食管胃结合部腺癌(adenocarcinoma of esophagogastric junction,AEG)是指位于食管、胃交界处的腺癌,肿瘤的中心位于食管胃解剖交界上下5 cm内,且肿瘤本身跨域或直接接触食管胃解剖交界线[1]。近年来,AEG的发病率呈明显上升趋势[2-4]。Siewert等[5]于1987年提出将AEG分为3型:Ⅰ型肿瘤的中心位置位于食管胃结合部(esophagogastric junction,EGJ)上方1~5 cm处;Ⅱ型肿瘤中心在EGJ上方1 cm至下方2 cm内;Ⅲ型肿瘤中心位于EGJ下方2~5 cm处。因该分型对外科手术策略的选择和综合治疗有较好的指导价值,2011年的美国国立综合癌症网络(National Comprehensive Cancer Network,NCCN)指南[6]、2020年的胃癌NCCN指南[7]及2019年中国专家共识[8]均建议使用Siewert分型。Siewert Ⅰ型AEG是西方国家中较常见的一种类型[9],Siewert Ⅱ型和Ⅲ型AEG在东方国家较常见[10]。我国Siewert Ⅱ/Ⅲ型的AEG患者大多处于中晚期,肿瘤范围较大[2, 11],外科手术治疗是可切除AEG的主要治疗方法,而准确的分型和临床分期是AEG术前评估的重要内容。Siewert Ⅲ型AEG由于其肿瘤部位偏下,胃下部淋巴结转移率较高,尤其是局部进展期AEG,手术方式推荐全胃切除[1]。参照日本胃癌治疗指南,肿瘤长径≤4 cm的Siewert Ⅲ型AEG可采用经腹近端胃大部切除术[12]。相较于研究证据强、争议少的Siewert Ⅲ型AEG患者,Siewert Ⅱ型因解剖位置的特殊性,其肿瘤浸润范围、淋巴结转移途径等生物学行为复杂,治疗策略、手术入路选择、手术切除范围及淋巴结清扫的规范至今尚无共识达成。因此,对于Siewert Ⅱ/Ⅲ型AEG,术前较为准确的影像学评估可为患者的手术方式选择和综合治疗提供有价值的信息。本研究通过回顾性分析Siewert Ⅱ/Ⅲ型AEG患者资料,探讨增强MRI检查对AEG的术前评估价值。
1 资料和方法 1.1 研究对象回顾性收集2018年1月1日至2020年5月30日我院80例经手术切除及病理证实的Siewert Ⅱ/Ⅲ型AEG患者资料。纳入标准:(1)经胃镜病理提示AEG;(2)入院后行根治性切除手术;(3)无胃肠道梗阻、穿孔、出血等严重并发症;(4)临床病理资料完整。排除标准:(1)T1早期或新辅助化疗后肿瘤完全缓解,影像学检查无法明确肿瘤位置;(2)术前影像学检查发现远处转移,如肝转移和腹膜后淋巴结转移;(3)患者配合欠佳,图像伪影显著影响诊断。
1.2 检查资料 1.2.1 影像学检查增强CT:采用320排CT机(型号Aqulion ONE,日本东芝医疗公司)进行检查,对比剂为碘普罗胺注射液(商品名:优维显,含碘370 mg/mL,德国拜耳公司),采用双通道高压注射器(型号XD2001,德国Ulrich公司)进行注射。患者禁食4 h以上,检查前30 min口服清水1 000 mL以充盈胃及十二指肠。常规先行CT平扫,然后经肘前静脉以3~4 mL/s速率团注对比剂0~100 mL,分别于注射开始后20~25、60~75、90~95 s行动脉期、静脉期和延迟期扫描。重建层厚为3 mm,并进行多平面重建(multi-planner reformation,MPR)。
增强MRI:采用3.0 T MRI仪(型号MAGNETOM Skyra,德国Siemens公司)进行检查,18通道体部相控阵线圈和嵌入式脊柱线圈用于接收磁共振信号。采用二维T2加权成像(T2 weighted imaging,T2WI)快速自旋回波叠加刀锋伪影校正技术和呼吸触发,以减少运动伪影;采用呼吸触发单次自旋回波平面技术、三维T1加权成像(T1 weighted imaging,T1WI)容积内插体部检查(volume interpolated body examination,VIBE)和3期(动脉期、静脉期和延迟期)增强T1WI VIBE成像;并进行弥散加权成像(diffusion weighted imaging,DWI)。主要成像参数见表 1。增强动脉期、静脉期和延迟期T1WI图像分别在注射对比剂钆喷替酸葡甲胺(北京北陆药业股份有限公司)后30、60和90 s获得,对比剂按0.2 mL/kg使用高压注射器以2 mL/s注射,然后以相同的速率注射20 mL盐水冲洗。
|
|
表 1 MRI检查的主要成像参数 Tab 1 Main imaging parameters of MRI |
1.2.2 影像学诊断
所有图像被传输到后处理工作站进行分析。由2位主治医师通过盲法分别对术前增强CT及术前增强MRI图像进行独立观察及结果分析、测量、诊断,并由1名副主任医师复核。EGJ线的确定:冠状面或MPR重建后,食管、胃大弯侧的解剖交界点(角切迹)与对应胃小弯侧的食管胃交界点的连线。对于增强CT检查,由横断位联合MPR重建图像,于肿瘤最大层面测量肿瘤中心与EGJ的距离进行Siewert分型。依照Siewert分型标准,Ⅱ型为肿瘤中心在EGJ上方1 cm至下方2 cm内的病灶,Ⅲ型为肿瘤中心位于EGJ下方2~5 cm处的病灶[5]。食管壁局限性增厚>3 mm或结节状增厚并增强后强化的病灶诊断为食管受侵。淋巴结短径>1 cm且增强后可见强化即诊断为淋巴结转移。对于增强MRI检查,在T2WI快速自旋回波序列及冠状面增强T1WI图像联合DWI序列,于肿瘤最大层面测量肿瘤中心与EGJ的距离进行Siewert分型,将食管壁局限性增厚>3 mm、DWI弥散受限且增强后强化的病灶诊断为食管受侵。淋巴结短径>1 cm、增强可见强化且DWI弥散受限时诊断为淋巴结转移。采用胃肠外科医师常规选择的日本食管学会(Japan Esophageal Society,JES)标准进行淋巴结分组。
