2. 新疆医科大学第一附属医院昌吉分院呼吸与危重症医学科, 昌吉 831100;
3. 复旦大学附属华东医院呼吸与危重症医学科, 上海 200040;
4. 上海市浦东新区人民医院呼吸与危重症医学科, 上海 201200;
5. 上海市普陀区人民医院呼吸与危重症医学科, 上海 200060;
6. 上海交通大学医学院附属瑞金医院呼吸与危重症医学科, 上海 200020;
7. 上海交通大学医学院附属新华医院呼吸与危重症医学科, 上海 200092;
8. 上海中医药大学附属曙光医院宝山分院呼吸与危重症医学科, 上海 200940;
9. 上海交通大学医学院附属同仁医院呼吸与危重症医学科, 上海 200336;
10. 复旦大学附属华山医院呼吸与危重症医学科, 上海 200040;
11. 同济大学附属上海市肺科医院呼吸与危重症医学科, 上海 200433;
12. 复旦大学附属浦东医院呼吸与危重症医学科, 上海 201300;
13. 同济大学附属同济医院呼吸与危重症医学科, 上海 200065;
14. 复旦大学附属上海市第五人民医院呼吸与危重症医学科, 上海 200040;
15. 上海交通大学医学院附属第九人民医院呼吸与危重症医学科, 上海 200011
2. Department of Respiratory and Critical Care Medicine, Changji Branch of the First Affiliated Hospital of Xinjiang Medical University, Changji 831100, Xinjiang Uygur Autonomous Region, China;
3. Department of Respiratory and Critical Care Medicine, Huadong Hospital Affiliated to Fudan University, Shanghai 200040, China;
4. Department of Respiratory and Critical Care Medicine, People's Hospital of Shanghai Pudong New Area, Shanghai 201200, China;
5. Department of Respiratory and Critical Care Medicine, People's Hospital of Putuo District, Shanghai 200060, China;
6. Department of Respiratory and Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200020, China;
7. Department of Respiratory and Critical Care Medicine, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China;
8. Department of Respiratory and Critical Care Medicine, Baoshan Branch of Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200940, China;
9. Department of Respiratory and Critical Care Medicine, Tongren Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200336, China;
10. Department of Respiratory and Critical Care Medicine, Huashan Hospital, Fudan University, Shanghai 200040, China;
11. Department of Respiratory and Critical Care Medicine, Shanghai Pulmonary Hospital, Tongji University, Shanghai 200433, China;
12. Department of Respiratory and Critical Care Medicine, Shanghai Pudong Hospital, Fudan University, Shanghai 201300, China;
13. Department of Respiratory and Critical Care Medicine, Tongji Hospital, Tongji University, Shanghai 200065, China;
