胶质瘤是起源于神经外胚层细胞或前体细胞的肿瘤,包括星形细胞瘤、少突胶质细胞瘤和室管膜瘤等,占成人脑原发恶性肿瘤的75%[1]。全世界每年有超过10万人被诊断为胶质瘤,其致死率和致残率均较高[2]。低级别胶质瘤(low-grade glioma,LGG)是指WHO分级中异柠檬酸脱氢酶(isocitrate dehydrogenase,IDH)突变的Ⅱ级和Ⅲ级胶质瘤[3],预后相对较好,但也可能进展为恶性程度更高、侵袭性更强的高级别胶质瘤。近年来分子遗传学和生物标志物方面的研究进展为LGG的精确诊断、个体化治疗及预后判断带来了新的希望和方向[4-5],找到可靠、有效的生物标志物对LGG的基础研究和临床治疗意义重大。
补体C3a受体1(complement C3a receptor 1,C3AR1)是G蛋白偶联受体家族成员,其特征是经典的7个跨膜结构域和较大的第2个细胞外环[6]。C3AR1参与固有免疫反应调节和病原体监测,其与乳腺癌[7]、神经发生[8]、脂类分解代谢[9]等病理生理过程密切相关。目前尚未见LGG与C3AR1有关的报道。本研究利用美国癌症基因组图谱(The Cancer Genome Atlas,TCGA)数据库中LGG相关的测序数据和临床信息进行生物信息学分析,运用R 4.0.1软件和基因表达谱数据动态分析(gene expression profiling interactive analysis,GEPIA)[10]工具分析C3AR1在不同肿瘤组织与正常组织中的表达差异、C3AR1与不同WHO分级LGG的相关性及对患者预后的影响。为了解肿瘤免疫细胞浸润情况,本研究进一步利用肿瘤免疫评估(Tumor Immune Estimation Resource,TIMER)数据库分析C3AR1与LGG中肿瘤浸润性免疫细胞的相关性及肿瘤浸润免疫细胞对患者预后的影响,通过对LGG中与C3AR1表达呈正相关的基因群进行京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)通路富集分析,确定C3AR1在LGG发生、发展及免疫细胞浸润中的潜在通路。
1 资料和方法 1.1 数据收集与预处理从TCGA数据库(https://portal.gdc.cancer.gov/)[11]筛选并下载529例LGG患者的临床信息和组织的基因表达谱数据,均为肿瘤组织,无正常组织。由于缺少正常组织对照,从TCGA数据库筛选并下载5例多形性胶质母细胞瘤患者正常脑组织的基因表达谱数据用于对照分析。检查下载数据的完整性,删去完全缺失临床信息或缺失生存状态、生存时间等关键数据的病例。将基因编码转换为美国基因命名委员会(HUGO Gene Nomenclature Committee,HGNC)的标准基因名称。
1.2 C3AR1基因在肿瘤组织和正常组织中的表达水平分析利用Oncomine数据库(https://www.oncomine.org/resource/login.html)[12]鉴定多种类型肿瘤组织中C3AR1基因的表达水平。筛选条件:P<0.05、差异倍数>1.5,基因排名前10%,数据类型为mRNA。比较分析TCGA数据库中部分肿瘤组织和正常组织中C3AR1基因的表达水平。
1.3 C3AR1基因在不同WHO分级LGG组织中的表达分析及与患者预后的关系利用癌症数据在线分析和挖掘网站UALCAN(http://ualcan.path.uab.edu/index.html)[13]对TCGA数据库中的LGG数据进行分析。计算不同WHO分级LGG组织样本中C3AR1基因的表达量。用GEPIA(http://gepia.cancer-pku.cn/index.html)对正常脑组织与LGG组织中C3AR1基因表达量进行对比分析,根据C3AR1基因表达量的中位数将病例分为高表达组和低表达组,并绘制生存曲线。
1.4 LGG患者临床特征和C3AR1基因表达水平对患者预后影响分析对病例数据的分类进行赋值转换,在用R 4.0.1软件对LGG患者临床特征和C3AR1基因表达水平进行单因素和多因素Cox回归分析,分析其对患者预后的影响。
1.5 肿瘤免疫细胞浸润与C3AR1基因表达的关系及对患者预后影响分析利用TIMER数据库(https://cistrome.shinyapps.io/timer/)[14]对LGG组织中C3AR1基因的表达水平与6种免疫细胞(B细胞、CD8+ T细胞、CD4+ T细胞、巨噬细胞、中性粒细胞、树突状细胞)浸润水平之间的相关性进行分析,并分析这6种免疫细胞在LGG组织中的浸润水平与患者总体预后的关系。
