2. 上海市生物医药技术研究院, 国家卫生健康委员会计划生育药具重点实验室, 上海 200032
2. NHC Key Lab of Reproduction Regulation (Shanghai Institute for Biomedical and Pharmaceutical Technologies), Fudan University, Shanghai 200032, China
卵母细胞的核成熟在形态学上表现为生发泡破裂(germinal vesicle breakdown,GVBD)和第一极体的排出[1]。研究发现,脑和生殖腺组织特异高表达泛素羧基末端水解酶L1(ubiquitin C-terminal hydrolase L1,UCHL1),其蛋白含量占可溶性蛋白提取物的1%~5%[2-3]。UCHL1是进化上高度保守的基因,在卵母细胞中高表达,并可能在卵母细胞成熟过程中发挥作用,如在猪卵母细胞中抑制UCHL1的作用后,细胞停滞在第1次减数分裂中后期[4]。本课题组前期对蟾蜍卵母细胞的离体研究结果表明,卵母细胞的成熟启动依赖一种泛素羧基末端水解酶,但进一步研究发现其成熟过程不依赖该酶的水解酶活性[5-6]。已有神经生物学领域的研究发现UCHL1的几个位点突变具有致病作用。例如,小鼠脑组织中UCHL1的第90位半胱氨酸突变为丝氨酸(C90S)后,其水解酶活性消失,但在细胞内仍表现出稳定单体泛素的作用[7];在散发性帕金森病家系中发现,UCHL1基因第277位的胞嘧啶突变为鸟嘌呤,导致UCHL1蛋白第93位异亮氨酸突变为甲硫氨酸(I93M)[8],进而引起帕金森病[9]。本研究拟通过观察UCHL1点突变蛋白与过量野生型UCHL1蛋白对离体卵母细胞成熟过程的影响,分析UCHL1在小鼠卵母细胞成熟中发挥的作用及作用方式。
1 材料和方法 1.1 实验动物、质粒载体和试剂3~4周龄雌性野生型ICR小鼠购于上海西普尔-必凯实验动物有限公司[动物生产许可证号:SCXK(沪)2013-0016],UCHL1(I93M)点突变C57雌鼠由本课题组张瑜制备[10]。pGEX4T-3-UCHL1载体与pGEX4T-3空载体由国家卫生健康委员会计划生育药具重点实验室保存,于-80℃冷冻保存含载体质粒的DH5α甘油菌。DH5α和DE3感受态菌、TOP10细菌、质粒抽提试剂盒及D2000 DNA分子量标准物(marker)购自天根生化科技(北京)有限公司,核固红染料、吐温-20、DNA回收试剂盒购自北京鼎国昌盛有限公司,DMEM/F12培养基购自美国ThermoFisher Scientific公司,异丙基硫代-β-D-半乳糖苷(isopropylthio-β-D-galactoside,IPTG)购自生工生物工程(上海)股份有限公司,氯仿、异丙醇购自北京化工厂,酵母抽提物(yeast extract)购自英国OXOID公司,牛血清白蛋白(bovine serum albumin,BSA)购自美国BBI Life Science公司,限制性内切酶、T4 DNA连接酶、高保真酶(DR010)、10×CutSmart限制性内切酶缓冲液购自宝生物工程(大连)有限公司。
1.2 原核重组蛋白技术制备UCHL1(I93M)和UCHL1(C90S)点突变蛋白用Primer Premier 5.0软件设计引物。UCHL1(I93M)编码序列引物:UCHL1-u5(5'-TAGTCGACGTGCCATCCGCGAAG- ATGCAG-3'),UCHL1-u3-mu(5'-ATCAACCCCAT-GGTACCACAGGAGTTTCC-3'),UCHL1-d5-mu-(5'-GGAAACTCCTGTGGTACCATGGGGTTG-AT-3'),UCHL1-d3(5'-TTGCGGCCGCAGACTT-AAGCTGCTTTGCAG-3');UCHL1(C90S)编码序列引物:UCHL1-u5(5'-TAGTCGACGTGCCATC-CGCGAAGATGCAG-3'),UCHL1-u3-mu(5'-AT-CAACCCGATGGTACCCGAGGAGTTTCC-3'),UCHL1-d5-mu(5'-GGAAACTCCTCGGGTACC-ATCGGGTTGAT-3'),UCHL1-d3(5'-TTGCGGC-CGCAGACTTAAGCTGCTTTGCAG-3')。引物序列中加下划线的碱基为突变位点。
