胶质瘤是最常见的原发性颅内肿瘤,目前化学治疗的有效率为30%~40%[1]。为了攻克胶质瘤,美国国家癌症研究所和国家人类基因组研究所于2006年通过基因组分析找到了胶质母细胞瘤最重要的3条信号调节通路,即Akt/哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)信号通路、视网膜母细胞瘤信号通路、P53肿瘤抑制通路[1]。mTOR通路抑制剂可与细胞内FK506结合蛋白12(FK506-binding protein 12,FKBP12)结合形成mTOR复合体1(mTOR complex 1,mTORC1),从而抑制mTOR信号通路[2]。mTOR信号通路抑制剂依维莫司已被用于治疗胶质瘤[3],但在临床工作中,依维莫司耐药常见,亟待解决这个问题以延长患者的无病生存期和总生存期[4]。建立以分子分型为基础、联合阻断多条信号通路的治疗体系,有望为更多的脑胶质瘤患者带来新的希望[5]。本研究通过高通量测序和药物敏感试验探讨联合阻断mTOR信号通路及旁路激活通路抑制胶质瘤生长的机制。
1 材料和方法 1.1 挑选胶质瘤细胞系选取5种常用的人胶质瘤细胞系U87、U251、U373、T98、LN229(均由本院神经外科实验室冻存并提供),超声破碎细胞后提取总蛋白,用蛋白质印迹法检测其mTOR蛋白表达水平,重复实验3次,确定后续实验所用细胞系。mTOR和β-actin(内参照)兔抗人单克隆抗体及山羊抗兔抗体均购自美国CST公司。
1.2 mTOR基因敲减细胞模型的建立构建慢病毒FUGW质粒载体[6],然后转染U87和U251细胞构建mTOR基因敲减细胞模型,以未转染的胶质瘤细胞为对照组。FUGW质粒载体均购自汉尹(上海)生物科技有限公司。
1.2.1 设计与合成引物根据美国Sigma公司的干扰靶点信息挑选出1个mTOR基因干扰靶点,序列为5′-CCGTCCCTACATGGATGAAAT-3′(小鼠,mTOR,NM020009)。在3′端加入终止片段TTTTTT,两端分别加入限制性内切酶AgeⅠ和EcoRⅠ的酶切位点黏性末端,中间加入loop环,由生工生物工程(上海)股份有限公司合成2对短发夹RNA(short hairpin RNA,shRNA)进行验证。shRNA引物序列如下:shRNA-1上游引物5′-GA-TCCCCGCAAAGATCTCATGGGCTTTTCAAG-AG-3′,下游引物5′-CTAGAAAAAGCAAAGATC-TCATGGGCTTTCTCTTGAAAAGCCCATGAGAT-CTTTGCGGG-3′;shRNA-2上游引物5′-GATCC-CCCCAGCCAATCATTCGCATTTTCAAGAGAA-ATGCGAATGATTGGCTGGTTTTT-3′,下游引物5′-CTAGAAAAACCAGCCAATCATTCGCATTTCT-CTTGAAAATGCGAATGATTGGCTGGGGG-3′。阳性对照(转染连接产物的感受态细胞)、阴性对照(用水溶液代替质粒DNA进行感受态细胞转染操作)由生工生物工程(上海)股份有限公司提供。
1.2.2 FUGW质粒载体双酶切将含FUGW质粒的大肠埃希菌菌液1.5 mL置于EP管内离心后弃上清液,将菌体沉淀悬浮于150 μL预冷的Tris-EDTA缓冲液内,室温放置10 min。加入200 μL碱裂解液,颠倒数次轻轻混匀,冰上放置5 min。加入150 μL乙酸钾溶液,冰浴放置15 min后离心,将上清液转至另一干净的EP管内。加入等体积的酚/氯仿饱和溶液,将上层水相溶液转移至另一EP管中。加入2倍体积的预冷无水乙醇,于冰上放置10 min后离心,弃去上清液,再加入1 mL预冷的70%乙醇后离心,弃去上清液,将DNA沉淀溶于20 μL Tris-EDTA缓冲液中。之后用相应的限制性内切酶进行双酶切,37 ℃酶切1 h。将酶切产物进行琼脂糖凝胶电泳,胶回收,并测定浓度。
1.2.3 质粒载体与目的片段的连接采用PCR扩增目的片段,并于37 ℃酶切目的基因约3 h。测定载体和目的片段的浓度,并按载体∶目的片段=1∶(3~10)的摩尔比计算载体和目的片段所需的体积。将FUGW质粒载体与目的片段在22 ℃连接60 min。
