2. 海军军医大学(第二军医大学)海军特色医学中心潜水与高气压医学研究室, 上海 200433;
3. 同济大学化学科学与工程学院普通化学教研室, 上海 200092
2. Department of Diving and Hyperbaric Medical Research, Naval Special Medical Center, Naval Medical University(Second Military Medical University), Shanghai 200433, China;
3. Department of General Chemistry, School of Chemical Science and Engineering, Tongji University, Shanghai 200092, China
骨缺损是常见临床病症,颌面部骨缺损直接影响咀嚼功能和美观。骨移植是修复骨缺损的重要方法,自体骨取材受限,异种骨移植材料因来源广而备受青睐。天然煅烧牛骨(calcined bovine bone,CBB)是从牛骨中提取的具有骨引导性的物质,其多孔结构为新骨长入提供了支架,是骨缺损修复的良好材料,研究发现国产CBB修复材料对骨缺损修复有一定作用[1]。碱性成纤维细胞生长因子(basic fibroblast growth factor,bFGF)可诱导多种细胞增殖与分化,对骨再生和骨缺损修复有促进作用[2]。高压氧(hyperbaric oxygen,HBO)可改善牙龈微循环,抑制实验性牙周炎炎症因子产生,促进骨再生[3]。骨保护素(osteoprotegerin,OPG)可抑制NF-κB受体活化因子配体的激活,刺激破骨细胞分化;成骨细胞还可通过分泌OPG抑制破骨细胞的产生,在骨改建中起促进作用[4]。有研究用粒细胞集落刺激因子动员从外周血单核细胞中分离的CD34+细胞治疗严重肢体缺血患者,结果显示出良好的疗效,提示CD34参与了缺血组织的愈合[5]。本研究观察了HBO联合bFGF对CBB修复骨缺损的作用,并初步探讨其与OPG、CD34表达变化的关系。
1 材料和方法 1.1 材料与仪器CBB(商品名:骼瑞;陕西瑞盛生物科技有限公司,生产批号:161202),bFGF(珠海亿胜生物制药有限公司,生产批号:20161208),医用胶原蛋白海绵(无锡贝迪生物工程股份有限公司,生产批号:20171217)。便携式高速涡轮牙钻机(上海品瑞医疗器械设备有限公司),i-CAT 17-19型CT检测系统(美国KaVo公司),Olympus IX 70型荧光显微镜(日本奥林巴斯株式会社)。OPG抗体、CD34抗体(武汉博士德生物工程有限公司),GTVisionTM Ⅱ型免疫组织化学染色试剂盒(广州深达生物制品技术有限公司),DAB显色液[基因科技(上海)股份有限公司],EM UV-6型超薄切片机(德国Leica公司)。
1.2 动物选择与分组SD大鼠32只[上海西普尔-必凯实验动物有限公司,实验动物生产许可证号:SCXK(沪)2013-0016],体重为250~300 g,分笼喂养,自由饮水和摄取饲料。动物随机分为两组:一组接受HBO治疗(HBO组,n=16),另一组不接受HBO治疗(NHBO组,n=16)。每组根据移植材料不同分为CBB组(n=8)、CBB联合bFGF组(CBBbF组,n=8),设置未移植的自身对照组(Con组,n=16)。本课题动物实验通过海军军医大学(第二军医大学)长海医院动物伦理委员会审批。