1.3 统计学处理应用SPSS 23.0软件进行统计学分析。计量资料以x±s表示;计数资料以例数和百分数表示。以病理结果为金标准,采用Kappa检验比较增强CT检查、增强MRI检查与病理结果之间的一致性。Kappa值≥0.75表示一致性较好,0.4<Kappa值<0.75表示一致性中等,0≤Kappa值≤0.4表示一致性较差;Kappa值<0表示不一致,其中Kappa值≤-0.75表示不一致性明显;-0.75<Kappa值<-0.4表示不一致性中等;-0.4≤Kappa值<0表示不一致性较弱。
2 结果 2.1 患者基本资料80例患者中2例因肝脏转移、2例因配合欠佳图像伪影显著排除,共76例入组。男69例、女7例,年龄为43~83岁,平均年龄为(65±8)岁。76例患者均在术前1周内行影像学检查,仅行增强CT检查27例,只行增强MRI检查9例,同时行增强CT及增强MRI检查(间隔1 d)40例,获得术前增强CT检查图像67例,术前增强MRI图像49例。76例患者均接受根治性切除手术,每例患者至少有1个确定的病灶;术后病理显示Siewert Ⅱ型40例、Siewert Ⅲ型37例;病理分期为T2N0~T4aN3b;病灶中心距EGJ线的距离为EGJ线上方0.20 cm至下方3.80 cm。
2.2 CT检查特征与一致性分析67例患者行增强CT检查,男61例、女6例,年龄为43~83岁,中位年龄为70岁,病灶中心距EGJ线的距离为EGJ线上方0.20 cm至下方3.70 cm。EGJ的增强CT影像特征:EGJ局部管壁不均匀增厚,部分可见软组织肿块影;增强后病灶呈相对较明显强化,与正常胃壁分界处呈“斜坡样”改变;部分患者腹腔内可见肿大淋巴结;MPR可较直观地显示病灶范围(图 1、2)。行增强CT检查的67例AEG患者病理结果示Siewert Ⅱ型35例、Siewert Ⅲ型32例,增强CT诊断结果示Siewert Ⅱ型32例,Siewert Ⅲ型35例。以病理结果为金标准,Siewert Ⅱ型的CT诊断正确率为80.0%(28/35),Siewert Ⅲ型诊断正确率为87.5%(28/32),Siewert Ⅱ型增强CT检查误诊率为20.0%(7/35),Siewert Ⅲ型误诊率为12.5%(4/32);一致性检验分析Kappa值为0.672,提示增强CT检查与病理结果一致性中等。

|
图 1 Siewert Ⅲ型AEG的术前增强CT图像 Fig 1 Preoperative enhanced CT images of Siewert type Ⅲ AEG A: Coronal plane image in arterial phase showed the EGJ (white line) and a slope-like appearance (white arrow) at the boundary between the tumor and the normal gastric wall; B: Transverse image in arterial phase showed the significantly thicken and stiff EGJ wall and a slope-like appearance (white arrow) at the boundary between the tumor and the normal gastric wall; C: Coronal plane image in portal phase showed multiple enlarged lymph nodes of lesser curvature of stomach (group 3), with the short diameter of the largest one being about 1.10 cm (white dotted arrow) and slight enhancement. AEG: Adenocarcinoma of esophagogastric junction; CT: Computed tomography; EGJ: Esophagogastric junction. |

|