14. Department of Respiratory and Critical Care Medicine, Shanghai Fifth People's Hospital, Fudan University, Shanghai 200040, China;
15. Department of Respiratory and Critical Care Medicine, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, China
慢性阻塞性肺疾病(chronic obstructive pulmonary disease,COPD)是以持续性气流受限为特征的一种常见慢性气道疾病,好发于中老年人,其发病率、病死率逐年增高,治疗手段有限,不仅严重威胁患者的生命健康,也给个人、家庭、社会带来沉重的经济负担[1-3],一直是呼吸科的研究重点。文献报道COPD患者合并症发生率高,个体差异大,可不同程度影响患者预后[4-6]。例如,心血管疾病、代谢性疾病、精神疾病、骨骼肌肉系统疾病等合并症可导致COPD患者的生活质量下降、急性加重及死亡风险增加[7-8],部分成为导致COPD患者全因死亡的独立预测因子,因此加强对COPD合并症的管理对改善患者预后有重要意义[9-12]。目前国内有关研究主要集中在急性加重期COPD患者,并认为合并症的种类和特征有所差异,但尚未见有关我国东部地区COPD合并症特点的研究报道。Charlson合并症指数(Charlson comorbidity index,CCI)是多种疾病合并症较为常用的评估工具之一[13],在国外被广泛应用,我国针对该指标在COPD中的应用报道较少。为进一步了解上海地区COPD患者合并症的发生情况,本研究对上海地区部分具有呼吸专科的二、三级甲等医院诊治的稳定期COPD患者开展了多中心、横断面调查,分析了稳定期COPD患者合并症的分布特征,借助CCI评分工具对合并症进行综合评估,并对主要合并症与疾病严重程度的相关性进行分析。
1 对象和方法 1.1 研究对象采用横断面调查设计,通过普查的方法,连续纳入上海地区具有呼吸专科的14家二、三级甲等医院呼吸与危重症医学科门诊2018年10月至2019年8月诊治的所有稳定期COPD患者,通过面对面填写病例报告表的形式收集患者的临床资料和33种合并症资料。纳入标准:(1)年龄≥40岁;(2)有吸烟史(吸烟指数≥10包年),或长期生物燃料或化学燃料接触史;(3)符合2017慢性阻塞性肺疾病全球倡议(global initiative for chronic obstructive lung disease,GOLD)COPD诊断标准[14]的稳定期COPD患者(纳入研究前6周内患者病情基本稳定且无急性加重[15]);(4)合并症处于稳定期;(5)签署研究知情同意书。本研究通过复旦大学附属华东医院伦理委员会审批(2018K127)。
1.2 临床资料采集与合并症判断收集患者性别、年龄、身高、体重、吸烟指数、COPD病程、慢性阻塞性肺疾病评估测试(chronic obstructive pulmonary disease assessment test,CAT)评分、改良英国医学研究学会呼吸困难指数(modified British Medical Research Council, mMRC)评分、圣乔治呼吸问卷(St. George respiratory questionnaire,SGRQ)评分、汉密尔顿焦虑量表(Hamilton anxiety scale,HAMA)评分、GOLD分组、6-min步行距离(6-min walking distance,6MWD)、6MWD测试前后(即刻)指脉氧饱和度(pulse blood oxygen saturation,SpO2)、一氧化氮呼气试验结果、肺功能指标、既往1年内急性加重次数、是否使用吸入药物治疗等资料。收集COPD患者8个类别共33种合并症(表 1),33种合并症的诊断均以既往明确病史及辅助检查结果符合最新诊断标准为依据。GOLD分组具体方法:以过去1年内发生0~1次急性加重未导致住院,且CAT评分<10分、mMRC评分0~1分者为A组;过去1年内发生0~1次急性加重未导致住院,且CAT评分≥10分或mMRC评分≥2分者为B组;过去1年内发生≥2次急性加重,或≥1次急性加重导致住院,且CAT评分<10分、mMRC评分0~1分者为C组;过去1年内发生≥2次急性加重,或≥1次急性加重导致住院,且CAT评分≥10分或mMRC评分≥2分者为D组。
|
|
