组蛋白去乙酰化酶(histone deacetylase,HDAC)是表观遗传调节因子,在染色体的结构修饰和基因表达调控中发挥着关键作用[1]。目前已发现HDAC有4大类18个亚型,Ⅰ类HDAC包括HDAC1、HDAC2、HDAC3、HDAC8,Ⅱ类包括HDAC4、HDAC5、HDAC6、HDAC7、HDAC9、HDAC10,Ⅲ类包括沉默信息调节因子(sirtuin,SIRT)1、SIRT2、SIRT3、SIRT4、SIRT5、SIRT6、SIRT7,Ⅳ类包括HDAC11[2]。在一些疾病中,HDAC过表达促使组蛋白去乙酰化,使组蛋白与DNA间的相互作用增强,从而抑制相关基因转录过程;HDAC抑制剂可通过提高组蛋白乙酰化水平促进基因转录,达到预防和治疗疾病的目的。在抗肿瘤药物开发方面,HDAC已经成为热门靶点,美国FDA已批准多个HDAC抑制剂类药物用于肿瘤治疗,如SAHA(vorinostat)用于治疗罕见皮肤T细胞淋巴瘤,romidepsin、belinostat和panobinostat用于治疗皮肤T细胞淋巴瘤、外周T细胞淋巴瘤,基于苯甲酰胺的Ⅰ类HDAC选择性抑制剂chidamide用于治疗复发性或耐药性外周T细胞淋巴瘤等[3-4]。
目前使用的靶向药物多为单一靶点药物,然而,大多数疾病尤其是肿瘤是多基因、多通路、多步骤发展的疾病,使用单一靶点药物治疗往往疗效欠佳且容易产生耐药性。相较于单药物-单靶点的治疗策略,联合给药可以同时使用多种药物、作用于多个靶点,疗效更好,但对患者的依从性要求较高,用药剂量需要严格控制,治疗较为复杂。多靶点药物是一个药物对应多个靶点,可同时作用于疾病的多条通路,与联合给药相比能够减少给药剂量、提高患者依从性,比单一靶点药物联合使用更为有效[5]。本文以抗阿尔茨海默病、抗真菌、抗肿瘤领域为代表,介绍基于HDAC的双靶点抑制剂的设计策略和研究进展。
1 基于HDAC的双靶点抑制剂的设计策略经典的HDAC抑制剂药效团模型可分为3部分:帽子(cap)部分,通常是一个疏水性和芳香族基团,能与HDAC活性口袋的表面识别区相互作用;Zn2+结合基团(zinc binding group,ZBG),与HDAC活性口袋底部的Zn2+螯合形成复合物,是关键药效团;连接体(linker),由连接ZBG和帽子部分的线性或环状疏水性结构组成(图 1A)。由于帽子部分可变性强,是HDAC抑制剂设计的主要位点,将帽子部分用其他抑制剂的分子骨架(药效团)替换,可以实现双靶点或多靶点抑制(图 1B)。若在HDAC抑制剂上连接作用于其他靶点的疏水性抑制剂,则可增强其与HDAC蛋白的结合,且同时实现对多条通路的抑制,进而发挥协同提高药效的作用[6]。

|
图 1 经典的HDAC抑制剂药效团模型(A)及基于HDAC的双靶点抑制剂设计策略(B) Fig 1 Pharmacophore model of classical HDAC inhibitor (A) and design strategy of dual-target inhibitor based on HDAC (B) HDAC: Histone deacetylase; ZBG: Zinc binding group. |
在基于HDAC的双靶点药物设计中,依据药物与靶点的作用方式,保留与靶点结合的基团,基于药物融合策略将暴露于溶剂区的基团替换,通过连接体与HDAC抑制剂的ZBG结构连接,得到双靶点抑制剂。对基于HDAC的双靶点抑制剂各部分的结构改造会对药效产生影响:帽子部分用不同药效团替换,可提升对相应靶酶的亲和力;连接体部分引入多样性结构,可以改善溶解性,提升成药性;ZBG部分引入不同Zn2+螯合基团,可提升对HDAC靶酶的抑制活性[7]。常见的帽子结构主要有以下6种:(1)激酶抑制剂;(2)细胞毒性化合物;(3)激素受体调节剂;(4)表观遗传调节剂;(5)天然产物;(6)其他抗肿瘤药物或制剂。基于该策略已经获得多个候选药物,并且在临床前研究中显示出很好的治疗效果[8],但目前尚未有药物上市。
2 抗阿尔茨海默病双靶点抑制剂 2.1 磷酸二酯酶5(phosphodiesterase 5,PDE-5)/HDAC双靶点抑制剂HDAC与记忆功能有关。Ⅰ类HDAC,特别是HDAC2,主要存在于细胞核,可抑制对学习和记忆重要的环磷酸腺苷反应元件结合蛋白(cAMP response element-binding protein,CREB)调节基因的转录,并且HDAC1可能具有神经保护作用。HDAC抑制剂使染色质结构松弛,激活转录,促进基因表达,调节突触的生成和突触可塑性,从而促进突触生长并发挥作用[9]。PDE-5抑制剂被认为是阿尔茨海默病的潜在治疗药物,可改善不同阿尔茨海默病动物模型的记忆表现、增强突触可塑性和认知能力,并在临床前研究中取得了令人鼓舞的结果[10]。
基于以上研究,有研究者提出PDE-5/HDAC双靶点抑制剂可能成为治疗阿尔茨海默病的有效策略[11-12]。Garcia-Osta课题组[13]发现了一种新型小分子化合物CM-414(图 2),可作为PDE-5/HDAC双靶点抑制剂,该化合物可缓解APP/PS1小鼠海马长期受损情况;用该化合物治疗阿尔茨海默病转基因模型Tg2576小鼠可防止突触破坏,降低脑β-淀粉样蛋白(amyloid β protein,Aβ)和磷酸化tau蛋白水平,减弱糖原合成酶激酶3β(glycogen synthase kinase 3β,GSK3β)活性,恢复海马体神经元树突棘密度,逆转其认知缺陷,并通过诱导基因表达促进突触传递。