1.3 颅骨缺损造模、修复与HBO治疗SD大鼠用3%戊巴比妥钠按1 mL/kg剂量于耳缘静脉注射麻醉,消毒后在顶骨处切开皮肤和肌肉,暴露颅骨,在距离颅骨分界线左右两侧1~2 mm处用便携式高速涡轮牙钻机在滴水降温下切割全层颅骨,形成2个直径为6 mm的圆形双皮质骨缺损(勿损伤脑膜)。一侧骨缺损处移植CBB或移植CBB后覆盖载bFGF胶原蛋白海绵(在6 mm×25 mm×2 mm胶原蛋白海绵上滴加4 375 U/mL的bFGF 300 μL,胶原蛋白海绵含bFGF 1 312 U);另一侧骨缺损不移植,用4-0丝线缝合伤口。术后每只动物每天肌内注射80万U青霉素,连续5 d。HBO组行HBO暴露2周(0.25 MPa,每次1 h,每周5次)。
1.4 锥形束CT检测大鼠骨缺损区骨密度(bone density,BD)HBO-CBB、HBO-CBBbF、NHBO-CBB和NHBO-CBBbF组在术后4周、8周时各处死2只大鼠,在术后12周时处死剩余4只大鼠;HBO-Con、NHBO-Con组在术后4周、8周时各处死4只大鼠,在术后12周时处死剩余8只大鼠。取大鼠颅骨标本,对骨缺损区标本进行锥形束CT扫描,将图像录入计算机,对手术部位骨缺损区的扫描图像各测定5个区域CT值,用以代表BD,求BD平均值[6]。
1.5 组织学观察处死大鼠后取实验部位颅骨标本行锥形束CT扫描后,用4%多聚甲醛溶液固定72 h,10% EDTA脱钙2个月,梯度乙醇脱水,石蜡包埋,5 μm厚度切片,行H-E染色。用HPILAS-1000高清晰度彩色病理图文分析系统对H-E染色切片进行观察,由同一位观察者对每张切片原缺损区随机选取3个100倍视野,测定新骨形成面积。按公式计算,新骨形成面积百分比(%)=(新骨形成面积/测定区域面积)×100%[6]。
1.6 大鼠骨缺损修复组织中OPG和CD34表达的测定按GTVisionTM Ⅱ型免疫组织化学染色试剂盒说明书检测大鼠骨缺损修复组织中OPG和CD34的表达情况,每个标本取连续的3张切片,所有切片均在同一染色条件下完成。染色呈黄色为阳性,比较各组染色阳性部位和强度差异。在显微镜(200×)下用图像分析系统(Vectra多色荧光系统,美国Perkin Elmer公司)测量阳性染色的光密度值,每张切片选择10个染色阳性视野测定OPG和CD34含量,计算其平均值。
1.7 统计学处理应用SPSS 16.0软件进行统计学分析。计量资料以x±s表示,两组间比较采用单因素方差分析。检验水准(α)为0.05。
2 结果 2.1 各组大鼠骨缺损区BD比较术后4、8和12周时HBO-CBB组和HBO-CBBbF组大鼠骨缺损区BD均大于HBO-Con组(P均<0.01),NHBO-CBB组和NHBO-CBBbF组均大于NHBO-Con组(P均<0.01);HBO-CBBbF组大鼠骨缺损区BD大于HBO-CBB组、NHBO-CBB组和NHBO-CBBbF组(P均<0.01);NHBO-CBBbF组大鼠骨缺损区BD大于NHBO-CBB组(P均<0.01)。见表 1。
|
|
表 1 术后4、8、12周时各组大鼠骨缺损区BD比较 Tab 1 Comparison of BDs in bone defect areas of rats in each group at 4, 8 and 12 weeks after operation |
2.2 各组大鼠骨缺损愈合和新骨形成情况
H-E染色结果(图 1)显示,术后4周时,HBO-CBB组、HBO-CBBbF组、NHBO-CBBbF组有少许新生骨,成骨细胞较多,血管形成明显增多;NHBO-CBB组新骨形成和血管形成较少。术后8周时,HBO-CBB组、HBO-CBBbF组、NHBO-CBBbF组新骨形成和血管数量多于NHBO-CBB组,HBO-Con组和NHBO-Con组血管数量较少。术后12周时,HBO-CBB组、HBO-CBBbF组和NHBO-CBBbF组新骨形成较多,残留移植物体积减小;HBO-CBBbF组新骨形成最多,残留物量最少;NHBO-CBB组虽有新骨形成,但结构紊乱;HBO-Con组和NHBO-Con组新骨形成较少;HBO-CBB组和HBO-CBBbF组炎症细胞数量少于其他组;HBO-CBBbF组和NHBO-CBBbF组血管数量多于HBO-Con组和NHBO-Con组。