点突变蛋白的构建方法参照文献[6],步骤简述如下:(1)配制LB液体培养基与LB固体培养基,铺板保存;(2)扩增菌液,离心;(3)抽提质粒,测定浓度后保存;(4)以pGEX4T-3-UCHL1质粒为模板,分别以UCHL1-u5和UCHL1-u3-mu、UCHL1-d5-mu和UCHL1-d3为引物进行分段PCR,构建UCHL1(C90S)和UCHL1(I93M)编码序列全长片段(点突变UCHL1片段);(5)酶切获得UCHL1点突变片段;(6)连接pGEX4T-3质粒与UCHL1点突变片段,转化TOP10细菌,扩增提取质粒,利用PCR方法进行验证;(7)挑选单克隆,进行预测序;(8)从测序正确的菌株中抽提质粒,转化DE3感受态菌,挑取单克隆扩大培养,保存甘油菌;(9)纯化蛋白并进行鉴定。获得的谷胱甘肽S-转移酶(glutathione S-transferase,GST)与UCHL1野生型或点突变蛋白的融合蛋白分别记为GST-UCHL1、GST-UCHL1(I93M)、GST-UCHL1(C90S)。
1.3 UCHL1点突变蛋白在小鼠卵母细胞成熟过程中的作用观察参照文献[11]方法获取与培养小鼠未成熟卵母细胞(生发泡期卵母细胞)。将获得的未成熟卵母细胞分为无注射无添加对照组、无注射添加GST-UCHL1组、注射PBS组、注射GST组、注射GST-UCHL1组、注射GST-UCHL1(I93M)组、注射GST-UCHL1(C90S)组、添加100 µmol/L 3-异丁基-1-甲基黄嘌呤(3-isobutyl-1-methylxanthine,IBMX;美国Sigma公司)组(阳性对照组),根据分组情况,通过显微注射技术将相应的蛋白或试剂注射到未成熟卵母细胞,或在体外培养基中添加相应的蛋白或试剂,每组均按蛋白添加剂量设0.1和0.2 pmol亚组(或相应的对照组)。培养3 h后,在显微镜下观察卵母细胞成熟情况(是否发生GVBD)。GVBD率(%)=(存活的卵母细胞数-生发泡期卵母细胞数)/存活的卵母细胞数×100%。
1.4 UCHL1(I93M)点突变小鼠生发泡期卵母细胞体外成熟率的观察取UCHL1(I93M)点突变小鼠与野生型小鼠各3只,参照文献[11]方法获取与培养小鼠生发泡期卵母细胞,培养3 h后观察卵母细胞成熟情况并计算GVBD率。每只小鼠各取6~7个生发泡期卵母细胞添加IBMX作为阳性对照。
1.5 统计学处理采用SPSS 21软件进行数据处理。计数资料以细胞数和百分数表示,组间比较采用χ2检验。检验水准(α)为0.05。
2 结果 2.1 UCHL1点突变蛋白重组表达与纯化通过分段PCR方法在UCHL1编码序列中引入点突变,成功扩增出小鼠UCHL1(C90S)和UCHL1(I93M)编码序列片段,并且在两端分别引入SalⅠ和NotⅠ重组内切酶位点。扩增的片段经过琼脂糖凝胶电泳,片段长度符合预期(650 bp,图 1A),利用凝胶DNA回收试剂盒回收DNA片段,紫外分光光度计检测其浓度为127 ng/μL。利用SalI和NotⅠ双酶切pGEX4T-3质粒与UCHL1点突变片段(图 1B),凝胶DNA回收后,测得空载体和目的片段DNA浓度分别为47和64 ng/mL。将经过双酶切的载体和UCHL1点突变片段连接构成重组表达质粒,转化TOP10细菌扩增质粒,提取质粒,利用PCR方法筛选重组质粒克隆,所选8个克隆均含有插入的UCHL1点突变片段(图 1C)。

|
图 1 UCHL1(C90S)编码序列扩增与重组表达质粒克隆的构建 Fig 1 Amplification of UCHL1 (C90S) coding sequence and construction of recombinant expression plasmid clone A: Electrophoresis of UCHL1 (C90S) coding sequence amplified bands; B: Electrophoresis detection of empty plasmid pGEX4T-3 and UCHL1 (C90S) coding sequence amplified fragments after double enzyme digestion; C: After the recombinant plasmid was transformed into TOP10 bacteria, the extracted plasmids from different clones were detected by PCR, and the positive clones successfully recombined were screened and initially identified. UCHL1: Ubiquitin C-terminal hydrolase L1; PCR: Polymerase chain reaction; M: DNA molecular marker. |
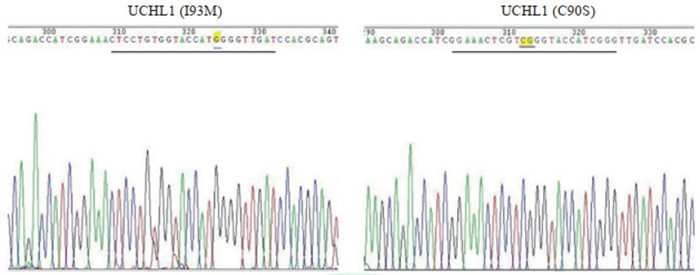