|
图 2 Garcia-Osta课题组[13]发现的抗阿尔茨海默病PDE-5/HDAC双靶点抑制剂 Fig 2 Anti-Alzheimer's disease PDE-5/HDAC dual-target inhibitor discovered by Garcia-Osta, et al[13] PDE-5: Phosphodiesterase 5; HDAC: Histone deacetylase; ZBG: Zinc binding group. |
2.2 GSK3 β/HDAC双靶点抑制剂
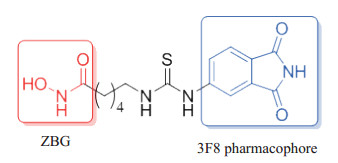
目前研究表明,通过对GSK3β和HDAC的双重抑制,可以发挥对神经的协同保护作用[14]。Milelli课题组[15]以GSK3β抑制剂3F8的邻苯二酰亚胺结构为帽子部分,通过连接体与HDAC6的ZBG连接,得到一个化合物(图 3),该化合物可同时作用于GSK3β和HDAC双靶点,对神经保护起到协同作用。研究结果发现其通过诱导增加组蛋白乙酰化并减少tau磷酸化从而发挥抗阿尔茨海默病作用,还具有促进神经形成和调节免疫的作用;进一步利用野生型斑马鱼模型进行评价,表明化合物无明显毒性,具有低分子量和高溶解度的优点,是有开发前景的候选药物。
|
图 3 Milelli课题组[15]合成的抗阿尔茨海默病GSK3β/HDAC双靶点抑制剂 Fig 3 Anti-Alzheimer's disease GSK3β/HDAC dual-target inhibitor synthesized by Milelli, et al[15] GSK3β: Glycogen synthase kinase 3β; HDAC: Histone deacetylase; ZBG: Zinc binding group. |
3 抗真菌双靶点抑制剂
临床上,白血病患者常因化疗导致抵抗力极度下降,面临真菌感染的风险。Sheng课题组[16]研究了一种新型治疗策略,利用Janus激酶(Janus kinase,JAK)2/HDAC双靶点抑制剂同时治疗白血病和侵袭性真菌感染,证实JAK2/HDAC双靶点抑制剂对血液细胞系有良好的抗增殖活性,并可与氟康唑协同治疗耐药性白念珠菌感染。最终筛选出的化合物(图 4)在异种移植瘤模型中表现出良好的体内抗肿瘤效果,显著延长了白血病小鼠存活时间,减少了白血病细胞向小鼠脾脏的浸润,同时与氟康唑协同治疗可延长被耐药白念珠菌感染小鼠的存活时间。这是首个可用于同时治疗白血病与真菌感染的小分子化合物,具有很好的研究前景。

|
图 4 Sheng课题组[16]合成的抗真菌JAK2/HDAC双靶点抑制剂 Fig 4 Anti-fungal JAK2/HDAC dual-target inhibitor synthesized by Sheng, et al[16] JAK2: Janus kinase 2; HDAC: Histone deacetylase. |
4 抗肿瘤双靶点抑制剂 4.1 肿瘤耐药逆转双靶点抑制剂
铂类药物是治疗实体瘤的主要方法。然而,由于耐药转运蛋白的过表达和DNA修复,肿瘤细胞对铂类药物的耐药性不断增强。Fu课题组[17]发现化合物CUDC-907(图 5)可作为一种新型双靶点抑制剂,可同时抑制HDAC和PI3K的活性。CUDC-907与顺铂联用对顺铂耐药癌细胞具有协同杀伤作用。在对铂类药物耐药的肿瘤细胞中,CUDC-907通过抑制ATP结合盒转运蛋白C亚族2(ATP-binding cassette transporter isoform C2,ABCC2)和DNA修复诱导细胞周期阻滞,细胞中铂类药物积累并且DNA-铂配合物形成增加[18]。上述结果表明CUDC-907可作为肿瘤联合化疗的耐药逆转剂,值得进一步研究。

|
图 5 Fu课题组[17]合成的肿瘤耐药逆转PI3K/HDAC双靶点抑制剂 Fig 5 PI3K/HDAC dual-target inhibitor for tumor resistance reversal synthesized by Fu, et al[17] PI3K: Phosphatidylinositol 3-kinase; HDAC: Histone deacetylase. |
4.2 JAK/HDAC双靶点抑制剂
JAK/信号转导与转录激活因子(signal transducer and activator of transcription,STAT)信号通路参与细胞增殖、细胞存活和细胞凋亡,这一通路是肿瘤靶向药物开发的重要靶点。HDAC抑制剂可降低STAT水平,使其与JAK抑制剂成为协同抑制的理想搭档。Dymock课题组[19]通过药效团融合策略将HDAC抑制剂SAHA与JAK2选择性抑制剂XL019的药效团相连,设计合成了一种同时抑制JAK/STAT通路和HDAC的双靶点抑制剂(图 6A)。该双靶点抑制剂在实体瘤细胞系和血液肿瘤细胞系中均有效,对JAK2和HDAC仍保持原有抑制活性与选择性,具有很好的研究前景。

|