1.2.4 慢病毒包装与细胞转染效果鉴定取感受态细胞解冻后加入10 μL连接产物,摇匀后在冰中放置30 min,之后放到预加热42 ℃的恒温水浴锅中热激90 s,快速将EP管转移到冰浴中冷却2~3 min。每管加900 μL溶菌肉汤(Luria-Bertani,LB)培养基(无抗生素),37 ℃摇床温育1 h使细菌复苏。取适量的转化菌液涂布于LB琼脂培养基进行抗性培养。挑选10个克隆进行菌液PCR初步鉴定,将初步鉴定为阳性的样品挑选2个克隆送测序公司进行测序鉴定。分别在U87和U251细胞中,对GFP转染效果进行比较。
1.3 U87和U251细胞mTOR基因敲减效果转染48 h后,分别取mTOR基因敲减的U87和U251细胞及对照细胞(相应的mTOR基因未敲减细胞),超声破碎细胞后提取总蛋白,用蛋白质印迹法检测mTOR蛋白表达水平,比较shRNA敲减mTOR基因的效果。重复实验4次。
1.4 高通量测序筛选差异表达基因应用TRIzol法分别提取mTOR基因敲减U87细胞及对照细胞(mTOR基因未敲减U87细胞)的总RNA,并使用DNA酶Ⅰ消化DNA。使用美国安捷伦公司2100型生物分析仪检测RNA的浓度、RNA完整值(RNA integrity number,RIN)、28S/18S和片段长度,以确定RNA的完整性。检测结果达标后,用带有Oligo(dT)的磁珠富集真核生物的mRNA,加入破碎缓冲液将mRNA打断成短片段,以mRNA为模板,用六碱基随机引物合成第1条cDNA链,然后加入缓冲液、dNTP、RNA酶H和DNA聚合酶Ⅰ合成第2条cDNA链,并纯化双链cDNA。再对纯化后的双链cDNA进行末端修复、加A尾并连接测序接头,最后进行PCR扩增。测序文库构建完成后,使用2100型生物分析仪对文库的插入片段范围进行检测,使用美国Applied Biosystems公司的一步实时荧光定量聚合酶链反应仪(StepOnePlus Real-Time PCR System)对文库的浓度进行定量,质检合格后,使用美国Illumina公司GA2000型基因组分析平台进行测序。测序由北京博云华康基因科技有限公司完成。
1.5 药物敏感试验检测信号通路抑制剂的抑制效果U87细胞用含体积分数为10% FBS的DMEM培养基,于37 ℃、体积分数为5% CO2、饱和湿度条件下常规培养,每2 d更换培养基1次,细胞融合度达80%时传代。将处于对数生长期的U87细胞制成1×105/mL的细胞悬液,无药培养24 h后加入100 μmol/L信号转导因子和转录激活因子3(signal transducers and activators of transcription 3,STAT3)通路抑制剂氢氯化物,继续培养30 h左右计算抑制率[7]。
1.6 CCK-8实验检测细胞增殖活性使用CCK-8检测试剂盒(上海语纯生物科技有限公司)检测U87细胞活性。设置空白组(STAT3通路抑制剂氢氯化物、培养基和CCK-8试剂)、阴性对照组(U87细胞、培养基和CCK-8试剂)及mTOR基因未敲减组、mTOR基因敲减组、mTOR基因敲减+氢氯化物作用72 h组,每组设5个复孔。U87细胞以每孔1×105个细胞、100 μL接种于96孔板,孵育6~48 h细胞贴壁后加入10 μL CCK-8试剂,培养1~4 h,待颜色变为橙色时取出96孔板,用酶标仪检测450 nm波长处的光密度(D)值。计算细胞活性:细胞活性(%)=(加药孔D值-空白孔D值)/(对照孔D值-空白孔D值)×100%。
1.7 统计学处理采用SPSS 20.0软件进行统计学分析。计量资料以x±s表示,多组间比较采用单因素方差分析;若方差齐两组间比较采用独立样本t检验,若方差不齐采用非参数秩和检验。检验水准(α)为0.05。
2 结果 2.1 筛选适合mTOR基因敲减的细胞系蛋白质印迹法检测结果(图 1)显示,mTOR在U87和U251细胞中的蛋白表达水平高于U373、T98和LN229细胞(分别为1.05±0.02、0.93±0.01、0.49±0.01、0.39±0.03、0.29±0.01),因而选择U87和U251细胞进行后续实验。
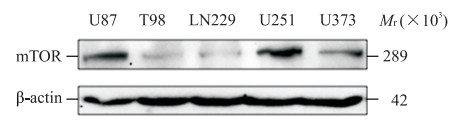
|
图 1 蛋白质印迹法检测5种人胶质瘤细胞系中mTOR的蛋白表达水平 Fig 1 Protein expression of mTOR in 5 human glioma cell lines detected by Western blotting mTOR: Mammalian target of rapamycin. |
2.2 GFP在转染FUGW质粒U87和U251细胞中的表达
明场中U87和U251细胞形态完整,与绿色荧光图相比形态吻合,说明质粒转染成功,所构建的质粒载体结果可靠(图 2)。