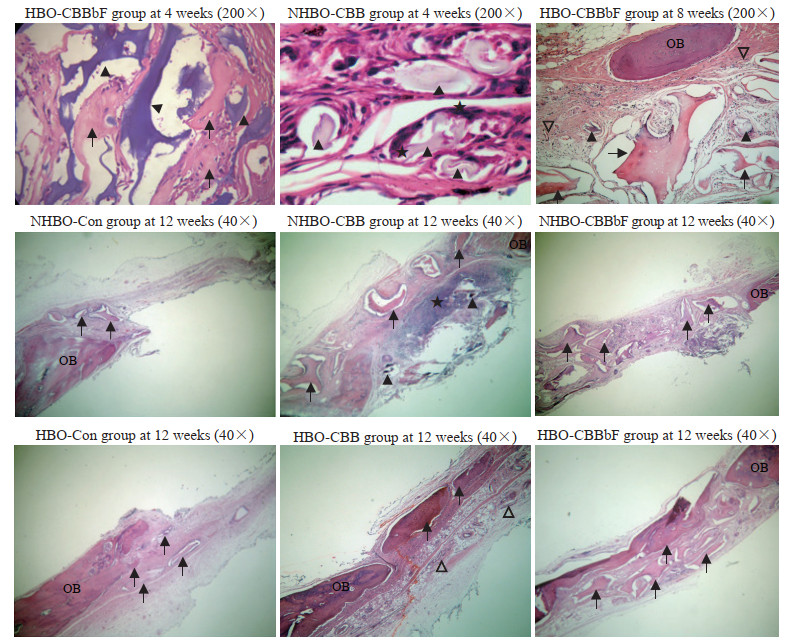
|
图 1
术后大鼠骨缺损修复组织H-E染色光镜观察
Fig 1
H-E staining photomicrographs of bone defect repair tissues of rats after operation
H-E: Hematoxylin-eosin; HBO: Hyperbaric oxygen; CBBbF: CBB combined with basic fibroblast growth factor; NHBO: Non-hyperbaric oxygen; CBB: Calcined bovine bone; Con: Control; OB: Old bone; ▲: Graft;  : New bone; ★: Inflammatory cell; ▽: Blood vessel. : New bone; ★: Inflammatory cell; ▽: Blood vessel.
|
由表 2可见,术后12周时,HBO-CBB组和HBO-CBBbF组的新骨形成面积百分比均大于HBO-Con组(P均<0.01),NHBO-CBB组和NHBO-CBBbF组的新骨形成面积百分比均大于NHBO-Con组(P均<0.01);HBO-CBB组、HBO-CBBbF组和NHBO-CBBbF组新骨形成面积百分比均大于NHBO-CBB组(P均<0.01)。
|
|
表 2 术后4、8、12周时各组大鼠新骨形成面积百分比比较 Tab 2 Comparison of the percentages of new bone formation areas of rats in each group at 4, 8 and 12 weeks after operation |
2.3 各组大鼠骨缺损修复组织中OPG和CD34表达的特点及水平
免疫组织化学染色结果(图 2、3)显示,在术后4、8、12周时,HBO-CBB组、HBO-CBBbF组和NHBO-CBBbF组大鼠骨缺损修复组织中OPG和CD34的表达较NHBO-CBB组、HBO-Con组和NHBO-Con组增高。术后12周时HBO-CBB组、HBO-CBBbF组、NHBO-CBB组和NHBO-CBBbF组大鼠骨缺损修复组织中OPG和CD34表达较术后8周降低。血管组织CD34表达呈强阳性,新骨和骨髓CD34和OPG表达呈阳性,成熟新骨CD34、OPG表达呈弱阳性,陈旧骨CD34、OPG表达呈弱阳性或阴性,移植材料周围OPG染色呈阳性。
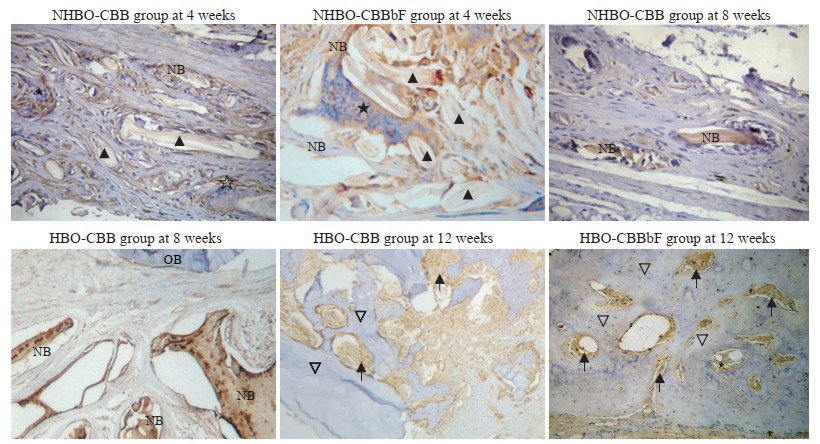
|
图 2
免疫组织化学染色检测术后大鼠骨缺损修复组织中OPG的表达(200×)
Fig 2
OPG expression in bone defect repair tissues of rats after operation detected by immunohistochemical staining (200×)
OPG: Osteoprotegerin; NHBO: Non-hyperbaric oxygen; CBB: Calcined bovine bone; CBBbF: CBB combined with basic fibroblast growth factor; HBO: Hyperbaric oxygen; NB: New bone; OB: Old bone. ▲: Graft; ★: Inflammatory cell; ▽: Mature new bone;  : Bone marrow. : Bone marrow.