图 6 抗肿瘤JAK/HDAC双靶点抑制剂 Fig 6 Anti-tumor JAK/HDAC dual-target inhibitors A: A JAK/HDAC dual-target inhibitor synthesized by Dymock, et al[19]; B: A JAK/HDAC dual-target inhibitor synthesized by Zhang, et al[20]; C: A JAK/HDAC dual-target inhibitor synthesized by Dymock, et al[21]; D: A JAK/HDAC dual-target inhibitor synthesized by Dymock, et al[22]. JAK: Janus kinase; HDAC: Histone deacetylase; ZBG: Zinc binding group. |
Zhang课题组[20]通过药效团融合策略将JAK抑制剂药效团与HDAC抑制剂的ZBG相连,合成了一系列新的嘧啶-2-氨基吡唑异羟肟酸衍生物类JAK/HDAC双靶点抑制剂,其中一种化合物(图 6B)对JAK2和HDAC6均有较强活性,IC50值在纳摩尔水平。尤其在JAK2 V617F基因突变的HEL细胞(人红白细胞白血病细胞系)中,该化合物比SAHA与ruxolitinib(第1个应用于临床的特异性JAK1/2抑制剂)联合给药组具有更好的抗增殖和促凋亡活性。在小鼠体内药代动力学实验中,该化合物腹腔给药后具有良好的生物利用度;在体外实验中,该化合物显示出良好的抗肿瘤活性,且没有明显毒性。Dymock课题组[21]同样将JAK1/2抑制剂ruxolitinib的药效团与HDAC抑制剂SAHA的药效团拼合,设计合成了JAK/HDAC双靶点抑制剂。通过优化吡唑和异羟肟酸酯之间的连接体,优选出一种化合物(图 6C),其对JAK1和HDAC1、HDAC2、HDAC3、HDAC6、HDAC10的IC50值均小于20 nmol/L,对JAK1和HDAC11的效应剂量小于100 nmol/L,并且对JAK家族均具有较好的选择性。
Dymock课题组[22]将JAK2/FMS样酪氨酸激酶3(FMS-like tyrosine kinase 3,FLT3)抑制剂pacritinib与HDAC抑制剂SAHA的药效团进行融合制备了一类JAK/HDAC双靶点抑制剂,通过结构优化得到一种化合物(图 6D)。该研究结果显示,在急性髓系白血病细胞系HL-60、红白血病细胞系HEL92.1.7和急性T细胞白血病细胞系Jurkat中,该化合物可同时对JAK、HDAC2、HDAC6和HDAC10产生抑制,具有较好的抗肿瘤效果。
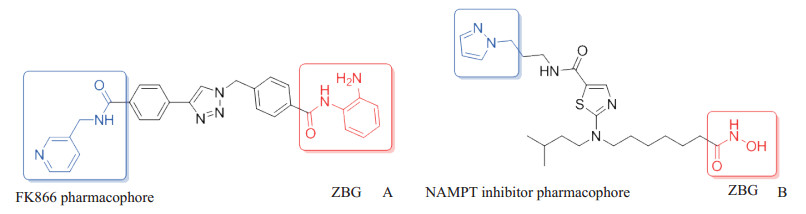
4.3 烟酰胺磷酸核糖转移酶(nicotinamidephosphoribosyl transferase,NAMPT)/HDAC双靶点抑制剂NAMPT和HDAC分别是肿瘤代谢和表观遗传学的2个重要靶点,基于此,Sheng课题组[23]通过药效团融合策略,根据NAMPT和HDAC抑制剂的结构特征,将FK866(NAMPT抑制剂)与SAHA的药效团融合,设计合成了一类可同时抑制NAMPT和HDAC的小分子,而后通过基于迭代结构的药物设计、化学合成和生物测定,优选出一种化合物(图 7A),其对NAMPT(IC50=31 nmol/L)和HDAC1(IC50=55 nmol/L)均具有良好抑制活性,能有效诱导细胞凋亡和自噬,并在HCT116裸鼠移植瘤模型中表现出优异的体内抗肿瘤活性。该研究团队还利用相同策略优选得到另一种化合物(图 7B),其对NAMPT(IC50=15 nmol/L)和HDAC1(IC50=2 nmol/L)的抑制作用更强,在HCT116裸鼠移植瘤模型中表现出比对照药物SAHA和FK866更优秀的抗肿瘤活性[24]。该研究证实设计合成同时对代谢和表观遗传学起作用的双靶点抑制剂是可行的,为研发多靶点抗肿瘤药物提供了有效的策略。
|
图 7 抗肿瘤NAMPT/HDAC双靶点抑制剂 Fig 7 Anti-tumor NAMPT/HDAC dual-target inhibitors A: A NAMPT/HDAC dual-target inhibitor synthesized by Sheng, et al[23]; B: Another NAMPT/HDAC dual-target inhibitor synthesized by Sheng, et al[24]. NAMPT: Nicotinamide phosphoribosyl transferase; HDAC: Histone deacetylase; ZBG: Zinc binding group. |
4.4 DNA/HDAC双靶点抑制剂
