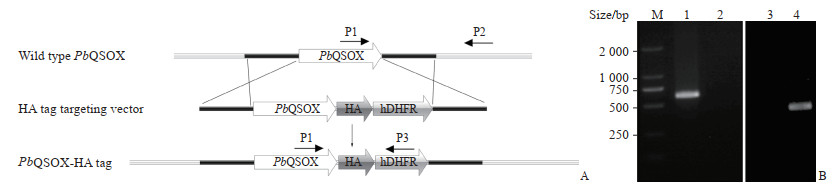
将线性化的PbQSOX-HA标签型打靶质粒电转进入成熟的裂殖体后,经过24 h发育,线性化的质粒与疟原虫基因组进行了同源重组。PbQSOX基因末端加入了HA标签基因,同时基因组也整合了药物压力选择基因人二氢叶酸还原酶(human dihydrofolate reductase, hDHFR)等(图 4A)。经乙胺嘧啶药物选择后,我们获取了耐药型疟原虫。经克隆传代后,PCR结果显示在PbQSOX末端成功加入了HA标签(图 4B)。
|
图 4 PbQSOX-HA标签位点特异性同源重组的PCR鉴定引物模式图及PCR鉴定结果 Fig 4 Schematic representation of PCR identification primers and PCR identification results for PbQSOX-HA tag site-specific homologous recombination A: Schematic representation of PCR identification primers for PbQSOX-HA tag site-specific homologous recombination. Primers P1 and P2 were used for diagnostic PCR of the wild type locus, while primers P1 and P3 were used for confirming PbQSOX-HA tag of parasite. B: PCR identification results. M: DNA marker; 1: Wild type genomic DNA was amplified by primers P1 and P2 (668 bp); 2: Wild type genomic DNA was amplified with primers P1 and P3 (425 bp); 3: PbQSOX-HA tag genomic DNA was amplified with primers P1 and P2 (668 bp); 4: PbQSOX-HA tag genomic DNA was amplified with primers P1 and P3 (425 bp). PbQSOX: Plasmodium berghei quiescin sulfhydryl oxidase; PCR: Polymerase chain reaction; hDHFR: Human dihydrofolate reductase. |
2.3 不同阶段PbQSOX-HA标签型疟原虫的分离纯化
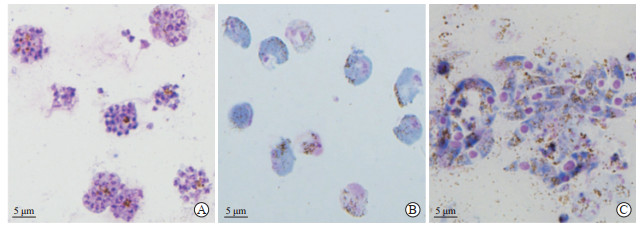
将疟原虫血症达到5%~10%的小鼠血液进行培养,培养后的PbQSOX-HA标签型疟原虫多处于裂殖体阶段,经55% Nycodenz密度梯度液离心纯化后得到了较纯的成熟裂殖体;给予疟原虫感染后的小鼠磺胺嘧啶饮用水处理,小鼠血液经48% Nycodenz密度梯度液离心后得到了纯化的配子体;疟原虫经过24 h培养并经62% Nycodenz密度梯度液离心后,获得了纯化的动合子。分离纯化后的疟原虫见图 5。
|
图 5 纯化后的PbQSOX-HA标签型裂殖体(A)、配子体(B)与动合子(C) Fig 5 Purified schizonts (A), gametocytes (B) and ookinetes (C) of PbQSOX-HA tag parasites Giemsa staining. PbQSOX: Plasmodium berghei quiescin sulfhydryl oxidase. |
2.4 PbQSOX蛋白表达阶段的鉴定
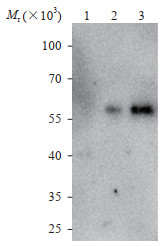
