2. 中国人民解放军联勤保障部队904医院心血管内科, 无锡 214044
2. Department of Cardiovasology, No. 904 Hospital of Joint Logistics Support Force of PLA, Wuxi 214044, Jiangsu, China
炎症在动脉粥样硬化的各个阶段都发挥着重要作用,从脂质条纹的形成到斑块破裂及随后的血栓形成,炎症反应都扮演着重要的角色。CRP是重要的炎症因子之一,已成为心血管事件的独立预测指标[1-2]。CRP可以直接作用于动脉粥样硬化斑块内各种细胞从而促进动脉粥样硬化形成,如CRP可以通过与白细胞免疫球蛋白γ Fc片段低亲和力受体Ⅱ(low affinity immunoglobulin gamma Fc receptor Ⅱ,FcgRⅡ;即CD32)及人主动脉内皮细胞CD32和CD64结合促进这一过程[3-4]。除了这些细胞表面受体,CRP还对低密度脂蛋白和高密度脂蛋白具有亲和力[5-6],从而影响脂蛋白功能。
高密度脂蛋白胆固醇(high-density lipoprotein-cholesterol,HDL-C)是冠状动脉粥样硬化性心脏病(以下简称冠心病)的一个独立保护因素,其可以通过多种机制预防动脉粥样硬化。而载脂蛋白A1(apolipoprotein A1,ApoA1)作为HDL-C颗粒的主要蛋白质成分,在抗动脉粥样硬化中起着重要作用[7-8]。有研究指出,相比HDL-C,血清ApoA1对冠心病发生风险的影响更加显著[9]。ApoA1主要通过胆固醇逆转运及抑制炎症反应来发挥其抗动脉粥样硬化作用,其抗炎作用已经在许多实验研究中得到证实[8, 10]。
尽管目前国内外关于炎症、脂质与冠心病的关系已有广泛研究,但尚未见将CRP与ApoA1这2个体现炎症与脂质代谢的因素联合起来探讨两者与冠心病相关性的报道。从理论上来说,CRP与ApoA1比值(CRP/ApoA1)一定程度上可以同时反映体内的氧化应激、炎症状况及脂质代谢紊乱情况。CRP作为炎症标志物,其与冠心病的发生和发展密切有关[11],而ApoA1具有抗氧化、抗炎、抗动脉粥样硬化作用,与冠状动脉病变程度呈负相关[12],因此CRP/ApoA1可能会更好地反映冠心病病程中的炎症因素及血脂代谢紊乱。本研究以Gensini积分、病变血管支数作为冠状动脉病变的评估标准,分析CRP/ApoA1与Gensini积分及病变血管支数的相关性,探讨CRP/ApoA1对冠状动脉病变的评估价值。
1 资料和方法 1.1 一般资料采用回顾性分析方法,连续选择2018年12月至2019年12月因胸痛在中国人民解放军联勤保障部队904医院心血管内科住院治疗的637例患者。根据美国心脏协会/美国心脏病学会冠心病诊断指南[13],以任一主要冠状动脉如左主干、左前降支、左回旋支、右冠状动脉或其主要分支(血管直径>1.5 mm的对角支、钝缘支、左室后支、后降支等)狭窄≥50%作为冠心病的诊断标准。纳入标准:(1)年龄为30~85岁,因胸痛入院择期行冠状动脉造影检查;(2)神志清楚,能自然交流沟通,无严重神经及精神类疾病;(3)入院后规范服用阿司匹林联合替格瑞洛/氯吡格雷双联抗血小板治疗。排除标准:(1)有陈旧性心肌梗死、经皮冠状动脉介入治疗或冠状动脉旁路移植术史的患者;(2)ST段抬高型心肌梗死或急诊行冠状动脉造影检查者;(3)有缺血性脑卒中病史者;(4)伴有肺栓塞、主动脉夹层、急/慢性肾炎或其他系统病变者;(5)合并血液系统疾病、恶性肿瘤或自身免疫性疾病者;(6)合并急/慢性感染性疾病者。本研究通过中国人民解放军联勤保障部队904医院伦理委员会审批。