表 1 8个类别33种合并症 Tab 1 Thirty-three kinds of comorbidities in 8 major categories |
1.3 合并症特征分析
根据CAT或mMRC评分,将研究对象分为多症状(CAT评分≥10分或mMRC评分≥2分)与少症状(CAT评分<10分且mMRC评分0~1分)组。按照既往1年内急性加重次数,将研究对象分为频繁急性加重(frequent exacerbation,FE;过去1年内急性加重≥2次,或≥1次急性加重导致住院)与非频繁急性加重(non-frequent exacerbation,NFE;过去1年内未出现急性加重,或出现1次急性加重未导致住院)[16]组。计算患者CCI评分[13]:(1)合并症评分。合并冠状动脉疾病、轻微肝脏疾病、消化性溃疡疾病、充血性心力衰竭、脑血管疾病、糖尿病、慢性肺部疾病、结缔组织疾病、周围性血管疾病计1分,偏瘫、痴呆、5年内任何肿瘤、中重度肾脏疾病、白血病、糖尿病伴器官损害、淋巴瘤计2分,中至重度肝脏疾病计3分,转移性实体肿瘤计6分。(2)年龄评分。从50岁起计1分,每增加10岁加1分。CCI评分为合并症评分与年龄评分之和。以CCI评分4分[17]为界,将研究对象分为CCI高分(≥4分)与低分(<4分)组。
1.4 统计学处理应用SPSS 21.0软件进行统计学分析。呈正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验。非正态分布的计量资料以中位数(下四分位数,上四分位数)表示,两组间比较采用Mann-Whitney U检验。计数资料以例数和百分数表示,两组间比较采用χ2检验。合并症与COPD严重程度的相关性分析采用单因素和多因素逐步向前法logistic回归分析。检验水准(α)为0.05。
2 结果 2.1 患者一般临床特征本研究共收集601例稳定期COPD患者,其中二级甲等医院251例,三级甲等医院350例,剔除病例资料不全患者69例,最终纳入532例。二级甲等医院214例,分别为上海市浦东新区人民医院93例、上海市普陀区人民医院71例、上海交通大学医学院附属同仁医院24例、复旦大学附属浦东医院12例、复旦大学附属上海市第五人民医院14例;三级甲等医院318例,分别为海军军医大学(第二军医大学)长海医院93例、复旦大学附属华东医院52例、上海交通大学医学院附属瑞金医院44例、上海交通大学医学院附属新华医院42例、上海中医药大学附属曙光医院宝山分院34例、同济大学附属上海市肺科医院20例、复旦大学附属华山医院19例、同济大学附属同济医院8例、上海交通大学医学院附属第九人民医院6例。
532例稳定期COPD患者年龄为43~95岁,平均年龄为(70.44±8.98)岁;男472例(88.7%),女60例(11.3%);吸烟指数为30(20,42)包年;BMI为(23.32±3.41)kg/m2;COPD病程为0~50年,平均病程为(8.00±7.59)年,过去1年内急性加重次数为1(0,2)次。CAT评分为15(10,23)分;mMRC评分为(1.86±1.00)分;SGRQ评分为(40.34±19.09)分;HAMA评分为10(6,12)分。6MWD为(314.57±88.06)m;6MWD测试前SpO2为(95.27±3.07)%,测试后SpO2为(93.31±3.53)%;吸入支气管扩张剂后第1秒用力呼气容积/用力肺活量(forced expiratory volume in the first second/forced vital capacity,FEV1/FVC)为(55.94±10.99)%,第1秒用力呼气容积占预计值的百分比(forced expiratory volume in the first second as a percentage of the predicted,FEV1%pred)为(46.63±16.88)%。正在使用吸入药物治疗者441例(82.9%),其中吸入长效胆碱能拮抗剂(long-acting muscarinic antagonist,LAMA)患者92例(20.9%),联合使用吸入糖皮质激素(inhaled corticosteroid,ICS)与长效β受体激动剂(long-acting β receptor agonist,LABA)患者175例(39.7%),联合使用ICS、LABA与LAMA患者171例(38.8%),联合使用LABA与LAMA患者3例(0.7%);未使用吸入药物治疗者91例(17.1%,91/532)。GOLD分组中A组80例(15.0%),B组185例(34.8%),C组14例(2.6%),D组253例(47.6%)。多症状组438例(82.3%),少症状组94例(17.7%);FE组267例(50.2%),NFE组265例(49.8%);CCI高分组248例(46.6%),CCI低分组284例(53.4%)。