为了进一步明确挑选细菌单克隆中重组质粒构建是否正确,提取单克隆细菌质粒DNA,通过PCR扩增插入片段后送测序,测序结果提示载体与UCHL1点突变片段连接正确,所有编码序列及突变位点序列均正确(图 2)。经原核重组表达、鉴定、谷胱甘肽亲和柱纯化及电泳后,采用考马斯亮蓝染色法鉴定纯化结果,显示重组表达的UCHL1融合蛋白分子量大小符合预期(50 000,图 3),而且纯度符合显微注射技术初步研究的要求。
|
图 2 UCHL1(I93M)和UCHL1(C90S)编码序列测序结果 Fig 2 Sequencing results of UCHL1 (I93M) and UCHL1 (C90S) coding sequences The sequencing peak map of the calibration area is showed by the black horizontal line, and the mutated bases are underlined. UCHL1: Ubiquitin C-terminal hydrolase L1. |

|
图 3 原核重组表达GST-UCHL1融合蛋白的SDS-PAGE分析结果 Fig 3 Prokaryotic recombinant expression of GST-UCHL1 fusion protein analyzed by SDS-PAGE 1: GST-UCHL1; 2: GST-UCHL1 (C90S); 3: GST-UCHL1 (I93M). GST: Glutathione S-transferase; UCHL1: Ubiquitin C-terminal hydrolase L1; SDS-PAGE: Sodium dodecyl sulphate-polyacrylamide gel electrophoresis; M: Protein molecular marker. |
2.2 向细胞内显微注射或在培养基中添加UCHL1蛋白或其点突变体对卵母细胞GVBD率的影响
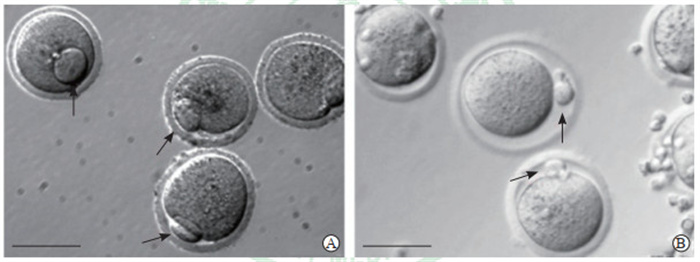
分别向未成熟卵母细胞内显微注射0.1 pmol或0.2 pmol融合蛋白GST-UCHL1、GST-UCHL1(I93M)、GST-UCHL1(C90S),以显微注射GST与PBS作为对照,培养3 h后,观察各组GVBD的发生情况。本实验0.1 pmol剂量采取3次独立重复实验,0.2 pmol剂量采取2次独立重复实验,综合数据后进行统计分析。由表 1可见,显微注射GST-UCHL1、GST-UCHL1(I93M)、GST-UCHL1(C90S)、GST、PBS各组之间GVBD率差异无统计学意义(P>0.05)。同时,在培养基中添加GST-UCHL1组GVBD率与无注射无添加对照组相比差异也无统计学意义(P>0.05)。说明UCHL1野生型及其C90S和I93M点突变蛋白的显微注射,以及在培养基中添加UCHL1对于小鼠卵母细胞GVBD率均没有显著影响。注射PBS组GVBD率低于无注射无添加对照组(P<0.05),说明显微注射操作可能对于卵母细胞的GVBD产生了影响,导致GVBD率偏低。此外,本研究还在注射0.2 pmol GST-UCHL1(C90S)融合蛋白组观察到,少量生发泡期卵母细胞体外发育为MⅡ期卵母细胞后呈现极体较对照组偏大的现象(图 4),因数量较少未进行统计学分析。
|
|
表 1 各组卵母细胞培养3 h后GVBD率的比较 Tab 1 Comparison of GVBD rates of oocytes in each group after 3 h of culture |
|
图 4 两组GV期卵母细胞体外成熟后极体大小比较 Fig 4 Comparison of polar body sizes of GV oocytes after in vitro maturation between 2 groups A: GV oocytes progressed to M Ⅱ stage oocytes in vitro after the injection of GST-UCHL1 (C90S) fusion protein; B: GV oocytes progressed to M Ⅱ stage oocytes in vitro without the injection as control group. The arrows indicate the polar body, and the scale bar is 50 μm. GV: Germinal vesicle; GST: Glutathione S-transferase; UCHL1: Ubiquitin C-terminal hydrolase L1. |
2.3 UCHL1(I93M)点突变小鼠卵母细胞体外GVBD率