1.6 与C3AR1基因表达呈正相关基因群的KEGG通路富集分析利用UALCAN进行相关分析得到与LGG组织中C3AR1基因表达呈正相关的基因群,再利用DAVID数据库(https://david.ncifcrf.gov/home.jsp)[15]进行KEGG通路富集分析,取富集倍数排名前10且P<0.01的通路,用R 4.0.1软件绘制气泡图。
1.7 统计学处理应用SPSS 22.0和R 4.0.1软件进行分析。计量资料以中位数(下四分位数,上四分位数)表示,采用非参数Mann-Whitney U检验;计数资料以例数和百分数表示。用Kaplan-Meier法绘制患者生存曲线,生存率的比较采用log-rank检验;用单因素和多因素Cox回归分析LGG患者临床特征和C3AR1基因表达水平对预后的影响;采用Spearman偏相关分析法分析C3AR1基因表达与肿瘤免疫浸润细胞的关系。检验水准(α)为0.05。
2 结果 2.1 组织样本的一般情况共514个LGG组织样本纳入本硏究,均含有基因表达谱测序数据和临床信息。其中星形细胞瘤组织样本194个(37.7%)、少突胶质细胞瘤组织样本191个(37.2%)、少突星形细胞瘤组织样本129个(25.1%);WHOⅡ级LGG 249例(48.4%),WHOⅢ级LGG 265例(51.6%);总生存时间为0~6 423 d,中位时间为674.0(400.5,1 227.0)d。
2.2 C3AR1基因在肿瘤组织和正常组织中的表达水平利用Oncomine数据库进行分析的结果表明,C3AR1基因在5个脑和中枢神经系统肿瘤数据集中明显高表达(P<0.01,差异倍数>1.5);在膀胱肿瘤、结直肠肿瘤、肺肿瘤、黑色素瘤等数据集中低表达;在胃肿瘤、头颈部肿瘤、肾脏肿瘤、肝肿瘤、胰腺肿瘤、肉瘤等数据集中高表达。为了进一步评估C3AR1基因在LGG组织中的表达,我们利用TCGA数据库的RNA测序数据分析了C3AR1基因在LGG组织和多形性胶质细胞瘤、膀胱癌、乳腺癌、胆囊癌、头颈鳞状细胞癌等肿瘤组织中的表达。与正常组织相比,LGG组织中C3AR1基因表达升高(P<0.05,图 1),与多形性胶质细胞瘤中的结果相似。
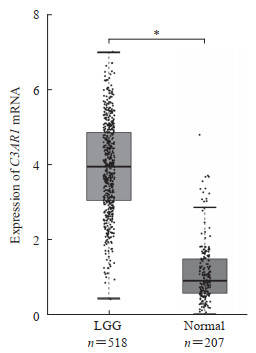
|
图 1 C3AR1 mRNA在LGG组织和正常组织中的表达 Fig 1 Expression of C3AR1 mRNA in LGG and normal tissues *P < 0.05. The data of 207 normal cases as controls were collected from GEPIA. Of 518 LGG samples, 514 cases had LGG grade and 4 cases had no LGG grade. C3AR1: Complement C3a receptor 1; LGG: Low-grade glioma. |
2.3 C3AR1基因在不同WHO分级LGG组织中的表达及与患者预后的关系
根据UALCAN计算得到不同WHO分级LGG组织样本中C3AR1基因的表达量,C3AR1基因在WHOⅡ级LGG组织中的表达量低于WHOⅢ级LGG组织,差异有统计学意义(P<0.05,图 2)。生存分析结果显示C3AR1基因低表达组(n=257)的生存预后优于高表达组(n=257),差异有统计学意义(HR=1.7,95% CI 1.1~2.4,P=0.003 6,图 3)。

|
图 2 C3AR1 mRNA在不同WHO分级LGG组织中的表达 Fig 2 Expression of C3AR1 mRNA in different WHO grades of LGG tissues *P < 0.05. C3AR1: Complement C3a receptor 1; LGG: Low-grade glioma; WHO: World Health Organization; TPM: Transcripts per million. |

|