|
图 2 绿色荧光蛋白在转染FUGW质粒的人胶质瘤细胞系U87和U251细胞中的表达(200×) Fig 2 Expression of green fluorescent protein in human glioma cell lines U87 and U251 transfected with FUGW plasmid (200×) |
2.3 U87和U251细胞中mTOR基因敲减效果
蛋白质印迹法检测结果(图 3)显示,在U87和U251细胞中,采用shRNA-1和shRNA-2敲减mTOR基因表达后,mTOR蛋白表达水平均较对照组下降(U87细胞:0.32±0.02、0.49±0.01 vs 0.84±0.01;U251细胞:0.56±0.01、0.72±0.01 vs 0.99±0.01),差异均有统计学意义(P均<0.01)。结合镜下观察到的细胞生长情况,最终选择生长状态较好且mTOR基因敲减效果更好的U87细胞进行后续实验。

|
图 3 蛋白质印迹法检测人胶质瘤细胞系U87(A)和U251(B)细胞的mTOR基因敲减效果 Fig 3 Knockdown efficiency of mTOR gene in human glioma cell lines U87 (A) and U251 (B) detected by Western blotting shNC: Normal control cells; shRNA-1/2: The cells that mTOR gene was knocked down by shRNA-1 or shRNA-2. mTOR: Mammalian target of rapamycin; shRNA: Short hairpin RNA |
2.4 差异表达基因的筛选与验证
共选取9个U87细胞样本(U87MG-shRNA-1_1、U87MG-shRNA-1_2、U87MG-shRNA-1_3、U87MG-shRNA-2_1、U87MG-shRNA-2_2、U87MG-shRNA-2_3、U87MG-shNC_1、U87MG-shNC_2、U87MG-shNC_3)进行高通量测序,筛选出24 528个新转录本和1 906个差异表达基因。火山图(图 4)横坐标的数值绝对值越大说明2种样品间的表达倍数差异越大,纵坐标数值越大说明差异表达越显著、筛选得到的差异表达基因越可靠;红色的点代表上调差异表达基因,蓝色的点代表下调差异表达基因,黑色的点代表非差异表达基因。聚类热图(图 5)中各列代表不同的样品,各行代表不同的基因;颜色深浅代表基因在样品中的表达量,即log2(FPKM+1),其中FPKM表示每千个碱基的转录每百万映射读取的片段数(fragments per kilobase of exon model per million mapped fragments,FPKM)。
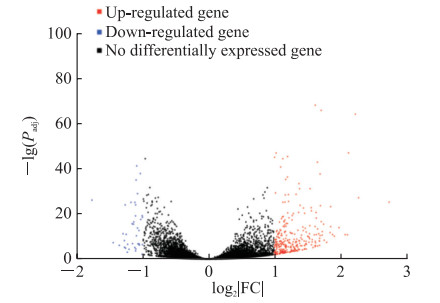
|
图 4 mTOR基因敲减U87细胞中差异表达基因火山图 Fig 4 Volcano map of differentially expressed genes in mTOR-knockdown U87 cells mTOR: Mammalian target of rapamycin; Padj: P value adjusted for multiple testing using Benjamini-Hochberg to estimate the false discovery rate; FC: Fold change. |
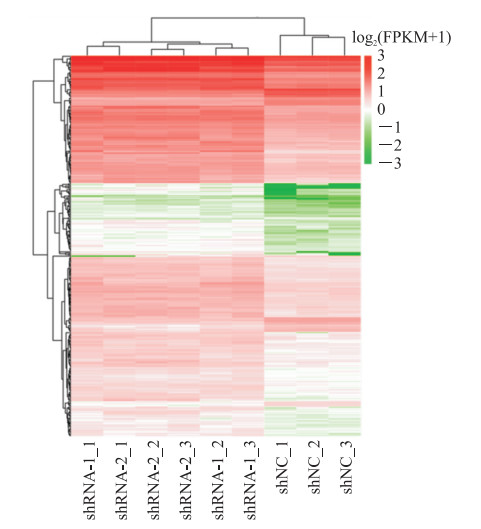
|
图 5 mTOR基因敲减U87细胞中差异表达基因聚类热图 Fig 5 Clustering heatmap of differentially expressed genes in mTOR-knockdown U87 cells shNC: Normal control cells; shRNA-1/2: The cells that mTOR gene was knocked down by shRNA-1 or shRNA-2. _1, _2 and _3 represent different samples. mTOR: Mammalian target of rapamycin; shRNA: Short hairpin RNA; FPKM: Fragments per kilobase of exon model per million mapped fragments. |