2.2 532例稳定期COPD患者的合并症及CCI评分分布33种合并症中共收集到28种。393例(73.9%)患者存在合并症,合并症总数为1 005例次,人均合并症数量为2(0,3)种。发生率>10%的合并症有肺动脉高压症(34.2%,182/532)、高血压(27.1%,144/532)、支气管哮喘(25.4%,135/532)、代谢综合征(22.9%,122/532)、过敏性鼻炎(10.9%,58/532),见图 1。按类别分析,发生率居前4位的是慢性肺部疾病(41.5%,221/532)、过敏性疾病(34.4%,183/532)、心血管疾病(32.3%,172/532)、代谢性疾病(26.5%,141/532),其余为消化系统慢性病(10.9%,58/532)、神经系统疾病(7.1%,38/532)、恶性肿瘤(5.6%,30/532)、其他疾病(4.5%,24/532)。532例患者的CCI评分为0~13分,平均(3.60±1.57)分。
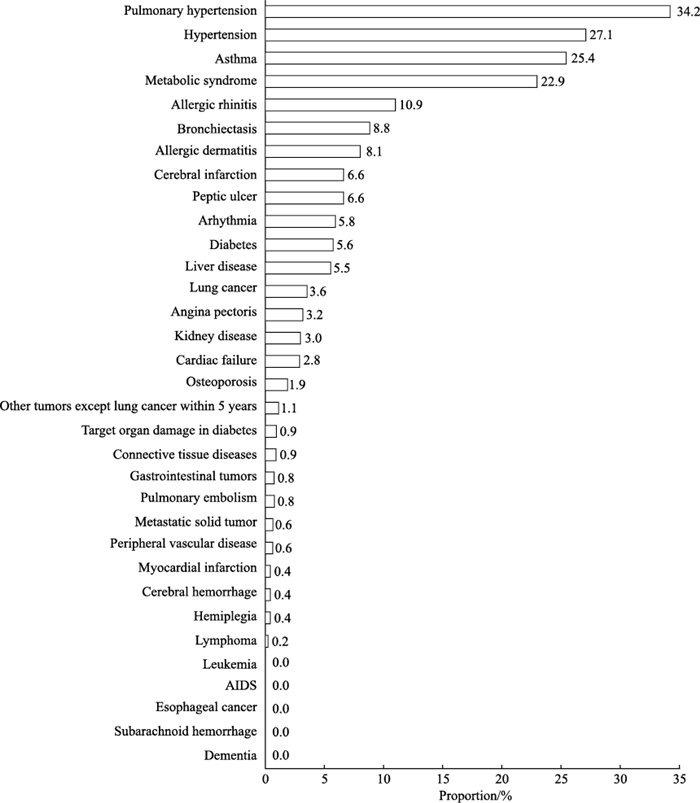
|
图 1 532例稳定期COPD患者33种合并症分布 Fig 1 Distribution of 33 kinds of comorbidities in 532 stable COPD patients COPD: Chronic obstructive pulmonary disease; AIDS: Acquired immune deficiency syndrome. |
2.2.1 多症状组与少症状组稳定期COPD患者合并症及CCI评分分布
见表 2,多症状组与少症状组稳定期COPD患者的CCI评分、有合并症患者比例、人均合并症数量比较差异均无统计学意义(P均>0.05);多症状组合并肺动脉高压症的患者比例低于少症状组,差异有统计学意义(χ2=6.749,P=0.009),其余合并症发生率在两组间比较差异均无统计学意义(P均>0.05);按类别分析,多症状组合并过敏性疾病的患者比例低于少症状组,差异有统计学意义(χ2=10.693,P=0.001),其余各系统疾病发生率在两组间比较差异均无统计学意义(P均>0.05)。单因素logistic回归分析显示,合并肺动脉高压症的稳定期COPD患者多为少症状组患者(OR=0.551,95% CI 0.351~0.867,P=0.010)。
|
|
表 2 多症状组与少症状组稳定期COPD患者合并症比较 Tab 2 Comparison of comorbidities in stable COPD patients between multi-symptom and few-symptom groups |
2.2.2 FE组与NFE组稳定期COPD患者合并症及CCI评分分布
见表 3,FE组与NFE组稳定期COPD患者的CCI评分、有合并症患者占比与人均合并症数量比较差异均无统计学意义(P均>0.05);28种合并症中,FE组合并支气管扩张症、肝脏疾病、肾脏疾病的患者比例均高于NFE组(χ2=5.128、4.326、4.062,P=0.024、0.038、0.044),而合并肺动脉高压症、支气管哮喘、代谢综合征的患者比例均低于NFE组(χ2=8.922、8.643、14.138,P=0.003、P=0.003、P<0.01);按类别分析,FE组合并过敏性疾病、代谢性疾病的患者比例均低于NFE组(χ2=11.832、12.182,P=0.001、P<0.01),合并其他疾病(周围血管疾病、肾脏疾病、结缔组织病、骨质疏松症)的患者比例高于NFE组(χ2=4.285,P=0.038)。见表 4,多因素logistic回归分析显示,合并支气管扩张症的稳定期COPD患者易发生FE(OR=2.127,95% CI 1.114~4.060,P=0.022),合并支气管哮喘和/或代谢综合征的稳定期COPD患者发生FE的风险较低(OR=0.608,95% CI 0.399~0.924,P=0.020;OR=0.524,95% CI 0.339~0.809,P=0.004)。
|
|
表 3 FE组与NFE组稳定期COPD患者合并症比较 Tab 3 Comparison of comorbidities in stable COPD patients between FE and NFE groups |
|
|
表 4 合并症与稳定期COPD频繁急性加重的logistic回归分析 Tab 4 Logistic regression analysis of comorbidities and frequent exacerbation of stable COPD |