图 3 高、低表达C3AR1的LGG患者生存曲线 Fig 3 Survival curves of LGG patients with high- and low-expression C3AR1 The dotted lines represent the 95% confidence bound. LGG: Low-grade glioma; C3AR1: Complement C3a receptor 1; TPM: Transcripts per million; HR: Hazard ratio; CI: Confidence interval. |
2.4 LGG患者临床特征和C3AR1基因表达水平对预后的影响
如表 1所示,单因素Cox回归分析结果显示,患者年龄、LGG WHO分级和C3AR1基因表达水平与患者总体预后有关(P均<0.01);多因素Cox回归分析结果显示,患者年龄、LGG WHO分级和C3AR1基因表达水平是LGG患者的独立预后因素(P均<0.01)。
|
|
表 1 LGG患者临床特征和C3AR1基因表达水平对预后影响的Cox回归分析 Tab 1 Cox regression analyses of influence of clinical features and expression level of C3AR1 on prognosis of LGG patients |
2.5 肿瘤免疫细胞浸润与C3AR1基因表达的关系及对患者预后的影响
Spearman偏相关分析发现,C3AR1基因的表达水平与LGG的肿瘤纯度[16]呈负相关(rs=-0.396,P<0.01),与肿瘤免疫细胞浸润水平呈正相关:B细胞(rs=0.671,P<0.01),CD8+ T细胞(rs=0.234,P<0.01),CD4+ T细胞(rs=0.819,P<0.01),巨噬细胞(rs=0.783,P<0.01),中性粒细胞(rs=0.840,P<0.01),树突状细胞(rs=0.841,P<0.01)。CD4+ T细胞、中性粒细胞和树突状细胞的浸润水平与C3AR1基因表达水平间的偏相关系数都在0.8以上,具有极强的正相关关系。
不同免疫细胞的浸润水平与LGG患者生存预后关系的分析发现,B细胞、T细胞、巨噬细胞、中性粒细胞、树突状细胞的浸润水平均会影响LGG患者的总体预后(P均<0.05,图 4)。

|
图 4 不同免疫细胞浸润水平的LGG患者生存曲线 Fig 4 Survival curves of LGG patients with different immune cell infiltration levels A: B cell; B: CD8+ T cell; C: CD4+ T cell; D: Macrophage; E: Neutrophil; F: Dendritic cell. LGG: Low-grade glioma. |
2.6 与C3AR1基因表达呈正相关基因群的KEGG通路富集分析结果
由于C3AR1基因的高表达是LGG患者生存预后的危险因素,我们利用UALCAN进行相关分析得到与LGG组织中C3AR1基因表达呈正相关的基因共1 658个,并利用DAVID数据库进行KEGG通路富集分析。结果发现,富集倍数排名前10且P<0.01的通路主要有干扰素γ介导的信号通路、金黄色葡萄球菌感染、Ig/主要组织相容性复合体(major histocompatibility complex,MHC)保守位点、Ig样Cl型结构域、MHC Ⅰ/Ⅱ类抗原识别蛋白、连接肽特定区域、Toll样受体信号通路、内质网膜腔侧的组成部分、α-2特定区域、α-1特定区域等,见图 5。
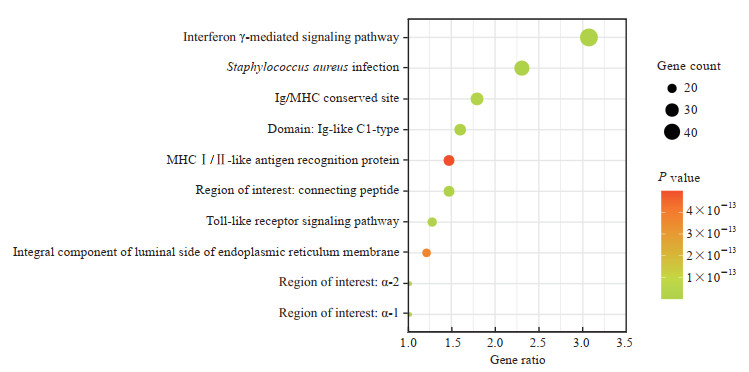
|
图 5 KEGG通路富集分析的前10个与C3AR1基因表达呈正相关的基因 Fig 5 Enrichment analysis of KEGG pathway showing top 10 genes positively correlated with C3AR1 expression KEGG: Kyoto Encyclopedia of Genes and Genomes; C3AR1: Complement C3a receptor 1; Ig: Immunoglobin; MHC: Major histocompatibility complex. |