qRT-PCR检测结果(图 6)显示,在mTOR基因敲减后缺刻基因(Notch gene,NOTCH)3、活化T细胞核因子c4(nulcear factor of activated T cells c4,NFATC4)、血小板源性生长因子受体β(platelet-derived growth factor receptor beta,PDGFRB)和C-C基序趋化因子配体20(C-C motif chemokine ligand 20,CCL20)的mRNA表达水平均较对照组升高(P均<0.05)。
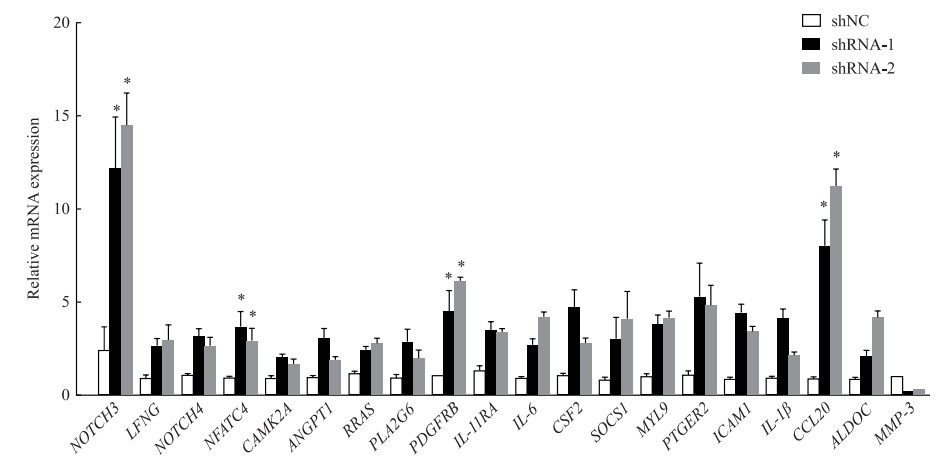
|
图 6 qRT-PCR检测mTOR基因敲减U87细胞中上调基因的mRNA表达水平 Fig 6 mRNA expression of up-regulated genes in mTOR-knockdown U87 cells detected by qRT-PCR shNC: Normal control cells; shRNA-1/2: The cells that mTOR gene was knocked down by shRNA-1 or shRNA-2. *P < 0.05 vs shNC group. n=4, x±s. qRT-PCR: Quantitative real-time polymerase chain reaction; mTOR: Mammalian target of rapamycin; shRNA: Short hairpin RNA; NOTCH3: Notch gene 3; LFNG: Lunatic fringe; NOTCH4: Notch gene 4; NFATC4: Nuclear factor of activated T cells c4; CAMK2A: Calcium/calmodulin-dependent protein kinaseⅡalpha; ANGPT1: Angiopoietin 1; RRAS: Recombinant human related Ras; PLA2G6: Phospholipase A2 group Ⅵ; PDGFRB: Platelet-derived growth factor receptor beta; IL-11RA: Interleukin 11 receptor alpha; IL-6: Interleukin 6; CSF2: Colony-stimulating factor 2; SOCS1: Suppressor of cytokine signaling 1; MYL9: Myosin light chain 9; PTGER2: Prostaglandin E receptor 2; ICAM1: Intercellular cell adhesion molecule 1; IL-1β: Interleukin 1 beta; CCL20: C-C motif chemokine ligand 20; ALDOC: Aldolase C; MMP3: Matrix metalloproteinase 3. |
2.5 旁路激活通路抑制剂的筛选
在高通量测序筛选出的24 528个新转录本和1 906个差异表达基因中,选取log2|FC|[FC为差异倍数(fold change)]排在前12位的mTOR基因敲减U87细胞中表达上调的基因进行通路分析,结果显示它们位于9条旁路激活通路。12个基因是WNT1诱导分泌蛋白2(WNT1-induced secreted protein 2,WISP2)、NOTCH3、CCL20、肿瘤坏死因子α诱导蛋白2(tumor necrosis factor alpha-inducible protein 2,TNFAIP2)、PDGFRB、NFATC4、重组人相关Ras基因(recombinant human related Ras,RRAS)、Epstein-Barr病毒诱导基因3(Epstein-Barr virus induced gene 3,EBI3)、6-磷酸果糖-2-激酶/果糖-2, 