|

|
图 3
免疫组织化学染色检测术后大鼠骨缺损修复组织中CD34的表达(200×)
Fig 3
CD34 expression in bone defect repair tissues of rats after operation detected by immunohistochemical staining (200×)
NHBO: Non-hyperbaric oxygen; Con: Control; CBB: Calcined bovine bone; CBBbF: CBB combined with basic fibroblast growth factor; HBO: Hyperbaric oxygen; OB: Old bone; NB: New bone.  : Blood vessel; ▽: Mature new bone. : Blood vessel; ▽: Mature new bone.
|
用光密度值反映大鼠骨缺损修复组织中OPG、CD34的表达水平发现,术后4、8、12周时,HBO-CBB组和HBO-CBBbF组大鼠骨缺损修复组织中OPG和CD34的表达水平均较HBO-Con组升高(P均<0.01),HBO-CBBbF组大鼠骨缺损修复组织中OPG和CD34的表达水平均较HBO-CBB组、NHBO-CBB组和NHBO-CBBbF组升高(P<0.05,P<0.01),NHBO-CBB组和NHBO-CBBbF组大鼠骨缺损修复组织中OPG、CD34的表达水平均较NHBO-Con组升高(P<0.05,P<0.01);术后8周时各组大鼠骨缺损修复组织中OPG和CD34表达水平高于术后4周和术后12周时。见表 3、表 4。
|
|
表 3 术后4、8、12周时各组大鼠骨缺损修复组织中OPG光密度值比较 Tab 3 Comparison of absorbance values of OPG in bone defect repair tissues of rats in each group at 4, 8 and 12 weeks after operation |
|
|
表 4 术后4、8、12周时各组大鼠骨缺损修复组织中CD34光密度值比较 Tab 4 Comparison of absorbance values of CD34 in bone defect repair tissues of rats in each group at 4, 8 and 12 weeks after operation |
3 讨论
据报道,美国每年有600例骨折患者,其中5%因骨折愈合欠佳而残疾[7],因此探讨骨缺损修复方法有着重要意义。我们既往研究发现,异种骨移植物Bio-Oss骨粉和Bio-Gide膜联合可促进骨再生,对牙周骨缺损修复有良好效果[8]。有研究显示CBB(骼瑞骨粉)有良好骨传导性,可引导新骨形成,促进骨缺损愈合,对颅骨和牙槽骨缺损修复有一定作用[1, 9];CBB对拔牙后颌骨缺损修复效果达93%以上,在24周时能有效保持拔牙后牙槽骨宽度和高度,其作用与Bio-Oss骨移植材料相似[1]。富血小板纤维蛋白(platelet rich fibrin,PRF)可分泌bFGF等多种生长因子诱导新骨形成,CBB联合PRF对兔颅骨缺损修复有明显疗效[6]。
CBB骨粉是天然牛骨经专利技术脱脂、脱蛋白、低温煅烧制成,主要成分为羟基磷灰石结晶,具有天然骨小梁及管腔系统,保留了天然骨多孔结构,其较大内表面积适宜细胞因子复合和细胞长入,生物相容性好。本研究发现,NHBO-CBBbF组和NHBO-CBB组在术后4、8和12周时BD和新骨形成面积百分比均大于对照组(NHBO-Con组),表明CBB作为支架材料有利于细胞成骨;NHBO-CBB组在12周时骨缺损处有新骨形成,表明CBB用于6 mm颅骨缺损有较好疗效。
胶原具有多孔结构,可在体内降解,提高细胞渗透性,利用胶原作为载体可使bFGF维持于骨缺损处缓慢释放,发挥生物学作用。Nakamura等[2]发现,bFGF联合胶原支架可促进大鼠水平骨缺损修复和骨再生。我们前期研究结果显示bFGF对人牙周骨缺损修复有显著疗效[10]。本研究用医用胶原作为bFGF载体观察到NHBO-CBBbF组大鼠在术后4、8和12周时骨缺失区的BD大于NHBO-CBB组,新骨形成面积百分比在术后8周和12周时大于NHBO-CBB组,表明用胶原海绵作为bFGF载体可促进CBB修复骨缺损的疗效。bFGF作用的发挥与其激活下游信号、促进血管再生、增加成骨细胞增殖和分化等有关[11]。