2.3 CCI高分组与低分组稳定期COPD患者临床特征比较
CCI高分组稳定期COPD患者年龄、吸烟指数、COPD病程、人均合并症数量、HAMA评分和呼出气一氧化氮(fractional exhaled nitric oxide,FeNO)水平均高于CCI低分组(Z=-13.852,P<0.01;Z=-2.123,P=0.034;Z=2.710,P=0.007;Z=-11.743,P<0.01;Z=-2.495,P=0.013;Z=-2.249,P=0.025),CAT评分、吸入支气管舒张剂前FEV1、FVC、用力肺活量占预计值的百分比(forced vital capacity as a percentage of the predicted,FVC%pred)与吸入支气管舒张剂后FVC均低于CCI低分组(Z=-2.079,P=0.038;t=3.811,P<0.01;t=5.075,P<0.01;t=2.028,P=0.043;t=3.176,P=0.002)。两组患者过去1年内急性加重次数比较差异无统计学意义(P>0.05)。见表 5。
|
|
表 5 CCI高分组与低分组稳定期COPD患者临床特征比较 Tab 5 Comparison of clinical features of stable COPD patients between high- and low-CCI groups |
3 讨论
根据本研究结果,上海地区部分二、三级甲等医院呼吸科就诊的稳定期COPD患者平均年龄为(70.44±48.98)岁,有合并症患者比例高达73.9%,人均合并症超过1种。在28种合并症中肺动脉高压症、高血压、支气管哮喘、代谢性综合征所占比例较高,提示上海地区稳定期COPD患者合并症发生率高且仍以老年人常见的心血管疾病、慢性呼吸系统疾病、代谢性疾病等慢性疾病为主。
我国北方地区的2项COPD合并症研究发现,急性加重期COPD患者合并症发生率均达80%以上,高于本组稳定期COPD患者(73.9%),并以肺部感染、心血管疾病、糖尿病多见[18-19]。河北、广西等地区有研究显示COPD患者焦虑抑郁发生率高达40%以上[20-21]。新疆地区COPD合并症以肺结核(46.2%)、冠心病(45.8%)、糖尿病(18.7%)和支气管扩张症(15.4%)为主[22]。国外一项研究显示,住院COPD患者合并呼吸道感染、心血管疾病、精神疾病、肌肉骨骼疾病、恶性肿瘤和糖尿病比例占全部疾病的1/5以上[23]。本研究结果显示,上海地区稳定期COPD患者除心血管疾病、糖尿病高发外,肺动脉高压症、支气管哮喘、代谢综合征的发生率也较高,提示COPD患者合并症存在地域差异,在临床询问病史时需兼顾地区差异以有侧重地采集患者临床资料,从而更全面地了解患者的健康状况并给予更准确的干预。
肺动脉高压症及高血压分别提示肺循环及体循环血管功能异常,两者的发生率在上海地区稳定期COPD患者合并症中居前2位。有学者提出内皮细胞和微血管病变在COPD发病中扮演了重要角色[24]。Wouters和Franssen[25]研究发现,即使是轻度COPD患者肺循环血流量也显著降低;在具有正常肺活量但肺弥散功能下降的健康吸烟者中发现内皮微粒水平增高,其可能来自凋亡的内皮细胞[26]。吸烟可早期引起内皮细胞凋亡、细胞功能紊乱,从而影响循环系统导致相应病变[5, 27],而内皮功能障碍可能是COPD患者存在多种合并症的潜在机制之一[28]。本研究发现上海地区稳定期COPD患者合并肺动脉高压症的比例高达30%以上,且少症状组及NFE组患者发生率较高,提示肺循环血管病变可出现在COPD患者早期阶段,早期针对内皮功能异常进行干预将有助于改善COPD预后。
Maio等[29]的一项针对20岁以上患者的大型研究表明,有烟草暴露和/或生物燃料、汽车尾气、雾霾天气等环境暴露者COPD、支气管哮喘和过敏性鼻炎的患病率是无暴露史者的至少2倍,本研究结果显示COPD合并过敏性疾病的患者占全部COPD患者的34.4%,推测烟草暴露和环境暴露可能是导致COPD患者过敏性疾病发生率高的外部因素之一。部分以嗜酸性粒细胞增高为表型的COPD与支气管哮喘有共同的炎症通路,两者同时存在的概率并不低[30],研究认为使用ICS可使此类人群的症状得到很好的控制[31]。本研究中,COPD合并支气管哮喘的总患病率为25.4%(135/532),且在少症状组及NFE组占比偏高,可能的原因是多数稳定期COPD患者长期规范使用吸入药物且大多数含ICS,并且对ICS治疗敏感,使病情控制稳定。