HDAC不仅是基因表达中的关键因素,而且在DNA修复中也起关键作用。Yuan课题组[25]将苯丁酸氮芥和泰克地那林(tacedinaline,选择性Ⅰ型HDAC抑制剂)的药效团部分结合,设计合成了一个DNA/HDAC双靶点抑制剂(图 8A)。研究结果表明,该化合物主要抑制HDAC3,可诱导A375肿瘤细胞凋亡并使细胞G2/M期阻滞,在测试的6种肿瘤细胞系中均显示出优秀的抗肿瘤活性,其IC50值为3.1~14.2 μmol/L,比对照药苯丁酸氮芥和泰克地那林更有效。该课题组还设计合成了另一个DNA/HDAC双靶点抑制剂——异羟肟酸苯丁酸氮芥衍生物(图 8B)。该化合物由苯丁酸氮芥和SAHA的核心结构组成,对HDAC1、HDAC2和HDAC6均有抑制活性,并且对HDAC1和HDAC2的抑制程度高于HDAC6。研究结果显示该化合物对肿瘤细胞有较强的抗增殖活性,IC50值为3.2~6.2 µmol/L,其抗增殖活性是苯丁酸氮芥的5.0~18.3倍。该化合物还能够抑制A375细胞集落形成,并有效诱导A375细胞凋亡,将细胞周期阻滞在G2/M期,可以作为抗肿瘤先导物进一步优化[26]。

|
图 8 抗肿瘤DNA/HDAC双靶点抑制剂 Fig 8 Anti-tumor DNA/HDAC dual-target inhibitors A: A DNA/HDAC dual-target inhibitor synthesized by Yuan, et al[25]; B: Another DNA/HDAC dual-target inhibitor synthesized by Yuan, et al[26]. HDAC: Histone deacetylase; ZBG: Zinc binding group. |
4.5 PI3K/HDAC双靶点抑制剂
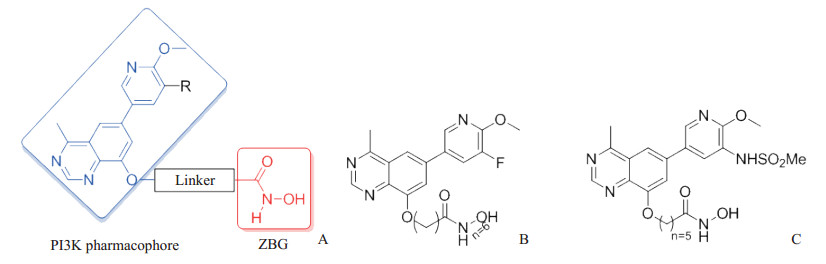
PI3K在肿瘤细胞的生长、繁殖、抑制中发挥着重要作用,抑制PI3K能够抑制肿瘤细胞生长[27-28]。Xu课题组[29]通过将喹唑啉PI3K抑制剂药效团和异羟肟酸HDAC抑制剂药效团进行杂交发现了一系列新型PI3K/HDAC双重抑制剂(图 9A),通过活性测试发现2种化合物(图 9B、9C)对PI3K和HDAC的抑制均在纳摩尔水平,并表现出广谱的抗肿瘤活性;在HCT116和HGC-27异种移植瘤体内抗肿瘤活性的实验中发现,其中一种化合物(图 9B)表现出良好体内抗肿瘤活性。
|
图 9 Xu课题组[29]设计合成的抗肿瘤PI3K/HDAC双靶点抑制剂 Fig 9 Anti-tumor PI3K/HDAC dual-target inhibitors synthesized by Xu, et al[29] A: Design of PI3K/HDAC dual-target inhibitors; B, C: Two PI3K/HDAC dual-target inhibitors. PI3K: Phosphatidylinositol 3-kinase; HDAC: Histone deacetylase; ZBG: Zinc binding group. |
Wang课题组[30]证明PI3K/HDAC双靶点抑制剂CUDC-907可诱导肿瘤细胞弥漫性大B细胞淋巴瘤细胞凋亡。致癌基因Myc的上调是人类肿瘤中常见的驱动因素,已知PI3K和Myc协同促进多种B细胞淋巴瘤的存活与生长。研究表明,抑制Myc的上游调节因子,如HDAC和PI3K,可以降低Myc蛋白水平并抑制Myc驱动的肿瘤生长。经化合物CUDC-907处理后,PI3K下游靶标包括AKT、PRAS40、S6和4EBP1的磷酸化程度降低,组蛋白乙酰化增加,Myc蛋白水平降低。体内实验证实化合物CUDC-907对多种Myc驱动的肿瘤类型具有抗肿瘤活性,且无明显毒副作用。研究结果表明,双靶点HDAC和PI3K抑制剂化合物CUDC-907是靶向Myc的有效药物,为开发Myc依赖性肿瘤的抗肿瘤药物提供了有效策略[31]。
4.6 拓扑异构酶Ⅰ(topoisomeraseⅠ,TopoⅠ)/HDAC双靶点抑制剂已有研究证实,TopoⅠ抑制剂和HDAC抑制剂同时作用于肿瘤细胞时具有协同抗肿瘤作用[32]。基于此,Dallavalle课题组[33]设计了新型双靶点抑制剂,同时靶向抑制TopoⅠ和HDAC。研究者将喜树碱和SAHA药效团结合,进行结构优化,实验结果表明喜树碱A环存在酚羟基且连接链长度合适(n=5)时,得到的化合物(图 10)在血液肿瘤和间皮瘤细胞系中表现出广谱的抗肿瘤活性,IC50值在纳摩尔水平。该TopoⅠ/HDAC双靶点抑制剂显示出了比HDAC抑制剂SAHA和TopoⅠ抑制剂伊立替康更强的活性,并表现出良好的耐受性和低毒性。这种双靶点抑制剂不仅可以同时从2种不同的途径攻击肿瘤细胞,而且可以在不增加伊立替康剂量的情况下提高疗效。因此,可以用于治疗喜树碱类药物或HDAC抑制剂易感的肿瘤。

|
图 10 Dallavalle课题组[33]合成的抗肿瘤TopoⅠ/HDAC双靶点抑制剂 Fig 10 Anti-tumor TopoⅠ/HDAC dual-target inhibitor synthesized by Dallavalle, et al[33] TopoⅠ: TopoisomeraseⅠ; HDAC: Histone deacetylase. |
4.7 哺乳动物雷帕霉素靶蛋白(mammalian
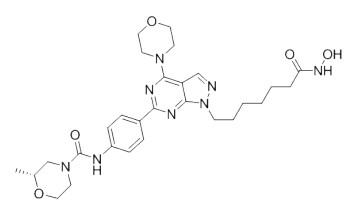