Yamamoto等[12]发现在挫伤后3~7 d用HBO暴露可促进血管形成。本实验结果显示,在术后4周时HBO-CBBbF组大鼠骨缺损修复组织中OPG和CD34表达水平高于NHBO-CBBbF组和HBO-CBB组,表明在暴露早期HBO对骨缺损修复组织中OPG和CD34表达有促进作用,HBO联合bFGF的作用更明显。Zhou等[13]报道,HBO可改善大鼠脊髓损伤后血管内皮生长因子(vascular endothelial growth factor,VEGF)含量,减少缺氧诱导因子1α(hypoxia inducible factor 1α,HIF-1α)表达,提高毛细血管密度,促进富血小板血浆对骨缺损的修复作用[14]。还有研究表明,HBO可刺激体内血管再生干细胞的生长和分化[15],对幼鼠和成年小鼠骨再生有促进作用[16]。本研究发现在术后4、8和12周时HBO-CBB和HBO-CBBbF组大鼠骨缺损区BD和新骨形成面积百分比均大于对照组(HBO-Con组),且成骨细胞数量和血管数量增加,HBO-CBBbF组BD和新骨形成面积百分比大于HBO-CBB组,HBO-CBB组和HBO-CBBbF炎症细胞数量减少,表明HBO有骨缺损修复作用,联合bFGF后疗效更好。
Mulawarmanti等[17]报道,HBO治疗可降低大鼠血清CRP水平,增强糖尿病牙周炎组织中OPG表达,减少破骨细胞数量。研究表明HBO治疗可调节股骨头坏死患者血清OPG/NF-κB受体活化因子配体水平,提升血清OPG表达[18]。Cheung等[19]报道,HBO可促进脐带血造血干/祖细胞归巢,并影响脐带血CD34+细胞分化、增殖和体外迁移。研究还发现HBO可增加碱性磷酸酶活性,促进胶原和Runt相关转录因子2(Runt-related transcription factor 2,Runx-2)mRNA表达,加速成骨细胞分化和骨形成[20]。本研究发现,大鼠颅骨缺损修复后接受HBO治疗或HBO联合bFGF治疗可提高术后4、8和12周时OPG和CD34表达水平,在术后8周时OPG和CD34表达水平增高、血管数量增多,与Oh等[21]的研究结果一致。本研究表明,CBB对骨缺损修复有一定疗效,HBO暴露可加快bFGF和CBB对骨缺损的修复愈合,其机制与HBO促进OPG和CD34表达有关。
HBO促进骨愈合的机制尚不清楚。研究发现,HBO暴露可增加损伤组织细胞中氧水平,使活性氧分子和活性氮分子增加,通过调控HIF-1α等途径促进内皮细胞、巨噬细胞等对VEGF和bFGF的分泌,使组织中生长因子增加,促进新生血管再生[22]。Sunkari等[23]发现HBO可激活HIF-1,改善糖尿病小鼠的伤口愈合;HBO还可增加矿化物沉积,并通过增加Runx2表达促进骨缺损处新骨形成,加速骨愈合[24];促进兔骨缺损区血管生成,使VEGF蛋白表达增加,对骨愈合起正向调控作用[25]。这些研究结果证明HBO对骨缺损修复的有效性,但HBO如何通过HIF-1调控骨再生尚需进一步研究。
| [1] |
崔妮, 秦瑞峰, 侯锐, 丁宇翔, 张林林, 王晓娟, 等. 天然煅烧骨修复材料用于拔牙后颌骨缺损修复的多中心临床研究[J]. 实用口腔医学杂志, 2015, 31: 81-84. |
| [2] |
NAKAMURA S, ITO T, OKAMOTO K, MIMA T, UCHIDA K, SIDDIQUI Y D, et al. Acceleration of bone regeneration of horizontal bone defect in rats using collagen-binding basic fibroblast growth factor combined with collagen scaffolds[J]. J Periodontol, 2019, 90: 1043-1052. DOI:10.1002/JPER.18-0674 |
| [3] |