图 2 Siewert Ⅱ型AEG的术前增强CT图像 Fig 2 Preoperative enhanced CT images of Siewert type Ⅱ AEG A: Coronal plane image in portal phase showed that the distance from the tumor center to the EGJ (white line) was measured, the tumor obviously straddled EGJ and invaded the lower esophagus, the boundary between the tumor and the normal gastric wall was slope-like (white arrow), and enlarged lymph nodes in abdominal cavity (white dotted arrow) were observed; B: Transverse plane image showed that the tumor had a slightly low density (black arrow), with multiple enlarged lymph nodes around the cardia and lesser curvature of the stomach (groups 1-3), the short diameter of the largest one was about 1.08 cm (white dotted arrow); C: Transverse plane image in portal phase showed that the tumor focus was markedly enhanced (black arrow), and the enlarged lymph nodes showed heterogeneous enhancement (white dotted arrow). AEG: Adenocarcinoma of esophagogastric junction; CT: Computed tomography; EGJ: Esophagogastric junction. |
2.3 MRI检查特征与一致性分析
49例患者行增强MRI检查,男44例、女5例,年龄为48~83岁,中位年龄68岁,病灶中心距EGJ线的距离为EGJ线下方0.10~3.20 cm。AEG的增强MRI影像特征:EGJ局限性不均匀管壁增厚、软组织占位并信号异常,病灶T1WI呈稍低信号、T2WI呈稍高信号、DWI呈较高信号,增强后病灶相对显著强化,与正常胃壁分界处呈“断崖样”改变(图 3、4);部分患者腹腔内可见肿大淋巴结,T2WI呈稍高信号,DWI呈较高信号(图 5)。行增强MRI检查的49例AEG患者病理结果示Siewert Ⅱ型27例、Siewert Ⅲ型22例,增强MRI诊断结果示Siewert Ⅱ型30例、Siewert Ⅲ型19例。以病理结果为金标准,Siewert Ⅱ型的MRI诊断正确率为96.3%(26/27),Siewert Ⅲ型正确率为81.8%(18/22),Siewert Ⅱ型的MRI检查误诊率为3.7%(1/27),Siewert Ⅲ型误诊率为18.2%(4/22),一致性检验分析Kappa值为0.791,提示增强MRI检查与病理结果一致性较好。
|
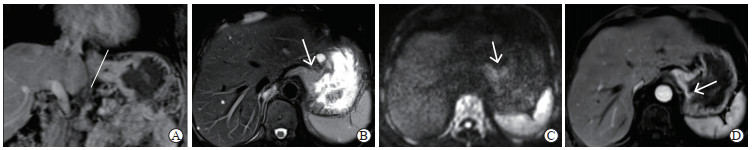
图 3 Siewert Ⅲ型AEG的术前增强MRI图像 Fig 3 Preoperative enhanced MRI images of Siewert type Ⅲ AEG A: Coronal plane image in portal phase showed that the wall of the EGJ was markedly thickened and rigid, and the distance from the tumor center to the EGJ (white line) could be measured; B: Transverse plane T2 weighted imaging showed that tumor lesions had slightly high signal (white arrow), and tumor and normal gastric wall was clearly demarcated; C: Transverse diffusion weighted imaging showed that the tumor had relatively high signal (white arrow) and the range was clear; D: Transverse plane image in portal phase showed that the tumor was enhanced, the boundary between the enhancement of the tumor and the normal gastric wall was cliff-like, and the boundary of the tumor was clear (white arrow). AEG: Adenocarcinoma of esophagogastric junction; MRI: Magnetic resonance imaging; EGJ: Esophagogastric junction. |
|
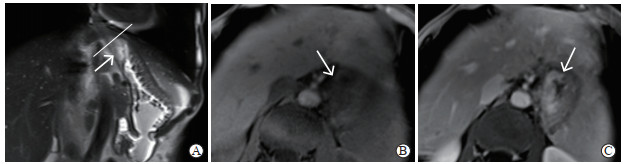