本研究多因素logistic回归分析显示,合并支气管扩张症是COPD患者FE的危险因素。Martinez-Garcia和Miravitlles[32]研究指出,无论是支气管扩张症并发不完全可逆的气流受限后临床具有COPD慢性炎症特征,还是COPD的特殊表型支气管扩张样改变,均能导致患者反复感染、FE、生活质量下降及肺功能受损等,且发生率均高于未合并支气管扩张症的COPD患者。2016年一项meta分析也表明合并支气管扩张症的COPD患者死亡风险增加(OR=1.96,95% CI 1.04~3.70)[33]。目前有关COPD合并支气管扩张表型的研究尚少,本研究将高分辨CT检查符合支气管扩张症临床表现的患者均纳入合并症研究中,结果发现FE组稳定期COPD患者支气管扩张症合并率高,且与稳定期COPD患者FE的风险相关,进一步证明了支气管扩张症的管理有助于降低COPD远期风险。
CCI主要用于评价患者1年死亡率和疾病负担,其特点是年龄大、风险高的疾病CCI评分高,是综合评估高龄及具有复杂合并症患者疾病状态的一个较好工具。国内研究中大多将年龄评分、支气管扩张症及支气管哮喘排除在外,黄亚玲等[17]研究认为CCI高分(≥4分)对COPD患者1年内急性加重有显著影响,但该研究纳入患者较少(64例)。本研究中CCI包含年龄评分和合并症评分,通过分组分析发现CCI高分组稳定期COPD患者的年龄更大、吸烟指数更高、COPD病程更长、肺功能更差、合并症更多,提示COPD患者合并症对其病情进展有一定影响,有合并症的患者可能更易发生FE[17, 34],临床仍需重视COPD合并症的管理,以制订更有针对性的综合干预措施[35]。此外,本研究CCI高分组患者FeNO水平高,提示CCI评分高的患者气道炎症更严重,可能是ICS治疗的获益人群。平时对COPD患者进行CCI评分,或许可作为判断患者能否从ICS治疗获益的一个评价指标,但目前尚缺乏相关研究。
本研究的患者来源局限于呼吸科门诊,对于一些无症状或症状不明显的早期COPD患者未能及时获取,故样本选择存在一定偏倚。
综上所述,上海地区14家二、三级甲等医院就诊的稳定期COPD患者合并症发生率高且不受患者症状多少及是否FE影响,并以慢性肺部疾病、过敏性疾病、心血管疾病、代谢性疾病为主。CCI高分患者年龄更大、病程更长、肺功能更差。合并支气管扩张症可能是导致COPD患者FE的危险因素之一,及早发现并积极干预支气管扩张症有助于提高COPD管理水平和改善预后。
| [1] |
NEGEWO N A, GIBSON P G, WARK P A, SIMPSON J L, MCDONALD V M. Treatment burden, clinical outcomes, and comorbidities in COPD: an examination of the utility of medication regimen complexity index in COPD[J]. Int J Chron Obstruct Pulmon Dis, 2017, 12: 2929-2942. DOI:10.2147/COPD.S136256 |
| [2] |
ALOTAIBI N M, CHEN V, HOLLANDER Z, HAGUE C J, MURPHY D T, LEIPSIC J A, et al. Phenotyping COPD exacerbations using imaging and blood-based biomarkers[J]. Int J Chron Obstruct Pulmon Dis, 2018, 13: 217-229. DOI:10.2147/COPD.S152484 |
| [3] |
VIINANEN A, LASSENIUS M I, TOPPILA I, KARLSSON A, VEIJALAINEN L, IDÄNPÄÄNHEIKKILÄ J J, et al. The burden of chronic obstructive pulmonary disease (COPD) in Finland: impact of disease severity and eosinophil count on healthcare resource utilization[J]. Int J Chron Obstruct Pulmon Dis, 2019, 14: 2409-2421. DOI:10.2147/COPD.S222581 |
| [4] |
DIVO M, COTE C, DE TORRES J P, CASANOVA C, MARIN J M, PINTO-PLATA V, et al; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease[J]. Am J Respir Crit Care Med, 2012, 186: 155-161.