CHEN T L, XU B, LIU J C, LI S G, LI D Y, GONG G C, et al. Effects of hyperbaric oxygen on aggressive periodontitis and subgingival anaerobes in Chinese patients[J]. J Indian Soc Periodontol, 2012, 16: 492-497. DOI:10.4103/0972-124X.106880 |
| [4] |
CAWLEY K M, BUSTAMANTE-GOMEZ N C, GUHA A G, MACLEOD R S, XIONG J, GUBRIJ I, et al. Local production of osteoprotegerin by osteoblasts suppresses bone resorption[J/OL]. Cell Rep, 2020, 32: 108052. DOI: 10.1016/j.celrep.2020.108052.
|
| [5] |
DONG Z, CHEN B, FU W, WANG Y, GUO D, WEI Z, et al. Transplantation of purified CD34+ cells in the treatment of critical limb ischemia[J/OL]. J Vasc Surg, 2013, 58: 404-411. e3. DOI: 10.1016/j.jvs.2013.01.037.
|
| [6] |
陈铁楼, 张新海, 许兵, 徐东升, 王世峰, 秦卫民, 等. 富血小板纤维蛋白联合天然煅烧骨对颅骨缺损修复作用研究[J]. 口腔医学, 2018, 38: 970-975. |
| [7] |
BURCHARDT H. The biology of bone graft repair[J]. Clin Orthop Relat Res, 1983(174): 28-42. |
| [8] |
陈铁楼, 刘国勤, 赵海军, 陈骏, 张庆福, 张新海, 等. 异种骨移植物Bio-Oss胶原和Bio-Gide膜对牙周骨下袋影响的临床研究[J]. 临床口腔医学杂志, 2007, 23: 515-517. DOI:10.3969/j.issn.1003-1634.2007.09.001 |
| [9] |
何越, 田智泉, 王亮, 姚阳, 杨鹭, 黄文涛, 等. 天然煅烧骨修复材料(骼瑞)修复动物骨缺损的有效性研究[J]. 实用口腔医学杂志, 2015, 31: 167-170. DOI:10.3969/j.issn.1001-3733.2015.02.04 |
| [10] |
陈铁楼, 张新海, 秦卫民, 王晓曼, 江一峰, 任正波, 等. 天然煅烧骨粉与碱性成纤维细胞生长因子联合对牙周骨缺损修复作用研究[J]. 中国实用口腔科杂志, 2018, 11: 224-228. |
| [11] |
SAVCHENKO E, TEKU G N, BOZA-SERRANO A, RUSS K, BERNS M, DEIERBORG T, et al. FGF family members differentially regulate maturation and proliferation of stem cell-derived astrocytes[J/OL]. Sci Rep, 2019, 9: 9610. DOI: 10.1038/s41598-019-46110-1.
|
| [12] |
YAMAMOTO N, OYAIZU T, ENOMOTO M, HORIE M, YUASA M, OKAWA A, et al. VEGF and bFGF induction by nitric oxide is associated with hyperbaric oxygen-induced angiogenesis and muscle regeneration[J/OL]. Sci Rep, 2020, 10: 2744. DOI: 10.1038/s41598-020-59615-x.