1.2 研究分组依据Gensini积分和冠状动脉造影检查结果,将所有入选患者分为冠状动脉正常组(Gensini积分为0分,n=118)、冠状动脉粥样硬化组(Gensini积分为1~18分但不满足冠心病诊断,n=109)和冠心病组(Gensini积分为1~180分且符合冠心病诊断标准,n=306)。根据Gensini积分,将冠心病患者分为轻度病变组(Gensini评分为1~<30分,n=177)和重度病变组(Gensini评分为30~180分,n=129)[14]。依据冠状动脉主支及分支的病变血管支数,将冠心病患者分为冠状动脉单支血管病变组(n=147)、冠状动脉双支血管病变组(n=90)、冠状动脉3支及以上血管病变组(n=69)。
1.3 Gensini积分评定冠状动脉造影检查均于入院3 d内完成,由至少2名经验丰富的高级职称医师评估冠状动脉病变情况。Gensini积分评定标准:(1)对左主干、左前降支、回旋支和右冠状动脉等血管的病变程度进行定量评定,无任何异常评为0分,狭窄≤25%计1分,狭窄26%~50%计2分,狭窄51%~75%计4分,狭窄76%~90%计8分,狭窄91%~99%计16分,狭窄100%计32分。(2)各支血管得分乘以相应系数为其Gensini积分,其中左主干病变得分×5;左前降支近段得分×2.5,中段得分×1.5,远段得分×1;第一对角支得分×1;左室后侧支得分×0.5;右冠状动脉近、中、远段和后降支得分均×1。(3)患者各支血管积分之和为该患者冠状动脉病变程度的最终Gensini积分[15]。
1.4 观察指标收集患者入院时临床资料,包括性别、年龄、高血压史、糖尿病史、吸烟史等。所有入选患者入院后清晨空腹时采集肘静脉血,检测血常规,采用全自动生化仪(美国Beckman公司)检测总胆固醇、HDL-C、低密度脂蛋白胆固醇(low-density lipoprotein-cholesterol,LDL-C)、ApoA1、血肌酐、CRP、白蛋白、同型半胱氨酸等,并计算C反应蛋白/白蛋白比值(C reactive protein to albumin ratio,CAR)和CRP/ApoA1。
1.5 统计学处理应用SPSS 20.0软件进行统计学分析。计量资料若服从正态分布以x±s表示,两组间比较采用独立样本t检验;若不服从正态分布以中位数(下四分位数,上四分位数)表示,两组间比较采用两样本Mann-Whitney U检验。计数资料以例数和百分数表示,组间比较采用χ2检验。将患者资料中连续性变量进行二分类后纳入单因素logistic回归分析,以P<0.05为标准筛选出相关影响因素纳入多因素logistic二元回归分析,得到冠心病及重度病变的独立危险因素,并通过ROC曲线评价多因素模型的区分度,采用Hosmer-Lemeshow拟合优度检验评价模型的校准度。采用Spearman相关性分析评估CRP/ApoA1与Gensini积分的相关性。应用MedCalc 15.2.2软件生成CRP、CAR、ApoA1、CRP/ApoA1预测冠心病、冠心病重度病变、冠状动脉3支及以上血管病变的ROC曲线,采用DeLong非参数法比较各指标的AUC值,分析它们对冠状动脉重度病变的预测价值。检验水准(α)为0.05。