分别取3只UCHL1(I93M)点突变小鼠与3只野生型小鼠的生发泡期卵母细胞进行培养,3 h后两组卵母细胞GVBD率差异无统计学意义(P>0.05,表 2)。该结果进一步表明UCHL1(I93M)点突变不会影响小鼠卵母细胞的成熟。
|
|
表 2 UCHL1(I93M)点突变小鼠与野生型小鼠GV期卵母细胞GVBD率比较 Tab 2 Comparison of GVBD rates of GV oocytes between UCHL1 (I93M) mutant and wild-type mice |
3 讨论
本实验以添加IBMX的培养基为阳性对照,该药物可以非选择性抑制磷酸二酯酶,有效抑制去除颗粒细胞卵后母细胞的GVBD。本实验结果提示添加过量UCHL1蛋白、注射有致病作用的UCHL1(I93M)突变蛋白及注射去泛素化水解酶活性的UCHL1(C90S)突变蛋白均不会影响剥离颗粒细胞后卵母细胞的体外GVBD率。
本课题组前期研究中以泛素-7-氨基-4-甲基香豆素(ubiquitin C-terminal 7-amino-4-methylcoumarin,UB-AMC)作为底物进行体外实验,验证了重组获得的小鼠GST-UCHL1具有水解酶活性[6],并且通过体外实验发现C90S位点突变会导致UCHL1水解UB-AMC的能力丧失。也有研究发现,一种针对细胞周期蛋白的人泛素载体蛋白酶突变体(C114S)可以竞争性结合野生型蛋白酶的生理底物[12]。由于GST融合蛋白具有增加外源蛋白可溶性的特点[13],借助GST-UCHL1融合蛋白研究UCHL1的活性与功能是可行的。有研究者在体内与体外实验中发现,UCHL1通过泛素化途径降低了微管蛋白聚合成微管的能力[14],而微管细胞骨架对于卵母细胞成熟过程中染色质的重组具有重要作用[15]。还有研究者发现,在过表达UCHL1小鼠的精子形成过程中,减数分裂的早期阶段(粗线期)存在发育阻滞[16]。在本研究中,添加外源UCHL1及其I93M、C90S突变体均没有影响卵母细胞的GVBD率。一项体外实验发现,UCHL1(I93M)突变体的添加会降低神经元内源性UCHL1水溶性[17],本实验中UCHL1(I93M)添加的量经估算已经超出了卵母细胞内源性UCHL1丰度,理论上讲,已能有效降低内源性UCHL1溶解性从而影响其生物学功能,但并没有观察到其对GVBD率的显著影响。有一种可能是,GST融合蛋白具有增加外源蛋白可溶性的特点,可能会抵消I93M突变蛋白溶解度降低作用。另外,本课题组制备的UCHL1(I93M)点突变小鼠生育力正常并且卵母细胞体外GVBD率与野生型小鼠无显著差异,也进一步证明了UCHL1(I93M)点突变蛋白对于游离的小鼠卵母细胞的体外成熟率并没有影响。在肿瘤细胞系的研究中发现,UCHL1参与肿瘤细胞的增殖、迁移、侵袭,且均依赖其酶活性[18-20]。本研究结果表明,通过显微注射添加去泛素化水解酶活性的UCHL1(C90S)蛋白,并没有影响小鼠卵母细胞去除颗粒细胞后的体外成熟率。综合以上实验结果,认为UCHL1过量或添加其I93M、C90S突变体,均不干扰小鼠游离的卵母细胞GVBD进程。
在本实验中还观察到,一些注射了UCHL1(C90S)蛋白的生发泡期卵母细胞在体外发育为MⅡ期卵母细胞后,其极体相较于未注射组偏大,提示与UCHL1水解酶活性丧失可能影响极体的形成。有研究指出,抑制泛素羧基末端水解酶活性会导致异常大的极体,其机制可能涉及纺锤体定位破坏或卵母细胞皮质微纤维骨架功能障碍[2]。UCHL1和相关的泛素羧基末端水解酶在极体向卵母细胞边缘的挤压过程中,可能参与调节含有肌动蛋白和肌球蛋白的微丝形成。秀丽隐杆线虫的早期胚胎中微丝控制的细胞分裂、分裂沟的形成和细胞极性的建立受泛素羧基末端水解酶CYK3的调控[21],CYK3蛋白包含哺乳动物泛素特异性蛋白酶(ubiquitin-specific protease,USP)11和USP32同源的泛素羧基末端水解酶结构域。最近一项研究发现,小分子泛素相关修饰物(small ubiquitin-related modifier,SUMO)通过Akt通路调控GVBD[22]。因为UCHL1与泛素分子的稳定性有关,所以虽然改变卵母细胞中UCHL1不影响GVBD进程,但是不排除其间接参与小鼠卵母细胞GVBD过程,而且其机制值得进一步研究。另一方面,UCHL1在小鼠卵母细胞成熟过程中可能并不占主导作用,其含量或功能变化很容易被其他泛素羧基末端水解酶代偿。
综上所述,本研究结果显示在小鼠卵母细胞中添加外源性UCHL1、有致病作用的UCHL1(I93M)突变体或水解酶活性丧失的UCHL1(C90S)突变体均不影响卵母细胞成熟的GVBD进程,UCHL1(I93M)点突变小鼠卵母细胞的GVBD率亦无异常改变,但UCHL1(C90S)突变可能影响极体的形成。本研究的不足之处在于只观察了生发泡期卵母细胞培养3 h后的GVBD率,今后需要细化到不同的时间点观察,同时可以构建UCHL1(C90S)点突变小鼠进一步明确UCHL1在卵母细胞成熟中的作用。