蛋白质印迹法检测结果显示,抗HA标签抗体可以识别配子体与动合子阶段分子量为60 000左右的蛋白,提示PbQSOX在疟原虫发育的配子体与动合子阶段表达(图 6)。
|
图 6 蛋白质印迹法分析PbQSOX蛋白的表达阶段 Fig 6 Western blotting analysis of PbQSOX protein expression stages 1: Schizonts; 2: Gametocytes; 3: Ookinetes. PbQSOX: Plasmodium berghei quiescin sulfhydryl oxidase. |
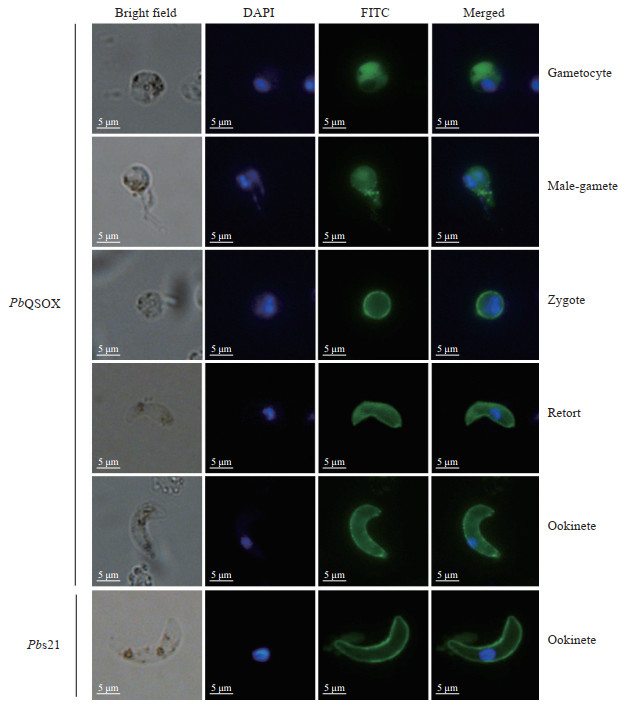
IFA结果显示,无论加入(图片未展示)或不加0.1% Triton X-100(图 7),抗HA标签抗体可识别配子体、雄配子、合子、retort与动合子表面抗原,显示明显的绿色荧光。蛋白质印迹法与IFA的结果一致,提示PbQSOX主要表达在配子体、雄配子、合子、retort与动合子表面。
|
图 7 间接免疫荧光实验分析PbQSOX的表达阶段 Fig 7 Indirect immunofluorescence assay analysis of PbQSOX expression stages Anti-Pbs21 monoclonal antibody served as a positive control. PbQSOX: Plasmodium berghei quiescin sulfhydryl oxidase; Pbs21: Plasmodium berghei ookinete surface antigen; DAPI: 4', 6-diamidino-2-phenylindole; FITC: Fluorescein isothiocyanate. |
3 讨论
QSOX可通过将还原性底物蛋白中的巯基氧化成二硫键来获得有活性的蛋白质,是促进生物体内蛋白质二硫键正确形成、促进蛋白质发挥生理活性的关键酶之一[12]。哺乳动物的QSOX1在不同的细胞中均有表达且功能各异。在胰腺癌和肾癌细胞系中过表达QSOX1可有效减少肿瘤细胞迁移[23]。QSOX1下调后可抑制血管平滑肌细胞的迁移和增殖[24]。在成纤维细胞和间充质干细胞中,QSOX1是一种免疫反应调节剂,可预防肺组织炎症反应和纤维化[25]。QSOX1高表达是乳腺癌预后不良的重要因素[19]。QSOX1对人肺成纤维细胞、豚鼠子宫内膜细胞和大鼠精囊细胞也有重要的调节作用[26]。此外,QSOX可能在朊病毒的形成过程中起一定的作用[27]。QSOX也在各种细胞器内表达,如线粒体、高尔基体、分泌颗粒、细胞核和细胞膜表面等[28]。哺乳动物的QSOX多位于高尔基复合体上,但在静止的成纤维细胞中表达上调并呈分泌模式,可参与细胞外基质组装[29]。此外,QSOX也存在于细胞外,如体外组织培养物[30]、血液和腺体分泌物中[31]。QSOX对寄生虫生长发育的作用及相关酶活性等也相继被报道[15, 22]。但QSOX在疟原虫中的表达阶段、表达特点及生物学功能目前尚不清楚。
随着疟原虫基因序列的相继公布,基因重组技术被广泛应用于疟原虫的基因功能、生物学行为及其与宿主的关系等方面的研究[32]。但将外源DNA导入疟原虫的细胞核内需要穿过4层细胞膜,常导致疟原虫的电转染效率较低。伯氏疟原虫成熟裂殖体中含有12~24个裂殖子,纯化后的裂殖体可以积聚大量的裂殖子,因此应用伯氏疟原虫纯化的成熟裂殖体进行电转染可明显提高基因敲除的效率[33]。同时,我们结合Amaxa公司开发的核转染技术[34],使用Lonza Nucleofector的U-033程序进行电转染,提高了转染效率,并获得了乙胺嘧啶耐药型疟原虫。经PCR鉴定显示,我们成功应用基因重组技术为PbQSOX蛋白加入了HA标签,获取了PbQSOX-HA标签型疟原虫,这为研究QSOX在伯氏疟原虫中的表达阶段及表达特点奠定了良好的实验基础。蛋白质印迹法和IFA检测结果表明,PbQSOX蛋白主要表达于配子体、雄配子、合子、retort与动合子,并不在裂殖体等无性生殖阶段表达;且IFA实验无论是否进行透膜处理,均可见细胞膜表面的绿色荧光,表明PbQSOX在细胞膜表面表达,这与信号肽和跨膜区预测的结果一致。