|
| [5] |
NEGEWO N A, GIBSON P G, MCDONALD V M. COPD and its comorbidities: impact, measurement and mechanisms[J]. Respirology, 2015, 20: 1160-1171. DOI:10.1111/resp.12642 |
| [6] |
HAN M K, AGUSTI A, CALVERLEY P M, CELLI B R, CRINER G, CURTIS J L, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD[J]. Am J Respir Crit Care Med, 2010, 182: 598-604. DOI:10.1164/rccm.200912-1843CC |
| [7] |
MOTEGI T, JONES R C, ISHII T, HATTORI K, KUSUNOKI Y, FURUTATE R, et al. A comparison of three multidimensional indices of COPD severity as predictors of future exacerbations[J]. Int J Chron Obstruct Pulmon Dis, 2013, 8: 259-271. |
| [8] |
BEEH K M, GLAAB T, STOWASSER S, SCHMIDT H, FABBRI L M, RABE K F, et al. Characterisation of exacerbation risk and exacerbator phenotypes in the POET-COPD trial[J/OL]. Respir Res, 2013, 14: 116. DOI: 10.1186/1465-9921-14-116.
|
| [9] |
SMITH M C, WROBEL J P. Epidemiology and clinical impact of major comorbidities in patients with COPD[J]. Int J Chron Obstruct Pulmon Dis, 2014, 9: 871-888. |
| [10] |
MANNI NO D M, T HOR N D, S W E NSE N A, HOLGUIN F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD[J]. Eur Respir J, 2008, 32: 962-969. DOI:10.1183/09031936.00012408 |
| [11] |
MACLAY J D, MACNEE W. Cardiovascular disease in COPD: mechanisms[J]. Chest, 2013, 143: 798-807. DOI:10.1378/chest.12-0938 |
| [12] |
SIKJÆR M G, LØKKE A, HILBERG O. The influence of psychiatric disorders on the course of lung cancer, chronic obstructive pulmonary disease and tuberculosis[J]. Respir Med, 2018, 135: 35-41. DOI:10.1016/j.rmed.2017.12.012 |
| [13] |
CHARLSON M E, POMPEI P, ALES K L, MACKENZIE C R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation[J]. J Chronic Dis, 1987, 40: 373-383. DOI:10.1016/0021-9681(87)90171-8 |
| [14] |
VOGELMEIER C F, CRINER G J, MARTINEZ F J, ANZUETO A, BARNES P J, BOURBEAU J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary[J/OL]. Eur Respir J, 2017, 49: 1700214. DOI: 10.1183/13993003.00214-2017.
|
| [15] |
SUN W L, WANG J L, JIA G H, MI W J, LIAO Y X, HUANG Y W, et al. Impact of obstructive sleep apnea on pulmonary hypertension in patients with chronic obstructive pulmonary disease[J]. Chin Med J (Engl), 2019, 132: 1272-1282. DOI:10.1097/CM9.0000000000000247 |
| [16] |
SUN L, CHEN Y, WU R, LU M, YAO W. Changes in definition lead to changes in the clinical characteristics across COPD categories according to GOLD 2017:a national cross-sectional survey in China[J]. Int J Chron Obstruct Pulmon Dis, 2017, 12: 3095-3102. DOI:10.2147/COPD.S142801 |
| [17] |
黄亚玲, 毛兵, 闵婕, 李官红, 郑玉琼, 吴丽华, 等. 慢性阻塞性肺疾病稳定期患者共患疾病与一年急性加重风险的关系研究[J]. 中华结核和呼吸杂志, 2018, 41: 349-354. DOI:10.3760/cma.j.issn.1001-0939.2018.05.009 |
| [18] |
沙悦, 方卫纲, 曾学军, 许文兵, 韩江娜. 慢性阻塞性肺疾病合并症的研究[J]. 国际呼吸杂志, 2013, 33: 986-989. |
| [19] |
李向欣, 张永祥, 余春晓, 乜庆荣, 胥振阳, 王春红, 等. 慢性阻塞性肺疾病住院患者发病因素及合并症调查[J]. 卫生软科学, 2017, 31: 58-61. |
| [20] |
齐佳华, 王红阳. 慢性阻塞性肺疾病合并焦虑、抑郁的情况调查[J]. 华北理工大学学报(医学版), 2018, 20: 118-122. |
| [21] |
刘丽华, 梁秋丽, 覃寿明. 慢性阻塞性肺病急性加重期合并焦虑抑郁的临床分析[J]. 华夏医学, 2014, 27: 60-64. |