6-二磷酸酶4(6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 4,PFKFB4)、N-myc下游调节基因1(N-myc downstream regulation gene 1,NDRG1)、果糖二磷酸醛缩酶C(fructose-bisphosphate aldolase C,ALDOC)、正五聚蛋白3(pentraxin 3,PTX3),log2|FC|分别为2.111、1.689、1.664、1.636、1.609、1.567、1.545、1.484、1.310、1.300、1.267、1.201,分别位于WNT、NOTCH、TNF-α、WNT、Ras、WNT、Ras、Janus激酶(Janus kinase,JAK)/STAT3、MAPK、WNT、缺氧诱导因子1(hypoxia-inducible factor 1,HIF-1)、cAMP旁路激活通路。通过查找文献,共找到45种针对以上9条旁路激活通路的抑制剂,其中HIF-1和cAMP通路上调表达基因的log2|FC|较低,因而选择其他7条通路进行后续实验。7种通路抑制剂的药物敏感试验所得细胞抑制率如表 1所示,在100 μmol/L浓度时,Ras、STAT3和WNT通路抑制剂对mTOR基因敲减U87细胞的抑制率比mTOR基因未敲减细胞高,而MAPK、TNF-α、JAK、NOTCH通路抑制剂的结果则相反。由于STAT3通路抑制剂100 μmol/L浓度的抑制率最高,因而选择STAT3通路抑制剂100 μmol/L进行后续实验。
|
|
表 1 17种通路抑制剂对mTOR基因敲减和未敲减U87细胞的药物敏感试验(抑制率) Tab 1 Sensitivity test of 7 pathway inhibitors in mTOR-knockdown and -unknockdown U87 cells (inhibition rate) |
2.6 mTOR基因敲减联合STAT3通路抑制剂对U87细胞增殖的影响
mTOR基因敲减后1~4 d,U87细胞的增殖活性均下降(P均<0.05),而联合使用STAT3通路抑制剂干预后U87细胞的增殖活性进一步下降(P均<0.05)。见图 7。
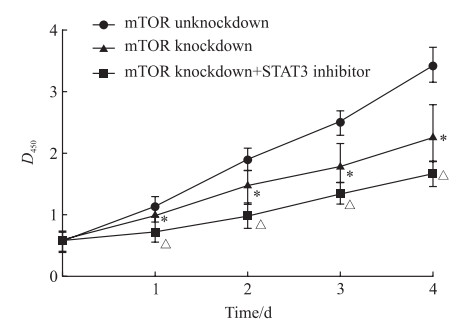
|
图 7 CCK-8实验检测mTOR基因敲减联合STAT3通路抑制剂干预对U87细胞增殖的影响 Fig 7 Effect of mTOR knockdown combined with STAT3 pathway inhibitor intervention on U87 cell proliferation detected by CCK-8 *P < 0.05 vs mTOR unknockdown group at same time point; △P < 0.05 vs mTOR knockdown group at same time point. n=4, x±s. CCK-8: Cell counting kit 8; mTOR: Mammalian target of rapamycin; STAT3: Signal transducers and activators of transcription 3. |
3 讨论
神经胶质瘤具有高度侵袭性,胶质瘤患者的中位生存期不超过3个月,术后辅助放化疗仅能延长生存期至12~16个月[8],其原因很大部分归结于化学治疗的耐药性,也部分因为瘤内基因异质性,即表达1种基因的肿瘤细胞被消除或1条信号转导通路受到抑制时,肿瘤细胞可通过表达其他基因或激活其他信号转导通路继续增殖[9-10]。
本课题组由化学治疗耐药性联想到联合阻断mTOR通路及其旁路激活通路减少耐药性的可行性,查询Clinical Trails官方网站(https://clinicaltrials.gov)中已登记的36项使用mTOR抑制剂依维莫司(或雷帕霉素和西罗莫司)联合其他通路抑制剂治疗胶质瘤的临床试验,如NCT00515086、NCT00387400、NCT00085566、NCT00107237、NCT01434602[11],发现多数取得不错的疗效[12],这些研究从临床角度证实了联合阻断mTOR通路及其旁路激活通路治疗胶质瘤的有效性。