3 讨论
胶质瘤是一种最常见、最致命的原发性脑肿瘤。胶质瘤的诊断主要依靠影像学检查,如CT和MRI,治疗主要依靠手术切除和辅助放化疗[17]。近年来,一些新的治疗方法,如免疫疗法[18]和光动力疗法[19]被采用,但患者预后仍不理想[3],可能与缺乏有效的诊断和治疗靶点有关。我们研究的C3AR1可能为LGG的诊断、治疗和预后判断提供潜在的分子靶点,C3AR1与肿瘤免疫细胞浸润关系的发现则有助于LGG免疫疗法的发展。
近年对于LGG预后生物标志物的研究逐步深入和丰富。IDH突变在LGG患者中的表达预示着良好的预后[20],而细胞周期蛋白依赖性激酶抑制剂2A和B(cyclin-dependent kinase inhibitor 2A/B,CDKN2A/B)纯合缺失近年被认为是IDH突变LGG中预后不良指标[21]。端粒酶RNA组分(telomerase RNA component,TERC)、端粒酶反转录酶(telomerase reverse transcriptase,TERT)、表皮生长因子受体(epidermal growth factor receptor,EGFR)、卷曲螺旋结构域蛋白26(coiled-coil domain-containing 26,CCDC26)、膜泡运输t-SNAREs同源物1A(vesicle transport through interaction with t-SNAREs homolog 1A,VTI1A)、锌指和BTB结构域蛋白16(zinc finger and BTB domain-containing 16,ZBTB16)、Pleckstrin同源样结构域家族B成员1(Pleckstrin homology-like domain family B member 1,PHLDB1)、聚合酶(RNA)Ⅲ(DNA定向)多肽B[polymerase(RNA)Ⅲ(DNA directed)polypeptide B,POLR3B]、电子转移黄素蛋白α多肽(electron-transfer-flavoprotein α polypeptide,ETFA)、肿瘤蛋白P53和端粒伸长解旋酶1调节因子(regulator of telomere elongation helicase 1,RTEL1)等基因突变与LGG发生风险密切相关[22-26]。分子标志物研究的进展也将促进脑肿瘤的脑脊液活检等新兴技术的发展[27],进一步在精确诊断、治疗的同时减少患者的痛苦。LGG的免疫治疗有了一定的发展,但由于肿瘤的异质性和有效分子靶标的缺乏,LGG的免疫治疗需要进一步研究[28]。
C3AR1是G蛋白偶联受体家族成员,在小胶质细胞等免疫细胞上表达,并与包括中枢神经系统炎症在内的各种免疫调节过程有关[29]。C3AR1最初被认为局限于固有免疫反应,在补体级联中发挥作用,但进一步研究发现C3AR1与乳腺肿瘤[30]、垂体激素释放[31]和神经发生[8]等均密切相关。有研究表明,肿瘤浸润免疫细胞是肿瘤患者的前哨淋巴结状态和预后生存的独立预测因子[32]。本研究首次在人类LGG患者队列中分析了C3AR1基因的表达,利用临床信息和RNA测序数据研究了C3AR1基因表达与LGG WHO分级预后的关系,并分析了C3AR1基因表达水平与LGG免疫细胞浸润的相关性及免疫细胞浸润对患者预后的影响。
本研究结果显示,C3AR1基因在LGG组织的表达高于正常组织(P<0.05),且其表达水平与肿瘤有关,在WHOⅡ级LGG组织中的表达量低于WHOⅢ级LGG组织(P<0.05),生存分析结果表明C3AR1基因表达水平与LGG的预后有关,高表达患者的预后可能不良。多因素Cox回归分析结果显示,C3AR1基因表达水平是LGG患者预后的独立危险因素(HR=1.877,P<0.01)。
本研究还发现LGG组织中C3AR1基因表达水平与LGG的肿瘤纯度呈负相关,与6种肿瘤免疫细胞(B细胞、CD8+ T细胞、CD4+ T细胞、巨噬细胞、中性粒细胞、树突状细胞)浸润水平呈正相关(P均<0.01)。肿瘤细胞的基因组变异可能会产生肿瘤抗原,被免疫系统识别为非自身成分,从而引发细胞免疫反应[33]。LGG在遗传学和免疫水平存在显著的异质性,找到合适的靶标仍是影响LGG免疫治疗的关键因素[28]。在既往研究中,利用RNA测序数据分析基因表达水平与浸润免疫细胞之间的关系时,相关系数多为不相关或弱、中度相关,强相关少见[34-35]。本研究中CD4+ T细胞、中性粒细胞和树突状细胞的浸润水平与C3AR1基因表达水平间的偏相关系数>0.8,与强正相关关系。提示C3AR1可能对肿瘤免疫有潜在的影响,其可能成为一种很有前途的肿瘤生物标志物。