target of rapamycin,mTOR)/HDAC双靶点抑制剂mTOR是细胞增殖和生长的重要因子,研究表明mTOR信号转导途径与细胞增殖密切相关,HDAC抑制剂与PI3K/mTOR抑制剂联合使用可提高肿瘤治疗效果[34-35]。基于此,Chen课题组[36]使用mTOR抑制剂的嘧啶-吡唑基药效团,加上HDAC帽子基团和作为ZBG的异羟肟酸,合成了一系列新型mTOR/HDAC双靶点抑制剂。其中,最优的先导化合物(图 11)对mTOR和HDAC1均具有强效抑制活性,IC50值分别为1.2 nmol/L和0.19 nmol/L。该化合物可阻滞肿瘤细胞周期于G0/G1期并诱导其凋亡,在MM1S骨髓瘤细胞异种移植瘤模型中可抑制肿瘤生长,但生物利用度相对较差。上述结果表明,该化合物有望开发成为治疗血液系统恶性肿瘤的双靶点抑制剂。
|
图 11 Chen课题组[36]合成的抗肿瘤mTOR/HDAC双靶点抑制剂 Fig 11 Anti-tumor mTOR/HDAC dual-target inhibitor synthesized by Chen, et al[36] mTOR: Mammalian target of rapamycin; HDAC: Histone deacetylase. |
4.8 其他双靶点抑制剂
除了上述提到的抗肿瘤双靶点抑制剂外,基于HDAC设计的抗肿瘤双靶点抑制剂还有很多,设计方法多为将作用在2个靶点的抑制剂药效团融合为一个化合物,如Ras/HDAC双靶点抑制剂(图 12A)[37]、吲哚胺2, 3-双加氧酶1(indoleamine 2, 3-dioxygenase 1,IDO1)/HDAC双靶点抑制剂(图 12B)[38]、血管内皮生长因子受体(vascular endothelial growth factor receptor,VEGFR)/HDAC双靶点抑制剂(图 12C)[39]、c-Met/HDAC双靶点抑制剂(图 12D)[40]、Bcl-2/HDAC双靶点抑制剂(图 12E)[41]、鼠双微体2(murine double minute 2,MDM2)/HDAC双靶点抑制剂(图 12F)[42]等,这些双靶点抑制剂均表现出较好的靶点抑制作用和抗肿瘤活性,有一定的开发价值。

|
图 12 其他基于HDAC设计的抗肿瘤双靶点抑制剂 Fig 12 Other anti-tumor dual-target inhibitors based on HDAC A: Ras/HDAC dual-target inhibitor[37]; B: Indoleamine 2, 3-dioxygenase 1/HDAC dual-target inhibitor[38]; C: Vascular endothelial growth factor receptor/HDAC dual-target inhibitor[39]; D: c-Met/HDAC dual-target inhibitor[40]; E: Bcl-2/HDAC dual-target inhibitor[41]; F: Murine double minute/HDAC dual-target inhibitor[42]. HDAC: Histone deacetylase. |
5 结语
为了克服单靶点药物缺陷,防止对治疗产生耐药性并增强协同效应,同时抑制2种或更多种靶标的药物比单一靶点药物具有更多优势。在HDAC表面疏水区域,连接相关分子药效团,设计双靶点抑制剂,对多条路径同时产生抑制作用,是增强药效的有效策略,并在多种疾病治疗中表现出良好的应用前景。
HDAC是开发药物的热门靶点,最近的研究进展发现HDAC抑制剂还可以靶向其他疾病,如神经疾病、糖尿病及其相关并发症、病毒性疾病和感染、心血管疾病、炎症和免疫调节障碍等[6]。虽然现有基于HDAC的双靶点抑制剂普遍显示出优秀的体外活性,但也存在体内活性欠佳、HDAC亚型选择性不佳等缺陷,多数仍处于临床研究阶段,尚未有基于HDAC的双靶点抑制剂上市。为避免毒性和不良反应,针对各亚型的HDAC选择性设计双靶点抑制剂将是下一步药物研发的重点。开发基于HDAC的双靶点抑制剂仍充满挑战,需要结合HDAC抑制剂发挥作用的生物学途径,减少药物融合后的毒性和不良反应,并改善药代动力学性质,最终实现HDAC双靶点抑制剂“1+1>2”的生物协同增效作用。
| [1] |
NEGMELDIN A T, PADIGE G, BIELIAUSKAS A V, PFLUM M K H. Structural requirements of HDAC inhibitors: SAHA analogues modified at the C2 position display HDAC6/8 selectivity[J]. ACS Med Chem Lett, 2017, 8: 281-286. DOI:10.1021/acsmedchemlett.6b00124 |
| [2] |
RAMIREZ-MEJIA G, GIL-LIEVANA E, URREGO-MORALES O, SOTO-REYES E, BERMÚDEZ-RATTONI F. Class Ⅰ HDAC inhibition improves object recognition memory consolidation through BDNF/TrkB pathway in a time-dependent manner[J/OL]. Neuropharmacology, 2021, 187: 108493. DOI: 10.1016/j.neuropharm.2021.108493.