目前尚无关于高通量测序寻找mTOR基因敲减后上调表达基因及信号通路的报道,但有很多学者开展了联合阻断mTOR信号通路及旁路激活通路的基础和临床研究[13-18]。Miyata等[15]研究发现STAT3通路通过壳多糖酶-3样蛋白-1(chitinase-3-like-1 protein,YKL-40)调节下游的mTOR信号通路,进一步联合STAT3通路抑制剂STX-0119和mTOR通路抑制剂雷帕霉素可有效抑制替莫唑胺耐药的胶质瘤细胞增殖。Shi等[16]通过MAPK通路抑制剂维莫非尼联合依维莫司有效延长了1例间变性少突胶质细胞瘤患者1年的预期寿命。Olmez等[17]联合使用美国FDA批准的首个细胞周期蛋白依赖性激酶4/6抑制剂帕博西尼(palbociclib)和依维莫司治疗胶质瘤,发现mTOR被阻断后Ras调节因子ERK会被激活,而帕博西尼可以阻断ERK进而引起胶质瘤细胞凋亡。Asby等[18]联合帕博西尼和mTOR通路抑制剂替西罗莫司成功引起弥漫内生性脑桥胶质瘤细胞发生不可逆的细胞周期停滞。
综上所述,本研究首次通过高通量测序验证联合阻断多条信号通路治疗胶质瘤的可行性,为目前临床上难以解决的耐药性提供了新思路。
| [1] |
HAAR C P, HEBBAR P, WALLACE G C, DAS A, VANDERGRIFT W A, SMITH J A, et al. Drug resistance in glioblastoma: a mini review[J]. Neurochem Res, 2012, 37: 1192-1200. DOI:10.1007/s11064-011-0701-1 |
| [2] |
JHANWAR-UNIYAL M, WAINWRIGHT J V, MOHAN A L, TOBIAS M E, MURALI R, GANDHI C D, et al. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship[J]. Adv Biol Regul, 2019, 72: 51-62. DOI:10.1016/j.jbior.2019.03.003 |
| [3] |
ULLRICH N J, PRABHU S P, REDDY A T, FISHER M J, PACKER R, GOLDMAN S, et al. A phase Ⅱ study of continuous oral mTOR inhibitor everolimus for recurrent, radiographic-progressive neurofibromatosis type 1-associated pediatric low-grade glioma: a Neurofibromatosis Clinical Trials Consortium study[J]. Neuro Oncol, 2020, 22: 1527-1535. DOI:10.1093/neuonc/noaa071 |
| [4] |
HEINZEN D, DIVÉ I, LORENZ N I, LUGER A L, STEINBACH J P, RONELLENFITSCH M W. Second generation mTOR inhibitors as a double-edged sword in malignant glioma treatment[J/OL]. Int J Mol Sci, 2019, 20: 4474. DOI: 10.3390/ijms20184474.
|
| [5] |
ZUREICK A H, MCFADDEN K A, MODY R, KOSCHMANN C. Successful treatment of a TSC2-mutant glioblastoma with everolimus[J/OL]. BMJ Case Rep, 2019, 12: e227734. DOI: 10.1136/bcr-2018-227734.
|
| [6] |
TAI A, FROELICH S, JOO K I, WANG P. Production of lentiviral vectors with enhanced efficiency to target dendritic cells by attenuating mannosidase activity of mammalian cells[J/OL]. J Biol Eng, 2011, 5: 1. DOI: 10.1186/1754-1611-5-1.