图 4 Siewert Ⅱ型AEG的术前增强MRI图像 Fig 4 Preoperative enhanced MRI images of Siewert type Ⅱ AEG A: Coronal T2 weighted imaging showed that the tumor was slightly high signal (white arrow) with a clear range, and the distance from the tumor center to the EGJ (white line) could be measured; B: Transverse T1 weighted imaging showed that the lesion was low signal (white arrow); C: Transverse plane image in portal phase showed that the tumor was enhanced, and tumor and normal gastric wall was clearly demarcated and the boundary was cliff-like (white arrow). AEG: Adenocarcinoma of esophagogastric junction; MRI: Magnetic resonance imaging; EGJ: Esophagogastric junction. |

|
图 5 Siewert Ⅱ型AEG患者胃小弯(第3组)肿大淋巴结术前增强MRI图像 Fig 5 Preoperative enhanced MRI images of enlarged lymph nodes on lesser curvature of stomach (group 3) in patients with Siewert type Ⅱ AEG A: Coronal plane image in portal phase clearly showed location of enlarged lymph nodes and short diameter (about 1.12 cm, white dotted arrow); B: Transverse diffusion weighted imaging showed enlarged lymph nodes with high signal and clear edge of the lesion (white dotted arrow); C: Transverse plane in portal phase showed that the enlarged lymph nodes were slightly and moderately enhanced, and the position of enlarged lymph nodes could be determined (white dotted arrow). AEG: Adenocarcinoma of esophagogastric junction; MRI: Magnetic resonance imaging. |
2.4 CT检查与MRI检查结果比较
同时行增强CT及增强MRI检查的AEG患者40例,男37例、女3例,年龄为55~79岁,中位年龄65岁;病灶中心距EGJ线的距离为EGJ线下方0.50~3.50 cm。病理结果示Siewert Ⅱ型20例、Siewert Ⅲ型20例;增强CT诊断结果示Siewert Ⅱ型18例、Siewert Ⅲ型22例;增强MRI诊断结果示Siewert Ⅱ型21例、Siewert Ⅲ型19例。一致性检验分析示增强CT检查与病理结果一致性中等(Kappa值=0.500),增强MRI检查与病理结果一致性较好(Kappa值=0.850),提示术前增强MRI检查对于AEG的Siewert分型与病理结果的一致性比术前增强CT检查高。
2.5 CT与MRI检查对淋巴结转移的诊断术前增强MRI检查提示的阳性转移淋巴结与术后病理结果一致性欠佳(Kappa值=0.115);增强CT检查提示的阳性转移淋巴结与术后病理结果无一致性(Kappa值=-0.129)。以病理结果为金标准,增强MRI检查提示阳性转移淋巴结分组区域的准确率为59.2%(29/49),高于增强CT检查的41.8%(28/67)。同时行增强CT及增强MRI检查的病例阳性转移淋巴结的分组区域准确率为62.5%(25/40)。