|
| [3] |
STENZEL K, HAMACHER A, HANSEN F K, GERTZEN C G W, SENGER J, MARQUARDT V, et al. Alkoxyurea-based histone deacetylase inhibitors increase cisplatin potency in chemoresistant cancer cell lines[J]. J Med Chem, 2017, 60: 5334-5348. DOI:10.1021/acs.jmedchem.6b01538 |
| [4] |
LUAN Y P, LI J, BERNATCHEZ J A, LI R S. Kinase and histone deacetylase hybrid inhibitors for cancer therapy[J]. J Med Chem, 2019, 62: 3171-3183. DOI:10.1021/acs.jmedchem.8b00189 |
| [5] |
DUAN Y C, JIN L F, REN H M, ZHANG S J, LIU Y J, XU Y T, et al. Design, synthesis, and biological evaluation of novel dual inhibitors targeting lysine specific demethylase 1(LSD1) and histone deacetylases (HDAC) for treatment of gastric cancer[J/OL]. Eur J Med Chem, 2021, 220: 113453. DOI: 10.1016/j.ejmech.2021.113453.
|
| [6] |
VAIDYA G N, RANA P, VENKATESH A, CHATTERJEE D R, CONTRACTOR D, SATPUTE D P, et al. Paradigm shift of "classical" HDAC inhibitors to "hybrid" HDAC inhibitors in therapeutic interventions[J/OL]. Eur J Med Chem, 2021, 209: 112844. DOI: 10.1016/j.ejmech.2020.112844.
|
| [7] |
BIERSACK B, POLAT S, HÖPFNER M. Anticancer properties of chimeric HDAC and kinase inhibitors[J/OL]. Semin Cancer Biol, 2020: S1044-S579X(20)30223-6. DOI: 10.1016/j.semcancer.2020.11.005.
|
| [8] |
RAMAIAH M J, TANGUTUR A D, MANYAM R R. Epigenetic modulation and understanding of HDAC inhibitors in cancer therapy[J/OL]. Life Sci, 2021, 277: 119504. DOI: 10.1016/j.lfs.2021.
|
| [9] |
FULLER N O, PIRONE A, LYNCH B A, HEWITT M C, QUINTON M S, MCKEE T D, et al. CoREST complex-selective histone deacetylase inhibitors show prosynaptic effects and an improved safety profile to enable treatment of synaptopathies[J]. ACS Chem Neurosci, 2019, 10: 1729-1743. DOI:10.1021/acschemneuro.8b00620 |
| [10] |
LIU L, XU H, DING S M, WANG D Y, SONG G Q, HUANG X F. Phosphodiesterase 5 inhibitors as novel agents for the treatment of Alzheimer's disease[J]. Brain Res Bull, 2019, 153: 223-231. DOI:10.1016/j.brainresbull.2019.09.001 |
| [11] |
SÁNCHEZ-ARIAS J A, RABAL O, CUADRADO-TEJEDOR M, DE MIGUEL I, PÉREZ-GONZÁLEZ M, UGARTE A, et al. Impact of scaffold exploration on novel dual-acting histone deacetylases and phosphodiesterase 5 inhibitors for the treatment of Alzheimer's disease[J]. ACS Chem Neurosci, 2017, 8: 638-661. DOI:10.1021/acschemneuro.6b00370 |
| [12] |
RABAL O, SÁNCHEZ-ARIAS J A, CUADRADO-TEJEDOR M, DE MIGUEL I, PÉREZ-GONZÁLEZ M, GARCÍA-BARROSO C, et al. Discovery of in vivo chemical probes for treating Alzheimer's disease: dual phosphodiesterase 5(PDE5) and classⅠhistone deacetylase selective inhibitors[J]. ACS Chem Neurosci, 2019, 10: 1765-1782. DOI:10.1021/acschemneuro.8b00648 |
| [13] |
CUADRADO-TEJEDOR M, GARCIA-BARROSO C, SÁNCHEZ-ARIAS J A, RABAL O, PÉREZ-GONZÁLEZ M, MEDEROS S, et al. A first-in-class small-molecule that acts as a dual inhibitor of HDAC and PDE5 and that rescues hippocampal synaptic impairment in Alzheimer's disease mice[J]. Neuropsychopharmacology, 2017, 42: 524-539. DOI:10.1038/npp.2016.163 |
| [14] |
SHARMA S, TALIYAN R. Synergistic effects of GSK-3β and HDAC inhibitors in intracerebroventricular streptozotocin-induced cognitive deficits in rats[J]. Naunyn Schmiedebergs Arch Pharmacol, 2015, 388: 337-349. DOI:10.1007/s00210-014-1081-2 |
| [15] |
DE SIMONE A, LA PIETRA V, BETARI N, PETRAGNANI N, CONTE M, DANIELE S, et al. Discovery of the first-in-class GSK-3β/HDAC dual inhibitor as disease-modifying agent to combat Alzheimer's disease[J]. ACS Med Chem Lett, 2019, 10: 469-474. DOI:10.1021/acsmedchemlett.8b00507 |