本研究对LGG组织中与C3AR1基因表达呈正相关的基因群进行了KEGG通路富集分析,以获知其主要产生生物学功能的通路。结果显示Ig/MHC保守位点、MHCⅠ/Ⅱ类抗原识别蛋白、Toll样受体信号通路等通路均有基因的富集,提示与C3AR1基因表达呈正相关的基因多可能通过这些通路调控LGG的免疫细胞浸润并影响患者的预后。
综上所述,C3AR1表达是LGG患者预后的潜在分子标志物。在LGG组织中由C3AR1调控的Ig/MHC保守位点、MHCⅠ/Ⅱ类抗原识别蛋白、Toll样受体信号通路等通路可能影响肿瘤免疫细胞浸润,进而影响肿瘤的发生、发展和患者预后。C3AR1作为LGG患者的独立预后因素,有成为治疗LGG分子靶标的潜力。
| [1] |
LAPOINTE S, PERRY A, BUTOWSKI N A. Primary brain tumours in adults[J]. Lancet, 2018, 392: 432-446. DOI:10.1016/S0140-6736(18)30990-5 |
| [2] |
FERLAY J, COLOMBET M, SOERJOMATARAM I, MATHERS C, PARKIN D M, PIÑEROS M, et al. Estimating the global cancer incidence and mortality in 2018:GLOBOCAN sources and methods[J]. Int J Cancer, 2019, 144: 1941-1953. DOI:10.1002/ijc.31937 |
| [3] |
YOUSSEF G, MILLER J J. Lower grade gliomas[J/OL]. Curr Neurol Neurosci Rep, 2020, 20: 21. DOI: 10.1007/s11910-020-01040-8.
|
| [4] |
REIFENBERGER G, WIRSCHING H G, KNOBBE-THOMSEN C B, WELLER M. Advances in the molecular genetics of gliomas-implications for classification and therapy[J]. Nat Rev Clin Oncol, 2017, 14: 434-452. DOI:10.1038/nrclinonc.2016.204 |
| [5] |
DE BLANK P, FOULADI M, HUSE J T. Molecular markers and targeted therapy in pediatric low-grade glioma[J]. J Neurooncol, 2020, 150: 5-15. DOI:10.1007/s11060-020-03529-1 |
| [6] |
RICKLIN D, REIS E S, MASTELLOS D C, GROS P, LAMBRIS J D. Complement component C3-the "Swiss Army Knife" of innate immunity and host defense[J]. Immunol Rev, 2016, 274: 33-58. DOI:10.1111/imr.12500 |
| [7] |
SONG G Q, HE L, YANG X L, YANG Y, CAI X M, LIU K, et al. Identification of aberrant gene expression during breast ductal carcinoma in situ progression to invasive ductal carcinoma[J/OL]. J Int Med Res, 2020, 48: 300060518815364. DOI: 10.1177/0300060518815364.