3 讨论胃镜、上消化道气钡双重造影、增强CT及增强MRI等影像学检查均有利于AEG的术前诊断。胃镜因观察和操作的限制易导致AEG漏诊,并且无法观察病灶与周围组织结构的关系。上消化道气钡双重造影对病灶范围的显示较模糊,不能准确显示病灶边界,特别是病灶的上界,且对早期病灶的显示与操作者的熟练度有关,常用于筛查,对于术前评估的价值欠佳。增强CT检查,特别是高分辨率增强CT检查,可判断AEG浸润的范围、深度、T分期、N分期及食管受侵犯的长度和范围,对AEG的术前评估具有很高的应用价值,对患者手术方式的选择和预后具有指导意义[13],是目前运用较为广泛的影像学手段。美国癌症联合委员会第8版TNM分期系统定义的EGJ交界线为解剖学EGJ交界线而非齿状线[1],为CT或MRI检查图像上判断EGJ位置提供了便利,本研究以此为基础划定EGJ交界线。增强CT检查联合后处理冠状面MPR图像可直接测量肿瘤中心距EGJ的距离,从而对AEG进行Siewert分型。但是增强CT检查对软组织的分辨率较低[14],主要根据食管及胃壁局限性僵硬、增厚及强化程度诊断AEG及判断食管受累范围,肿瘤分期较早、病灶显示欠清晰、食管受累不显著或食管下段水肿,以及强化的肿瘤组织与胃壁正常组织呈“斜坡样”分界,均会影响肿瘤边界的确定,特别是扩大了肿瘤病灶的边界,进而影响病灶中心点的判断、影响Siewert分型。本研究中行术前增强CT检查与病理Siewert分型结果不一致的11例患者中,因增强CT检查显示的肿瘤下界大于实际肿瘤界限,致使7例病理结果为Siewert Ⅱ型患者被诊断为Siewert Ⅲ型。
多项研究表明,增强MRI检查运用的T2WI、DWI及增强T1WI方案,包括横断面和冠状面扫描,可以提高胃癌特别是晚期胃癌患者T分期的准确性[15-18],评价胃癌患者T分期的总体准确率为73.3%~88.2%[15]。对于对比剂过敏的患者,DWI甚至可以作为评价T分期的替代方法[15]。近期,已有研究尝试用增强MRI检查对AEG进行诊断及分析,结果证明增强MRI检查可以准确反映AEG的位置及上界,对指导手术入路、保证切缘的充分性及提高手术根治率具有积极意义[19]。高分辨率增强MRI检查可以准确地评估肿瘤的侵犯深度,对评价AEG患者的T分期具有较高的诊断价值[20]。本研究结果显示,相较于增强CT检查的征象,除了局部食管、胃壁的僵硬、增厚外,T2WI序列肿瘤病灶呈稍高信号,DWI弥散序列呈高信号,增强T1WI强化与正常组织结构分界呈“断崖样”改变,更直观地显示了病灶的范围,既可以较清晰地显示上界侵犯食管的距离,也可以显示肿瘤下界与正常胃壁的分界,弥补了增强CT检查扩大病灶范围的缺陷,有效降低了将Siewert Ⅱ型AEG误诊为Siewert Ⅲ型的概率。本组增强MRI检查的病例中,仅1例Siewert Ⅱ型AEG被诊断为Siewert Ⅲ型。此外,由于增强MRI检查对软组织的分辨率高,在器官周围脂肪背景的衬托下,肿瘤与周围组织结构的关系显示更为清晰。本研究结果显示,术前增强MRI检查与病理结果Siewert分型结果的一致性相较于增强CT检查更高。
对于手术中的淋巴结清扫范围,Siewert Ⅲ型AEG推荐清扫淋巴结的重点在于腹腔,而目前的各类指南也均承认对Siewert Ⅱ型AEG的淋巴结清扫范围尚无共识形成[21-23],《Siewert Ⅱ型食管胃结合部腺癌腔镜手术治疗中国专家共识(2019版)》建议侵犯食管距离≥2 cm的Siewert Ⅱ型AEG须行下纵隔淋巴结清扫[8]。目前尚无准确判断胃癌淋巴结转移的方法[24]。虽然增强CT检查可直观地显示胃区域淋巴结,是胃癌术前N分期的主要手段,但目前国内外学者对于CT诊断胃癌单枚淋巴结转移尚没有形成统一且准确性高的标准,较广泛接受的诊断方法是将淋巴结短径>1 cm作为主要诊断阈值[25]。本研究中增强CT检查转移阳性淋巴结的诊断沿用此标准,结果显示增强CT检查提示的阳性转移淋巴结与术后病理结果无一致性,与王之龙等[26]的研究结果类似。本研究中增强MRI检查诊断转移阳性淋巴结的标准除短径标准外还需要满足DWI高信号的表现,结果显示术前增强MRI检查提示的阳性转移淋巴结与术后病理结果的一致性也欠佳,但对于阳性转移淋巴结的分组区域的准确率为59.2%(29/49),高于增强CT检查的41.8%(28/67);即便是同时行增强CT及增强MRI 2种检查,阳性淋巴结分组区域结果与病理结果对照的准确率也仅为62.5%(25/40)。故而增强MRI检查可能对手术淋巴结清扫范围的选择有一定的提示价值。
综上所述,增强MRI检查能在术前对Siewert Ⅱ/Ⅲ型AEG的分型及转移淋巴结分组区域提供更详细及可靠的信息,能较准确地显示肿瘤的上、下界,可为临床治疗方法、手术路径的选择及淋巴结清扫范围提供比增强CT检查更有利的证据,具有一定的术前评估价值。
| [1] |
袁勇, 陈心足, 胡建昆, 陈龙奇. 食管胃结合部腺癌外科治疗中国专家共识(2018年版)解读[J]. 中华胃肠外科杂志, 2019, 22: 101-106. |
| [2] |
LIU K, YANG K, ZHANG W, CHEN X, CHEN X, ZHANG B, et al. Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988-2012:a single-institution, high-volume experience in China[J]. Ann Surg, 2016, 263: 88-95. DOI:10.1097/SLA.0000000000001148 |
| [3] |
AJANI J A, D'AMICO T A, ALMHANNA K, BENTREM D J, CHAO J, DAS P, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2016, 14: 1286-1312. DOI:10.6004/jnccn.2016.0137 |