| [1] |
BRUNET S, MARO B. Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: integrating time and space[J]. Reproduction, 2005, 130: 801-811. DOI:10.1530/rep.1.00364 |
| [2] |
MTANGO N R, SUTOVSKY M, VANDEVOORT C A, LATHAM K E, SUTOVSKY P. Essential role of ubiquitin C-terminal hydrolases UCHL1 and UCHL3 in mammalian oocyte maturation[J]. J Cell Physiol, 2012, 227: 2022-2029. DOI:10.1002/jcp.22931 |
| [3] |
BHEDA A, GULLAPALLI A, CAPLOW M, PAGANO J S, SHACKELFORD J. Ubiquitin editing enzyme UCH L1 and microtubule dynamics: implication in mitosis[J]. Cell Cycle, 2010, 9: 980-994. DOI:10.4161/cc.9.5.10934 |
| [4] |
SUSOR A, ELLEDEROVA Z, JELINKOVA L, HALADA P, KAVAN D, KUBELKA M, et al. Proteomic analysis of porcine oocytes during in vitro maturation reveals essential role for the ubiquitin C-terminal hydrolase-L1[J]. Reproduction, 2007, 134: 559-568. DOI:10.1530/REP-07-0079 |
| [5] |
SUN Z G, KONG W H, ZHANG Y J, YAN S, LU J N, GU Z, et al. A novel ubiquitin carboxyl terminal hydrolase is involved in toad oocyte maturation[J]. Cell Res, 2002, 12: 199-206. DOI:10.1038/sj.cr.7290125 |
| [6] |
孙兆贵, 孔维华, 颜山, 顾正, 左嘉客. 蟾蜍泛素羧基末端水解酶(tUCH)以不依赖其UCH活性的方式参与卵母细胞成熟调控[J]. 实验生物学报, 2003, 36: 105-112. DOI:10.3321/j.issn:1673-520X.2003.02.004 |
| [7] |
NAKASHIMA R, GOTO Y, KOYASU S, KOBAYASHI M, MORINIBU A, YOSHIMURA M, et al. UCHL1-HIF-1 axis-mediated antioxidant property of cancer cells as a therapeutic target for radiosensitization[J]. Sci Rep, 2017, 7: 6879. DOI:10.1038/s41598-017-06605-1 |
| [8] |
LEROY E, BOYER R, AUBURGER G, LEUBE B, ULM G, MEZEY E, et al. The ubiquitin pathway in Parkinson's disease[J]. Nature, 1998, 395: 451-452. DOI:10.1038/26652 |
| [9] |
SHARMA A, LIU H D, TOBAR-TOSSE F, CHAND DAKAL T, LUDWIG M, HOLZ F G, et al. Ubiquitin carboxyl-terminal hydrolases (UCHs): potential mediators for cancer and neurodegeneration[J]. Int J Mol Sci, 2020, 21: 3910. DOI:10.3390/ijms21113910 |
| [10] |
张瑜, 孙兆贵. UCHL1基因缺陷细胞和小鼠模型的构建及其基因功能研究[D]. 上海: 复旦大学, 2018.