|
| [8] |
KLOS A, TENNER A J, JOHSWICH K O, AGER R R, REIS E S, KÖHL J. The role of the anaphylatoxins in health and disease[J]. Mol Immunol, 2009, 46: 2753-2766. DOI:10.1016/j.molimm.2009.04.027 |
| [9] |
CERO C, RAZZOLI M, HAN R J, SAHU B S, PATRICELLI J, GUO Z K, et al. The neuropeptide TLQP-21 opposes obesity via C3aR1-mediated enhancement of adrenergic-induced lipolysis[J]. Mol Metab, 2017, 6: 148-158. DOI:10.1016/j.molmet.2016.10.005 |
| [10] |
TANG Z F, LI C W, KANG B X, GAO G, LI C, ZHANG Z M. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses[J]. Nucleic Acids Res, 2017, 45(W1): W98-W102. DOI:10.1093/nar/gkx247 |
| [11] |
BLUM A, WANG P, ZENKLUSEN J C. SnapShot: TCGA-analyzed tumors[J/OL]. Cell, 2018, 173: 530. DOI: 10.1016/j.cell.2018.03.059.
|
| [12] |
RHODES D R, KALYANA-SUNDARAM S, MAHAVISNO V, VARAMBALLY R, YU J, BRIGGS B B, et al. Oncomine 3.0:genes, pathways, and networks in a collection of 18, 000 cancer gene expression profiles[J]. Neoplasia, 2007, 9: 166-180. DOI:10.1593/neo.07112 |
| [13] |
CHANDRASHEKAR D S, BASHEL B, BALASUBRAMANYA S A H, CREIGHTON C J, PONCE-RODRIGUEZ I, CHAKRAVARTHI B V S K, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses[J]. Neoplasia, 2017, 19: 649-658. DOI:10.1016/j.neo.2017.05.002 |
| [14] |
LI T W, FAN J Y, WANG B B, TRAUGH N, CHEN Q M, LIU J S, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells[J/OL]. Cancer Res, 2017, 77: e108-e110. DOI: 10.1158/0008-5472.Can-17-0307.
|
| [15] |
HUANG DA W, SHERMAN B T, LEMPICKI R A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources[J]. Nat Protoc, 2009, 4: 44-57. DOI:10.1038/nprot.2008.211 |
| [16] |
YOSHIHARA K, SHAHMORADGOLI M, MARTÍNEZ E, VEGESNA R, KIM H, TORRES-GARCIA W, et al. Inferring tumour purity and stromal and immune cell admixture from expression data[J/OL]. Nat Commun, 2013, 4: 2612. DOI: 10.1038/ncomms3612.
|
| [17] |
KONG Z R, YAN C R, ZHU R Z, WANG J R, WANG Y N, WANG Y, et al. Imaging biomarkers guided anti-angiogenic therapy for malignant gliomas[J]. Neuroimage Clin, 2018, 20: 51-60. DOI:10.1016/j.nicl.2018.07.001 |
| [18] |
KONG Z R, WANG Y, MA W B. Vaccination in the immunotherapy of glioblastoma[J]. Hum Vaccin Immunother, 2018, 14: 255-268. DOI:10.1080/21645515.2017.1388481 |
| [19] |
AKIMOTO J. [Ⅲ. Photodynamic therapy for malignant glioma][J]. Gan To Kagaku Ryoho, 2018, 45: 933-937. |
| [20] |
BUCKNER J, GIANNINI C, ECKEL-PASSOW J, LACHANCE D, PARNEY I, LAACK N, et al. Management of diffuse low-grade gliomas in adults-use of molecular diagnostics[J]. Nat Rev Neurol, 2017, 13: 340-351. |
| [21] |
REIS G F, PEKMEZCI M, HANSEN H M, RICE T, MARSHALL R E, MOLINARO A M, et al. CDKN2A loss is associated with shortened overall survival in lower-grade (World Health Organization grades Ⅱ-Ⅲ) astrocytomas[J]. J Neuropathol Exp Neurol, 2015, 74: 442-452. DOI:10.1097/NEN.0000000000000188 |
| [22] |
JENKINS R B, XIAO Y, SICOTTE H, DECKER P A, KOLLMEYER T M, HANSEN H M, et al. A low-frequency variant at 8q24.21 is strongly associated with risk of oligodendroglial tumors and astrocytomas with IDH1 or IDH2 mutation[J]. Nat Genet, 2012, 44: 1122-1125. DOI:10.1038/ng.2388 |
| [23] |
KINNERSLEY B, LABUSSIÈRE M, HOLROYD A, DI STEFANO A L, BRODERICK P, VIJAYAKRISHNAN J, et al. Genome-wide association study identifies multiple susceptibility loci for glioma[J/OL]. Nat Commun, 2015, 6: 8559. DOI: 10.1038/ncomms9559.