| [4] |
IMAMURA Y, WATANABE M, TOIHATA T, TAKAMATSU M, KAWACHI H, HARAGUCHI I, et al. Recent incidence trend of surgically resected esophagogastric junction adenocarcinoma and microsatellite instability status in Japanese patients[J]. Digestion, 2019, 99: 6-13. DOI:10.1159/000494406 |
| [5] |
SIEWERT J R, HÖLSCHER A H, BECKER K, GÖSSNER W. Cardia cancer: attempt at a therapeutically relevant classification[J]. Der Chir Zeitschrift Fur Alle Gebiete Der Oper Medizen, 1987, 58: 25-32. |
| [6] |
AJANI J A, BARTHEL J S, BENTREM D J, D'AMICO T A, DAS P, DENLINGER C S, et al. Esophageal and esophagogastric junction cancers[J]. J Natl Compr Canc Netw, 2011, 9: 830-887. DOI:10.6004/jnccn.2011.0072 |
| [7] |
NCCN. NCCN clinical practice guidelines in oncology-gastric cancer (version 2.2020)[EB/OL]. Fort Washington: NCCN, 2020[2020-05-13]. https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
|
| [8] |
中华医学会外科学分会腹腔镜与内镜外科学组. Siewert Ⅱ型食管胃结合部腺癌腔镜手术治疗中国专家共识(2019版)[J]. 中国实用外科杂志, 2019, 39: 1129-1135. |
| [9] |
FEITH M, STEIN H J, SIEWERT J R. Adenocarcinoma of the esophagogastric junction: surgical therapy based on 1602 consecutive resected patients[J]. Surg Oncol Clin N Am, 2006, 15: 751-764. DOI:10.1016/j.soc.2006.07.015 |
| [10] |
HOSOKAWA Y, KINOSHITA T, KONISHI M, TAKAHASHI S, GOTOHDA N, KATO Y, et al. Clinicopathological features and prognostic factors of adenocarcinoma of the esophagogastric junction according to Siewert classification: experiences at a single institution in Japan[J]. Ann Surg Oncol, 2012, 19: 677-683. DOI:10.1245/s10434-011-1983-x |
| [11] |
朱娟, 王少明, 陈茹, 李新庆, 魏文强. 胃癌筛查现状的研究进展[J]. 中华肿瘤杂志, 2020, 42: 603-608. DOI:10.3760/cma.j.cn112152-20191125-00759 |
| [12] |
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014(ver. 4)[J]. Gastric Cancer, 2017, 20: 1-19. DOI:10.1007/s10120-016-0622-4 |
| [13] |
赵晓君, 周忠洁, 张弦, 刘银, 严志汉. 多排螺旋CT检查在食管胃结合部腺癌术前评估中的应用价值[J]. 中华消化外科杂志, 2016, 15: 1118-1122. DOI:10.3760/cma.j.issn.1673-9752.2016.11.015 |
| [14] |
KIM J W, SHIN S S, HEO S H, CHOI Y D, LIM H S, PARK Y K, et al. Diagnostic performance of 64-section CT using CT gastrography in preoperative T staging of gastric cancer according to 7th edition of AJCC cancer staging manual[J]. Eur Radiol, 2012, 22: 654-662. DOI:10.1007/s00330-011-2283-3 |