| [22] |
翟润晴, 许西琳, 刘冬, 何小双, 刘敏, 李明磊. 慢性阻塞性肺疾病合并症的分布情况及相关危险因素分析[J]. 吉林医学, 2017, 38: 1451-1454. DOI:10.3969/j.issn.1004-0412.2017.08.022 |
| [23] |
GERSHON A S, MECREDY G C, GUAN J, VICTOR J C, GOLDSTEIN R, TO T. Quantifying comorbidity in individuals with COPD: a population study[J]. Eur Respir J, 2015, 45: 51-59. DOI:10.1183/09031936.00061414 |
| [24] |
VOELKEL N F, GOMEZ-ARROYO J, MIZUNO S. COPD/emphysema: the vascular story[J]. Pulm Circ, 2011, 1: 320-326. DOI:10.4103/2045-8932.87295 |
| [25] |
WOUTERS E F M, FRANSSEN F M. Chronic obstructive pulmonary disease: shifting the paradigm to the vasculature[J]. Am J Respir Crit Care Med, 2019, 199: 258-259. DOI:10.1164/rccm.201808-1542ED |
| [26] |
THOMASHOW M A, SHIMBO D, PARIKH M A, HOFFMAN E A, VOGEL-CLAUSSEN J, HUEPER K, et al. Endothelial microparticles in mild chronic obstructive pulmonary disease and emphysema. The multi-ethnic study of atherosclerosis chronic obstructive pulmonary disease study[J]. Am J Respir Crit Care Med, 2013, 188: 60-68. DOI:10.1164/rccm.201209-1697OC |
| [27] |
POLVERINO F, CELLI B R, OWEN C A. COPD as an endothelial disorder: endothelial injury linking lesions in the lungs and other organs? (2017 Grover Conference Series)[J/OL]. Pulm Circ, 2018, 8: 2045894018758528. DOI: 10.1177/2045894018758528.
|
| [28] |
DAIBER A, STEVEN S, WEBER A, SHUVAEV V V, MUZYKANTOV V R, LAHER I, et al. Targeting vascular (endothelial) dysfunction[J]. Br J Pharmacol, 2017, 174: 1591-1619. DOI:10.1111/bph.13517 |
| [29] |
MAIO S, BALDACCI S, CARROZZI L, PISTELLI F, SIMONI M, ANGINO A, et al. 18-yr cumulative incidence of respiratory/allergic symptoms/diseases and risk factors in the Pisa epidemiological study[J]. Respir Med, 2019, 158: 33-41. DOI:10.1016/j.rmed.2019.09.013 |
| [30] |
YANAGISAWA S, ICHINOSE M. Definition and diagnosis of asthma-COPD overlap (ACO)[J]. Allergol Int, 2018, 67: 172-178. DOI:10.1016/j.alit.2018.01.002 |
| [31] |
YUN J H, LAMB A, CHASE R, SINGH D, PARKER M M, SAFERALI A, et al; COPDGene and ECLIPSE Investigators. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease[J/OL]. J Allergy Clin Immunol, 2018, 141: 2037-2047.e10. DOI: 10.1016/j.jaci.2018.04.010.
|
| [32] |
MARTINEZ-GARCIA M A, MIRAVITLLES M. Bronchiectasis in COPD patients: more than a comorbidity?[J]. Int J Chron Obstruct Pulmon Dis, 2017, 12: 1401-1411. DOI:10.2147/COPD.S132961 |
| [33] |
DU Q, JIN J, LIU X, SUN Y. Bronchiectasis as a comorbidity of chronic obstructive pulmonary disease: a systematic review and meta-analysis[J/OL]. PLoS One, 2016, 11: e0150532. DOI: 10.1371/journal.pone.0150532.
|
| [34] |
NAZ I, SAHIN H, VAROL Y, KÖMÜRCÜOĞLU B. The effect of comorbidity severity on pulmonary rehabilitation outcomes in chronic obstructive pulmonary disease patients[J/OL]. Chron Respir Dis, 2019, 16: 1479972318809472. DOI: 10.1177/1479972318809472.
|
| [35] |
HILLAS G, PERLIKOS F, TSILIGIANNI I, TZANAKIS N. Managing comorbidities in COPD[J]. Int J Chron Obstruct Pulmon Dis, 2015, 10: 95-109. |
 2021, Vol. 42
2021, Vol. 42