成为TBV候选抗原的疟原虫关键条件之一是抗原需在有性生殖阶段的疟原虫表面表达[35],PbQSOX的表达特点提示其抗体可能具有传播阻断潜能,使其有望成为TBV候选抗原,这对于TBV疫苗的研发具有一定意义。同时,PbQSOX在配子体、雄配子、合子、retort与动合子多个阶段表达,表明抗PbQSOX抗体可能有能力在多个阶段阻止疟原虫的发育。例如,PbQSOX在配子体与雄配子表面表达,提示抗PbQSOX抗体可能阻碍配子体与配子的发育,干扰雌雄配子的结合,从而降低合子的形成数量;其次,PbQSOX表达于合子与动合子表面,提示抗PbQSOX抗体可影响动合子的成熟。鉴于PbQSOX的表达模式与定位特征,后续可对其生物学功能及其产生的抗体进行传播阻断能力验证,以期深入探讨PbQSOX成为TBV候选抗原的可能性。
| [1] |
World Health Organization. World malaria report 2018[M]. Geneva: WHO Press, 2018: 14-15.
|
| [2] |
刘怀鄂, 苏品璨, 陈熙, 李翠英, 吴艳瑞, 孙乐, 等. 常用抗间日疟药物抗药性机制研究进展[J]. 中国病原生物学杂志, 2018, 13: 1049-1051. |
| [3] |
GATTON M L, CHITNIS N, CHURCHER T, DONNELLY M J, GHANI A C, GODFRAY H C, et al. The importance of mosquito behavioural adaptations to malaria control in Africa[J]. Evolution, 2013, 67: 1218-1230. DOI:10.1111/evo.12063 |
| [4] |
COWMAN A F, HEALER J, MARAPANA D, MARSH K. Malaria: biology and disease[J]. Cell, 2016, 167: 610-624. DOI:10.1016/j.cell.2016.07.055 |
| [5] |
雷正龙, 王立英. 全国重点寄生虫病防治形势与主要任务[J]. 中国寄生虫学与寄生虫病杂志, 2012, 30: 1-5. |
| [6] |
张丽, 丰俊, 张少森, 夏志贵, 周水森. 2018年全国疟疾疫情特征及消除工作进展[J]. 中国寄生虫学与寄生虫病杂志, 2019, 37: 241-247. DOI:10.12140/j.issn.1000-7423.2019.03.001 |
| [7] |
HALL N, KARRAS M, RAINE J D, CARLTON J M, KOOIJ T W, BERRIMAN M, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses[J]. Science, 2005, 307: 82-86. DOI:10.1126/science.1103717 |
| [8] |
malERA Consultative Group on Vaccines. A research agenda for malaria eradication: vaccines[J/OL]. PLoS Med, 2011, 8: e1000398. DOI: 10.1371/journal.pmed.1000398.
|
| [9] |
ALONSO P L, BROWN G, AREVALO-HERRERA M, BINKA F, CHITNIS C, COLLINS F, et al. A research agenda to underpin malaria eradication[J/OL]. PLoS Med, 2011, 8: e1000406. DOI: 10.1371/journal.pmed.1000406.