|
| [24] |
SANSON M, HOSKING F J, SHETE S, ZELENIKA D, DOBBINS S E, MA Y, et al. Chromosome 7p11.2(EGFR) variation influences glioma risk[J]. Hum Mol Genet, 2011, 20: 2897-2904. DOI:10.1093/hmg/ddr192 |
| [25] |
STACEY S N, SULEM P, JONASDOTTIR A, MASSON G, GUDMUNDSSON J, GUDBJARTSSON D F, et al. A germline variant in the TP53 polyadenylation signal confers cancer susceptibility[J]. Nat Genet, 2011, 43: 1098-1103. |
| [26] |
WALSH K M, ANDERSON E, HANSEN H M, DECKER P A, KOSEL M L, KOLLMEYER T, et al. Analysis of 60 reported glioma risk SNPs replicates published GWAS findings but fails to replicate associations from published candidate-gene studies[J]. Genet Epidemiol, 2013, 37: 222-228. DOI:10.1002/gepi.21707 |
| [27] |
SIMONELLI M, DIPASQUALE A, ORZAN F, LORENZI E, PERSICO P, NAVARRIA P, et al. Cerebrospinal fluid tumor DNA for liquid biopsy in glioma patients' management: close to the clinic?[J/OL]. Crit Rev Oncol Hematol, 2020, 146: 102879. DOI: 10.1016/j.critrevonc.2020.102879.
|
| [28] |
SAMPSON J H, GUNN M D, FECCI P E, ASHLEY D M. Brain immunology and immunotherapy in brain tumours[J]. Nat Rev Cancer, 2020, 20: 12-25. |
| [29] |
BOOS L, CAMPBELL I L, AMES R, WETSEL R A, BARNUM S R. Deletion of the complement anaphylatoxin C3a receptor attenuates, whereas ectopic expression of C3a in the brain exacerbates, experimental autoimmune encephalomyelitis[J]. J Immunol, 2004, 173: 4708-4714. |
| [30] |
OPSTAL-VAN WINDEN A W, VERMEULEN R C, PEETERS P H, BEIJNEN J H, VAN GILS C H. Early diagnostic protein biomarkers for breast cancer: how far have we come?[J]. Breast Cancer Res Treat, 2012, 134: 1-12. |
| [31] |
FRANCIS K, LEWIS B M, AKATSU H, MONK P N, CAIN S A, SCANLON M F, et al. Complement C3a receptors in the pituitary gland: a novel pathway by which an innate immune molecule releases hormones involved in the control of inflammation[J]. FASEB J, 2003, 17: 2266-2268. |
| [32] |
AZIMI F, SCOLYER R A, RUMCHEVA P, MONCRIEFF M, MURALI R, MCCARTHY S W, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma[J]. J Clin Oncol, 2012, 30: 2678-2683. |
| [33] |
MATSUSHITA H, VESELY M D, KOBOLDT D C, RICKERT C G, UPPALURI R, MAGRINI V J, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting[J]. Nature, 2012, 482: 400-404. |
| [34] |
PAN J H, ZHOU H, COOPER L, HUANG J L, ZHU S B, ZHAO X X, et al. LAYN is a prognostic biomarker and correlated with immune infiltrates in gastric and colon cancers[J/OL]. Front Immunol, 2019, 10: 6. DOI: 10.3389/fimmu.2019.00006.
|
| [35] |
MILLER C P, THORPE J D, KORTUM A N, COY C M, CHENG W Y, OU YANG T H, et al. JAK2 expression is associated with tumor-infiltrating lymphocytes and improved breast cancer outcomes: implications for evaluating JAK2 inhibitors[J]. Cancer Immunol Res, 2014, 2: 301-306. |
 2021, Vol. 42
2021, Vol. 42