| [16] |
HUANG Y H, DONG G Q, LI H Q, LIU N, ZHANG W N, SHENG C Q. Discovery of Janus kinase 2(JAK2) and histone deacetylase (HDAC) dual inhibitors as a novel strategy for the combinational treatment of leukemia and invasive fungal infections[J]. J Med Chem, 2018, 61: 6056-6074. DOI:10.1021/acs.jmedchem.8b00393 |
| [17] |
WU C P, HSIEH Y J, HSIAO S H, SU C Y, LI Y Q, HUANG Y H, et al. Human ATP-binding cassette transporter ABCG2 confers resistance to CUDC-907, a dual inhibitor of histone deacetylase and phosphatidylinositol 3-kinase[J]. Mol Pharm, 2016, 13: 784-794. DOI:10.1021/acs.molpharmaceut.5b00687 |
| [18] |
TO K K W, FU L W. CUDC-907, a dual HDAC and PI3K inhibitor, reverses platinum drug resistance[J]. Invest New Drugs, 2018, 36: 10-19. DOI:10.1007/s10637-017-0501-9 |
| [19] |
CHU-FARSEEVA Y Y, MUSTAFA N, POULSEN A, TAN E C, YEN J J Y, CHNG W J, et al. Design and synthesis of potent dual inhibitors of JAK2 and HDAC based on fusing the pharmacophores of XL019 and vorinostat[J]. Eur J Med Chem, 2018, 158: 593-619. DOI:10.1016/j.ejmech.2018.09.024 |
| [20] |
LIANG X W, ZANG J, LI X Y, TANG S, HUANG M, GENG M Y, et al. Discovery of novel Janus kinase (JAK) and histone deacetylase (HDAC) dual inhibitors for the treatment of hematological malignancies[J]. J Med Chem, 2019, 62: 3898-3923. DOI:10.1021/acs.jmedchem.8b01597 |
| [21] |
YAO L B, MUSTAFA N, TAN E C, POULSEN A, SINGH P, DUONG-THI M D, et al. Design and synthesis of ligand efficient dual inhibitors of Janus kinase (JAK) and histone deacetylase (HDAC) based on ruxolitinib and vorinostat[J]. J Med Chem, 2017, 60: 8336-8357. DOI:10.1021/acs.jmedchem.7b00678 |
| [22] |
YANG E G, MUSTAFA N, TAN E C, POULSEN A, RAMANUJULU P M, CHNG W J, et al. Design and synthesis of Janus kinase 2(JAK2) and histone deacetlyase (HDAC) bispecific inhibitors based on pacritinib and evidence of dual pathway inhibition in hematological cell lines[J]. J Med Chem, 2016, 59: 8233-8262. DOI:10.1021/acs.jmedchem.6b00157 |
| [23] |
DONG G, CHEN W, WANG X, YANG X, XU T, WANG P, et al. Small molecule inhibitors simultaneously targeting cancer metabolism and epigenetics: discovery of novel nicotinamide phosphoribosyltransferase (NAMPT) and histone deacetylase (HDAC) dual inhibitors[J]. J Med Chem, 2017, 60: 7965-7983. DOI:10.1021/acs.jmedchem.7b00467 |
| [24] |
CHEN W, DONG G Q, WU Y, ZHANG W N, MIAO C Y, SHENG C Q. Dual NAMPT/HDAC inhibitors as a new strategy for multitargeting antitumor drug discovery[J]. ACS Med Chem Lett, 2018, 9: 34-38. DOI:10.1021/acsmedchemlett.7b00414 |
| [25] |
XIE R, TANG P, YUAN Q P. Rational design and characterization of a DNA/HDAC dual-targeting inhibitor containing nitrogen mustard and 2-aminobenzamide moieties[J]. Medchemcomm, 2018, 9: 344-352. DOI:10.1039/C7MD00476A |
| [26] |
XIE R, LI Y, TANG P, YUAN Q P. Rational design, synthesis and preliminary antitumor activity evaluation of a chlorambucil derivative with potent DNA/HDAC dual-targeting inhibitory activity[J]. Bioorg Med Chem Lett, 2017, 27: 4415-4420. DOI:10.1016/j.bmcl.2017.08.011 |
| [27] |
WU D, YAN Y Q, WEI T, YE Z Q, XIAO Y T, PAN Y Q, et al. An acetyl-histone vulnerability in PI3K/AKT inhibition-resistant cancers is targetable by both BET and HDAC inhibitors[J/OL]. Cell Rep, 2021, 34: 108744. DOI: 10.1016/j.celrep.2021.108744.