|
| [10] |
HOFFMAN S L, VEKEMANS J, RICHIE T L, DUFFY P E. The march toward malaria vaccines[J]. Am J Prev Med, 2015, 49(6 Suppl 4): S319-S333. |
| [11] |
TUJU J, KAMUYU G, MURUNGI L M, OSIER F H A. Vaccine candidate discovery for the next generation of malaria vaccines[J]. Immunology, 2017, 152: 195-206. DOI:10.1111/imm.12780 |
| [12] |
FREEDMAN R B, HIRST T R, TUITE M F. Protein disulphide isomerase: building bridges in protein folding[J]. Trends Biochem Sci, 1994, 19: 331-336. DOI:10.1016/0968-0004(94)90072-8 |
| [13] |
KODALI V K, THORPE C. Oxidative protein folding and the quiescin-sulfhydryl oxidase family of flavoproteins[J]. Antioxid Redox Signal, 2010, 13: 1217-1230. DOI:10.1089/ars.2010.3098 |
| [14] |
ALON A, HECKLER E J, THORPE C, FASS D. QSOX contains a pseudo-dimer of functional and degenerate sulfhydryl oxidase domains[J]. FEBS Lett, 2010, 584: 1521-1525. DOI:10.1016/j.febslet.2010.03.001 |
| [15] |
HAQUE S J, MAJUMDAR T, BARIK S. Redox-assisted protein folding systems in eukaryotic parasites[J]. Antioxid Redox Signal, 2012, 17: 674-683. DOI:10.1089/ars.2011.4433 |
| [16] |
LIMOR-WAISBERG K, BEN-DOR S, FASS D. Diversification of quiescin sulfhydryl oxidase in a preserved framework for redox relay[J/OL]. BMC Evol Biol, 2013, 13: 70. DOI: 10.1186/1471-2148-13-70.
|
| [17] |
KATCHMAN B A, ANTWI K, HOSTETTER G, DEMEURE M J, WATANABE A, DECKER G A, et al. Quiescin sulfhydryl oxidase 1 promotes invasion of pancreatic tumor cells mediated by matrix metalloproteinases[J]. Mol Cancer Res, 2011, 9: 1621-1631. DOI:10.1158/1541-7786.MCR-11-0018 |
| [18] |
MEBAZAA A, VANPOUCKE G, THOMAS G, VERLEYSEN K, COHEN-SOLAL A, VANDERHEYDEN M, et al. Unbiased plasma proteomics for novel diagnostic biomarkers in cardiovascular disease: identification of quiescin Q6 as a candidate biomarker of acutely decompensated heart failure[J]. Eur Heart J, 2012, 33: 2317-2324. DOI:10.1093/eurheartj/ehs162 |
| [19] |
POILLET L, PERNODET N, BOYER-GUITTAUT M, ADAMI P, BORG C, JOUVENOT M, et al. QSOX1 inhibits autophagic flux in breast cancer cells[J/OL]. PLoS One, 2014, 9: e86641. DOI: 10.1371/journal.pone.0086641.
|
| [20] |
SONG H, ZHANG B, WATSON M A, HUMPHREY P A, LIM H, MILBRANDT J. Loss of Nkx3.1 leads to the activation of discrete downstream target genes during prostate tumorigenesis[J]. Oncogene, 2009, 28: 3307-3319. DOI:10.1038/onc.2009.181 |
| [21] |
BASU S, LEONARD J C, DESAI N, MAVRIDOU D A, TANG K H, GODDARD A D, et al. Divergence of Erv1-associated mitochondrial import and export pathways in trypanosomes and anaerobic protists[J]. Eukaryot Cell, 2013, 12: 343-355. DOI:10.1128/EC.00304-12 |
| [22] |
TURTURICE B A, LAMM M A, TASCH J J, ZALEWSKI A, KOOISTRA R, SCHROETER E H, et al. Expression of cytosolic peroxiredoxins in Plasmodium berghei ookinetes is regulated by environmental factors in the mosquito bloodmeal[J/OL]. PLoS Pathog, 2013, 9: e1003136. DOI: 10.1371/journal.ppat.1003136.