| [15] |
LIU S, HE J, GUAN W, LI Q, YU H, ZHOU Z, et al. Added value of diffusion-weighted MR imaging to T2-weighted and dynamic contrast-enhanced MR imaging in T staging of gastric cancer[J]. Clin Imaging, 2014, 38: 122-128. DOI:10.1016/j.clinimag.2013.12.001 |
| [16] |
JOO I, LEE J M, KIM J H, SHIN C I, HAN J K, CHOI B I. Prospective comparison of 3T MRI with diffusion-weighted imaging and MDCT for the preoperative TNM staging of gastric cancer[J]. J Magn Reson Imaging, 2015, 41: 814-821. DOI:10.1002/jmri.24586 |
| [17] |
ARSLAN H, FATIH ÖZBAY M, ÇALLı İ, DOĞAN E, ÇELIK S, BATUR A, et al. Contribution of diffusion weighted MRI to diagnosis and staging in gastric tumors and comparison with multi-detector computed tomography[J]. Radiol Oncol, 2017, 51: 23-29. DOI:10.1515/raon-2017-0002 |
| [18] |
CAIVANO R, RABASCO P, LOTUMOLO A, D'ANTUONO F, ZANDOLINO A, VILLONIO A, et al. Gastric cancer: the role of diffusion weighted imaging in the preoperative staging[J]. Cancer Invest, 2014, 32: 184-190. DOI:10.3109/07357907.2014.896014 |
| [19] |
张习杰, 马鹏飞, 李祥, 曲金荣, 张宏凯, 卢俊, 等. MRI动态增强扫描在食管胃结合部腺癌上界定位中的应用[J]. 中华普通外科杂志, 2021, 36: 277-280. |
| [20] |
YUAN Y, CHEN L, REN S, WANG Z, CHEN Y, JIN A, et al. Diagnostic performance in T staging for patients with esophagogastric junction cancer using high-resolution MRI: a comparison with conventional MRI at 3 tesla[J/OL]. Cancer Imaging, 2019, 19: 83. DOI: 10.1186/s40644-019-0269-6.
|
| [21] |
MARIETTE C, PIESSEN G, BRIEZ N, GRONNIER C, TRIBOULET J P. Oesophagogastric junction adenocarcinoma: which therapeutic approach?[J]. Lancet Oncol, 2011, 12: 296-305. DOI:10.1016/S1470-2045(10)70125-X |
| [22] |
HASHIMOTO T, KUROKAWA Y, MORI M, DOKI Y. Surgical treatment of gastroesophageal junction cancer[J]. J Gastric Cancer, 2018, 18: 209-217. DOI:10.5230/jgc.2018.18.e28 |
| [23] |
周梦婷, 何文华, 吕农华. 2018年版韩国胃癌实践指南解读[J]. 中华消化杂志, 2020, 40: 212-216. |
| [24] |
闵丛丛, 张静, 王晔, 郭燕磊, 张贺军, 丁士刚. 术前预测胃癌淋巴结转移的分子标志物研究[J]. 中华消化杂志, 2020, 40: 373-379. |
| [25] |
中国临床肿瘤学会指南工作委员会. 中国临床肿瘤学会(CSCO)原发性胃癌诊疗指南2017.V1[M]. 北京: 人民卫生出版社, 2017: 1-8.
|
| [26] |
王之龙, 唐磊, 李艳玲, 李忠武, 王胤奎, 李子禹, 等. 食管胃结合部腺癌膈肌旁及下纵隔淋巴结转移的CT与病理对照研究[J]. 临床放射学杂志, 2021, 40: 522-527. |
 2021, Vol. 42
2021, Vol. 42