|
| [11] |
松迪, 孙兆贵. UCHL1突变导致小鼠生殖障碍及其分子机制[D]. 上海: 复旦大学, 2020.
|
| [12] |
TOWNSLEY F M, ARISTARKHOV A, BECK S, HERSHKO A, RUDERMAN J V. Dominant-negative cyclin-selective ubiquitin carrier protein E2-C/UbcH10 blocks cells in metaphase[J]. PNAS, 1997, 94: 2362-2367. DOI:10.1073/pnas.94.6.2362 |
| [13] |
KIMPLE M E, BRILL A L, PASKER R L. Overview of affinity tags for protein purification[J/OL]. Curr Protoc Protein Sci, 2013, 73: 9.9.1-9.9.23. DOI: 10.1002/0471140864.ps0909s36.
|
| [14] |
TOKUMOTO T. Nature and role of proteasomes in maturation of fish oocytes[J]. Int Rev Cytol, 1999, 186: 261-294. |
| [15] |
KIDANE D, SAKKAS D, NOTTOLI T, MCGRATH J, SWEASY J B. Kinesin 5B (KIF5B) is required for progression through female meiosis and proper chromosomal segregation in mitotic cells[ J/OL]. PLoS One, 2013, 8: e58585. DOI: 10.1371/journal.pone.0058585.
|
| [16] |
MATUSZCZAK E, TYLICKA M, KOMAROWSKA M D, DEBEK W, HERMANOWICZ A. Ubiquitin carboxyterminal hydrolase L1-physiology and pathology[J]. Cell Biochem Funct, 2020, 38: 533-540. DOI:10.1002/cbf.3527 |
| [17] |
SETSUIE R, WANG Y L, MOCHIZUKI H, OSAKA H, HAYAKAWA H, ICHIHARA N, et al. Dopaminergic neuronal loss in transgenic mice expressing the Parkinson's disease-associated UCH-L1 I93M mutant[J]. Neurochem Int, 2007, 50: 119-129. DOI:10.1016/j.neuint.2006.07.015 |
| [18] |
GU Y Y, YANG M, ZHAO M, LUO Q, YANG L, PENG H, et al. The de-ubiquitinase UCHL1 promotes gastric cancer metastasis via the Akt and Erk1/2 pathways[J]. Tumor Biol, 2015, 36: 8379-8387. DOI:10.1007/s13277-015-3566-0 |
| [19] |
XIANG T X, LI L L, YIN X D, YUAN C F, TAN C, SU X W, et al. The ubiquitin peptidase UCHL1 induces G0/G1 cell cycle arrest and apoptosis through stabilizing p53 and is frequently silenced in breast cancer[J/OL]. PLoS One, 2012, 7: e29783. DOI: 10.1371/journal.pone.0029783.
|
| [20] |
ZHONG J L, ZHAO M, MA Y M, LUO Q, LIU J, WANG J, et al. UCHL1 acts as a colorectal cancer oncogene via activation of the β-catenin/TCF pathway through its deubiquitinating activity[J]. Int J Mol Med, 2012, 30: 430-436. DOI:10.3892/ijmm.2012.1012 |
| [21] |
KAITNA S, SCHNABEL H, SCHNABEL R, HYMAN A A, GLOTZER M. A ubiquitin C-terminal hydrolase is required to maintain osmotic balance and execute actindependent processes in the early C. elegans embryo[J]. J Cell Sci, 2002, 115: 2293-2302. DOI:10.1242/jcs.115.11.2293 |
| [22] |
FEITOSA W B, MORRIS P L. SUMOylation regulates germinal vesicle breakdown and the Akt/PKB pathway during mouse oocyte maturation[J/OL]. Am J Physiol Cell Physiol, 2018, 315: C115-C121. DOI: 10.1152/ajpcell.00038.2018.
|
 2021, Vol. 42
2021, Vol. 42