|
| [28] |
LI J, QIAN C G, ZHOU Q Q, LI J W, LI K L, YI P Y. BEBT-908:a novel potent PI3K/HDAC inhibitor against diffuse large B-cell lymphoma[J]. Biochem Biophys Res Commun, 2017, 491: 939-945. DOI:10.1016/j.bbrc.2017.07.139 |
| [29] |
ZHANG K H, LAI F F, LIN S W, JI M, ZHANG J B, ZHANG Y, et al. Design, synthesis, and biological evaluation of 4-methyl quinazoline derivatives as anticancer agents simultaneously targeting phosphoinositide 3-kinases and histone deacetylases[J]. J Med Chem, 2019, 62: 6992-7014. DOI:10.1021/acs.jmedchem.9b00390 |
| [30] |
SUN K M, ATOYAN R, BOREK M A, DELLAROCCA S, SAMSON M E, MA A W, et al. Dual HDAC and PI3K inhibitor CUDC-907 downregulates MYC and suppresses growth of MYC-dependent cancers[J]. Mol Cancer Ther, 2017, 16: 285-299. DOI:10.1158/1535-7163.MCT-16-0390 |
| [31] |
MONDELLO P, DERENZINI E, ASGARI Z, PHILIP J, BREA E J, SESHAN V, et al. Dual inhibition of histone deacetylases and phosphoinositide 3-kinase enhances therapeutic activity against B cell lymphoma[J]. Oncotarget, 2017, 8: 14017-14028. DOI:10.18632/oncotarget.14876 |
| [32] |
CINCINELLI R, MUSSO L, ARTALI R, GUGLIELMI M, BIANCHINO E, CARDILE F, et al. Camptothecin-psammaplin A hybrids as topoisomeraseⅠand HDAC dual-action inhibitors[J]. Eur J Med Chem, 2018, 143: 2005-2014. DOI:10.1016/j.ejmech.2017.11.021 |
| [33] |
CINCINELLI R, MUSSO L, ARTALI R, GUGLIELMI M B, LA PORTA I, MELITO C, et al. Hybrid topoisomeraseⅠand HDAC inhibitors as dual action anticancer agents[J/OL]. PLoS One, 2018, 13: e0205018. DOI: 10.1371/journal.pone.0205018.
|
| [34] |
MENG W, WANG B C, MAO W W, WANG J J, ZHAO Y, LI Q F, et al. Enhanced efficacy of histone deacetylase inhibitor panobinostat combined with dual PI3K/mTOR inhibitor BEZ235 against glioblastoma[J]. Nagoya J Med Sci, 2019, 81: 93-102. |
| [35] |
PIAO J J, CHEN L Y, QUAN T H, LI L S, QUAN C J, PIAO Y S, et al. Superior efficacy of co-treatment with the dual PI3K/mTOR inhibitor BEZ235 and histone deacetylase inhibitor Trichostatin A against NSCLC[J]. Oncotarget, 2016, 7: 60169-60180. DOI:10.18632/oncotarget.11109 |
| [36] |
CHEN Y, YUAN X, ZHANG W H, TANG M H, ZHENG L, WANG F, et al. Discovery of novel dual histone deacetylase and mammalian target of rapamycin target inhibitors as a promising strategy for cancer therapy[J]. J Med Chem, 2019, 62: 1577-1592. DOI:10.1021/acs.jmedchem.8b01825 |
| [37] |
LING Y, WANG X M, WANG C N, XU C J, ZHANG W, ZHANG Y H, et al. Hybrids from farnesylthiosalicylic acid and hydroxamic acid as dual Ras-related signaling and histone deacetylase (HDAC) inhibitors: design, synthesis and biological evaluation[J]. ChemMedChem, 2015, 10: 971-976. DOI:10.1002/cmdc.201500019 |
| [38] |
FANG K, DONG G Q, LI Y, HE S P, WU Y, WU S C, et al. Discovery of novel indoleamine 2, 3-dioxygenase 1(IDO1) and histone deacetylase (HDAC) dual inhibitors[J]. ACS Med Chem Lett, 2018, 9: 312-317. DOI:10.1021/acsmedchemlett.7b00487 |
| [39] |
ZANG J, LIANG X W, HUANG Y X, JIA Y P, LI X Y, XU W F, et al. Discovery of novel pazopanib-based HDAC and VEGFR dual inhibitors targeting cancer epigenetics and angiogenesis simultaneously[J]. J Med Chem, 2018, 61: 5304-5322. DOI:10.1021/acs.jmedchem.8b00384 |
| [40] |
LU D, YAN J, WANG L, LIU H C, ZENG L M, ZHANG M M, et al. Design, synthesis, and biological evaluation of the first c-Met/HDAC inhibitors based on pyridazinone derivatives[J]. ACS Med Chem Lett, 2017, 8: 830-834. DOI:10.1021/acsmedchemlett.7b00172 |
| [41] |
ZHOU R L, FANG S Y, ZHANG M M, ZHANG Q S, HU J, WANG M P, et al. Design, synthesis, and bioactivity evaluation of novel Bcl-2/HDAC dual-target inhibitors for the treatment of multiple myeloma[J]. Bioorg Med Chem Lett, 2019, 29: 349-352. DOI:10.1016/j.bmcl.2018.12.052 |
| [42] |
HE S P, DONG G Q, WU S C, FANG K, MIAO Z Y, WANG W, et al. Small molecules simultaneously inhibiting p53-murine double minute 2(MDM2) interaction and histone deacetylases (HDACs): discovery of novel multitargeting antitumor agents[J]. J Med Chem, 2018, 61: 7245-7260. DOI:10.1021/acs.jmedchem.8b00664 |
 2021, Vol. 42
2021, Vol. 42