|
| [7] |
ASHIZAWA T, AKIYAMA Y, MIYATA H, IIZUKA A, KOMIYAMA M, KUME A, et al. Effect of the STAT3 inhibitor STX-0119 on the proliferation of a temozolomide-resistant glioblastoma cell line[J]. Int J Oncol, 2014, 45: 411-418. DOI:10.3892/ijo.2014.2439 |
| [8] |
CHINNAIYAN P, WON M, WEN P Y, ROJIANI A M, WERNER-WASIK M, SHIH H A, et al. A randomized phase Ⅱ study of everolimus in combination with chemoradiation in newly diagnosed glioblastoma: results of NRG Oncology RTOG 0913[J]. Neuro Oncol, 2018, 20: 666-673. DOI:10.1093/neuonc/nox209 |
| [9] |
SATHE A, CHALAUD G, OPPOLZER I, WONG K Y, VON BUSCH M, SCHMID S C, et al. Parallel PI3K, AKT and mTOR inhibition is required to control feedback loops that limit tumor therapy[J/OL]. PLoS One, 2018, 13: e0190854. DOI: 10.1371/journal.pone.0190854.
|
| [10] |
POORE B, YUAN M, ARNOLD A, PRICE A, ALT J, RUBENS J A, et al. Inhibition of mTORC1 in pediatric low-grade glioma depletes glutathione and therapeutically synergizes with carboplatin[J]. Neuro Oncol, 2019, 21: 252-263. DOI:10.1093/neuonc/noy150 |
| [11] |
FRANK M O, KOYAMA T, RHRISSORRAKRAI K, ROBINE N, UTRO F, EMDE A K, et al. Sequencing and curation strategies for identifying candidate glioblastoma treatments[J/OL]. BMC Med Genomics, 2019, 12: 56. DOI: 10.1186/s12920-019-0500-0.
|
| [12] |
MIKLJA Z, YADAV V N, CARTAXO R T, SIADA R, THOMAS C C, CUMMINGS J R, et al. Everolimus improves the efficacy of dasatinib in PDGFRα-driven glioma[J]. J Clin Invest, 2020, 130: 5313-5325. DOI:10.1172/JCI133310 |
| [13] |
MAHONEY S J, NARAYAN S, MOLZ L, BERSTLER L A, KANG S A, VLASUK G P, et al. A small molecule inhibitor of Rheb selectively targets mTORC1 signaling[J/OL]. Nat Commun, 2018, 9: 548. DOI: 10.1038/s41467-018-03035-z.
|
| [14] |
KALEY T J, PANAGEAS K S, PENTSOVA E I, MELLINGHOFF I K, NOLAN C, GAVRILOVIC I, et al. Phase Ⅰ clinical trial of temsirolimus and perifosine for recurrent glioblastoma[J]. Ann Clin Transl Neurol, 2020, 7: 429-436. DOI:10.1002/acn3.51009 |
| [15] |
MIYATA H, ASHIZAWA T, IIZUKA A, KONDOU R, NONOMURA C, SUGINO T, et al. Combination of a STAT3 inhibitor and an mTOR inhibitor against a temozolomide-resistant glioblastoma cell line[J]. Cancer Genomics Proteomics, 2017, 14: 83-91. DOI:10.21873/cgp.20021 |
| [16] |
SHI L L, ZOU Z W, DING Q, LIU Q, ZHOU H X, HONG X H, et al. Successful treatment of a BRAF V600E-mutant extracranial metastatic anaplastic oligoastrocytoma with vemurafenib and everolimus[J]. Cancer Biol Ther, 2019, 20: 431-434. DOI:10.1080/15384047.2018.1529115 |
| [17] |
OLMEZ I, BRENNEMAN B, XIAO A, SERBULEA V, BENAMAR M, ZHANG Y, et al. Combined CDK4/6 and mTOR inhibition is synergistic against glioblastoma via multiple mechanisms[J]. Clin Cancer Res, 2017, 23: 6958-6968. DOI:10.1158/1078-0432.CCR-17-0803 |
| [18] |
ASBY D J, KILLICK-COLE C L, BOULTER L J, SINGLETON W G, ASBY C A, WYATT M J, et al. Combined use of CDK4/6 and mTOR inhibitors induce synergistic growth arrest of diffuse intrinsic pontine glioma cells via mutual downregulation of mTORC1 activity[J]. Cancer Manag Res, 2018, 10: 3483-3500. DOI:10.2147/CMAR.S167095 |
 2021, Vol. 42
2021, Vol. 42