|
| [13] |
ZHOU Y, LIU X H, QU S D, YANG J, WANG Z W, GAO C J, et al. Hyperbaric oxygen intervention on expression of hypoxia-inducible factor-1α and vascular endothelial growth factor in spinal cord injury models in rats[J]. Chin Med J (Engl), 2013, 126: 3897-3903. |
| [14] |
CHEN T L, WANG S F, ZHANG X H, CHEN J, LIU J. Synergistic effects of hyperbaric oxygen combining with platelet rich plasma on bone defects repair: a mini-review[J/OL]. (2020-06-23)[2020-06-25]. J Dent Oral Disord, 2020, 6: 1140. https://austinpublishinggroup.com/dental-disorders/fulltext/jdod-v6-id1140.php.
|
| [15] |
MILOVANOVA T N, BHOPALE V M, SOROKINA E M, MOORE J S, HUNT T K, HAUER-JENSEN M, et al. Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo[J]. J Appl Physiol (1985), 2009, 106: 711-728. DOI:10.1152/japplphysiol.91054.2008 |
| [16] |
IZUMINO J, KAKU M, YAMAMOTO T, YASHIMA Y, KAGAWA H, IKEDA K, et al. Effects of hyperbaric oxygen treatment on calvarial bone regeneration in young and adult mice[J/OL]. Arch Oral Biol, 2020, 117: 104828. DOI: 10.1016/j.archoralbio.2020.104828.
|
| [17] |
MULAWARMANTI D, PARISIHNI K, WIDYASTUTI W. The impact of hyperbaric oxygen therapy on serum C-reactive protein levels, osteoprotegerin expression, and osteoclast numbers in induced-periodontitis diabetic rats[J]. Eur J Dent, 2020, 14: 404-409. |
| [18] |
VEZZANI G, QUARTESAN S, CANCELLARA P, CAMPORESI E, MANGAR D, BERNASEK T, et al. Hyperbaric oxygen therapy modulates serum OPG/RANKL in femoral head necrosis patients[J]. J Enzyme Inhib Med Chem, 2017, 32: 707-711. DOI:10.1080/14756366.2017.1302440 |
| [19] |
CHEUNG K Y, BERRY A, LI D, ALJITAWI O S. Hyperbaric oxygen treatment effects on in vitro cultured umbilical cord blood CD34+ cells[J]. Cytotherapy, 2018, 20: 87-94. DOI:10.1016/j.jcyt.2017.08.020 |
| [20] |
AL HADI H, SMERDON G R, FOX S W. Hyperbaric oxygen therapy accelerates osteoblast differentiation and promotes bone formation[J]. J Dent, 2015, 43: 382-388. DOI:10.1016/j.jdent.2014.10.006 |
| [21] |
OH S E, HU K S, KIM S. Eight-week healing of grafted calvarial bone defects with hyperbaric oxygen therapy in rats[J]. J Periodontal Implant Sci, 2019, 49: 228-236. |
| [22] |
THOM S R. Hyperbaric oxygen: its mechanisms and efficacy[J]. Plast Reconstr Surg, 2011, 127(Suppl 1): 131S-141S. |
| [23] |
SUNKARI V G, LIND F, BOTUSAN I R, KASHIF A, LIU Z J, YLÄ-HERTTUALA S, et al. Hyperbaric oxygen therapy activates hypoxia-inducible factor 1(HIF-1), which contributes to improved wound healing in diabetic mice[J]. Wound Repair Regen, 2015, 23: 98-103. |
| [24] |
ROCHA F S, GOMES MOURA C C, ROCHA RODRIGUES D B, ZANETTA-BARBOSA D, NAKAMURA HIRAKI K R, DECHICHI P. Influence of hyperbaric oxygen on the initial stages of bone healing[J]. Oral Surg Oral Med Oral Pathol Oral Radiol, 2015, 120: 581-587. |
| [25] |
FOK T C, JAN A, PEEL S A, EVANS A W, CLOKIE C M, SÁNDOR G K. Hyperbaric oxygen results in increased vascular endothelial growth factor (VEGF) protein expression in rabbit calvarial critical-sized defects[J]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2008, 105: 417-422. |
 2021, Vol. 42
2021, Vol. 42