|
| [23] |
HANAVAN P D, BORGES C R, KATCHMAN B A, FAIGEL D O, HO T H, MA C T, et al. Ebselen inhibits QSOX1 enzymatic activity and suppresses invasion of pancreatic and renal cancer cell lines[J]. Oncotarget, 2015, 6: 18418-18428. DOI:10.18632/oncotarget.4099 |
| [24] |
BORGES B E, APPEL M H, COFRÉ A R, PRADO M L, STECLAN C A, ESNARD F, et al. The flavo-oxidase QSOX1 supports vascular smooth muscle cell migration and proliferation: evidence for a role in neointima growth[J]. Biochim Biophys Acta, 2015, 1852: 1334-1346. DOI:10.1016/j.bbadis.2015.03.002 |
| [25] |
SUN X, ZHENG M, ZHANG M, QIAN M, ZHENG Y, LI M, et al. Differences in the expression of chromosome 1 genes between lung telocytes and other cells: mesenchymal stem cells, fibroblasts, alveolar type Ⅱ cells, airway epithelial cells and lymphocytes[J]. J Cell Mol Med, 2014, 18: 801-810. DOI:10.1111/jcmm.12302 |
| [26] |
WANG T E, LI S H, MINABE S, ANDERSON A L, DUN M D, MAEDA K I, et al. Mouse quiescin sulfhydryl oxidases exhibit distinct epididymal luminal distribution with segment-specific sperm surface associations[J]. Biol Reprod, 2018, 99: 1022-1033. |
| [27] |
ABSKHARON R, DANG J, ELFARASH A, WANG Z, SHEN P, ZOU L S, et al. Soluble polymorphic bank vole prion proteins induced by co-expression of quiescin sulfhydryl oxidase in E. coli and their aggregation behaviors[J/OL]. Microb Cell Fact, 2017, 16: 170. DOI: 10.1186/s12934-017-0782-x.
|
| [28] |
TURY A, MAIRET-COELLO G, PONCET F, JACQUEMARD C, RISOLD P Y, FELLMANN D, et al. QSOX sulfhydryl oxidase in rat adenohypophysis: localization and regulation by estrogens[J]. J Endocrinol, 2004, 183: 353-363. DOI:10.1677/joe.1.05842 |
| [29] |
ILANI T, ALON A, GROSSMAN I, HOROWITZ B, KARTVELISHVILY E, COHEN S R, et al. A secreted disulfide catalyst controls extracellular matrix composition and function[J]. Science, 2013, 341: 74-76. DOI:10.1126/science.1238279 |
| [30] |
COPPOCK D, KOPMAN C, GUDAS J, CINA-POPPE D A. Regulation of the quiescence-induced genes: quiescin Q6, decorin, and ribosomal protein S29[J]. Biochem Biophys Res Commun, 2000, 269: 604-610. DOI:10.1006/bbrc.2000.2324 |
| [31] |
ISRAEL B A, JIANG L, GANNON S A, THORPE C. Disulfide bond generation in mammalian blood serum: detection and purification of quiescin-sulfhydryl oxidase[J]. Free Radic Biol Med, 2014, 69: 129-135. DOI:10.1016/j.freeradbiomed.2014.01.020 |
| [32] |
TONKIN C J, VAN DOOREN G G, SPURCK T P, STRUCK N S, GOOD R T, HANDMAN E, et al. Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method[J]. Mol Biochem Parasitol, 2004, 137: 13-21. DOI:10.1016/j.molbiopara.2004.05.009 |
| [33] |
LEHMANN C, HEITMANN A, MISHRA S, BURDA P C, SINGER M, PRADO M, et al. A cysteine protease inhibitor of Plasmodium berghei is essential for exo-erythrocytic development[J/OL]. PLoS Pathog, 2014, 10: e1004336. DOI: 10.1371/journal.ppat.1004336.
|
| [34] |
JANSE C J, RAMESAR J, WATERS A P. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei[J]. Nat Protoc, 2006, 1: 346-356. DOI:10.1038/nprot.2006.53 |
| [35] |
MIURA K, TAKASHIMA E, DENG B, TULLO G, DIOUF A, MORETZ S E, et al. Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay[J]. Infect Immun, 2013, 81: 4377-4382. DOI:10.1128/IAI.01056-13 |
 2021, Vol. 42
2021, Vol. 42