2 结果 2.1 患者一般资料比较共入选533例患者,男348例、女185例,年龄为37~85岁,平均年龄为(64±9)岁。冠状动脉正常组118例(男56例,女62例)、冠状动脉粥样硬化组109例(男60例,女49例)、冠心病组306例(男232例,女74例)。见表 1,与冠状动脉正常组相比,冠心病组患者中男性占比较高,年龄较大,吸烟患者占比较高,合并糖尿病、高血压的患者占比均较高,血清HDL-C、ApoA1均较低(P均<0.01),白细胞计数、中性粒细胞计数、CRP、α1微球蛋白、β2微球蛋白、同型半胱氨酸、血肌酐、胱抑素C、CAR、CRP/ApoA1均较高,差异均有统计学意义(P均<0.01)。见表 2,与冠心病轻度病变组相比,冠心病重度病变组患者的年龄较大,糖尿病、吸烟患者占比均较高,血清HDL-C、ApoA1、白蛋白水平均较低,白细胞计数、中性粒细胞计数、血小板计数、LDL-C、载脂蛋白B、脂蛋白a、CRP、β2微球蛋白、胱抑素C、CAR、CRP/ApoA1均较高,差异均有统计学意义(P均<0.05)。
|
|
表 1 冠状动脉正常组与CHD组患者的临床资料比较 Tab 1 Comparison of clinical data between normal coronary artery group and CHD group |
|
|
表 2 CHD轻度病变组与重度病变组患者的临床资料比较 Tab 2 Comparison of clinical data between mild and severe CHD groups |
2.2 冠心病的影响因素分析
将冠心病组与冠状动脉正常组之间比较P<0.2的因素纳入单因素logistic回归分析,结果显示HDL-C、ApoA1是冠心病的保护因素(OR=0.429、0.428,P均<0.01),男性、年龄、高血压、糖尿病、吸烟、白细胞计数、中性粒细胞计数、CRP、β2微球蛋白、同型半胱氨酸、血肌酐、胱抑素C、CAR、CRP/ApoA1是冠心病的危险因素(OR=1.550~4.281,P均<0.05)。将单因素logistic回归分析差异有统计学意义(P<0.05)的因素纳入多因素logistic二元回归分析,共线性诊断提示CAR、CRP与CRP/ApoA1的方差膨胀因子均>10、容忍度<0.1,考虑本研究主要研究CRP/ApoA1,故去除CAR、CRP,将性别、高血压、糖尿病、吸烟、年龄、白细胞计数、中性粒细胞计数、HDL-C、ApoA1、白蛋白、β2微球蛋白、同型半胱氨酸、血肌酐、胱抑素C、CRP/ApoA1纳入多因素logistic二元回归模型,以筛选冠心病的独立影响因素。多因素logistic二元回归分析结果显示,男性(OR=2.967,95% CI 1.686~5.220,P<0.01)、年龄(OR=3.433,95% CI 2.048~5.753,P<0.01)、高血压(OR=1.844,95% CI 1.124~3.023,P=0.015)、糖尿病(OR=3.190,95% CI 1.607~6.330,P<0.01)、吸烟(OR=1.874,95% CI 1.042~3.371,P=0.036)、CRP/ApoA1(OR=2.171,95% CI 1.293~3.647,P<0.01)是冠心病的独立危险因素。模型区分度检验分析显示,预测模型的ROC AUC值为0.810(95% CI 0.765~0.856,P<0.01),AUC值>0.75,提示该预测模型的区分度较好(图 1A)。通过Hosmer-Lemeshow检验评价预测模型的校准度,结果显示χ2=11.077、P=0.197,提示模型预测值与实际观测值之间差异无统计学意义,说明预测模型有较好的校准能力(图 1B)。
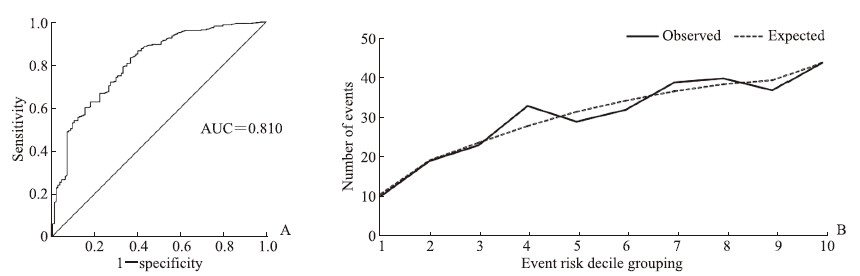
|
图 1 冠心病多因素logistic二元回归模型的区分度及校准度分析结果 Fig 1 Discrimination and calibration evaluation of multivariate logistic binary regression model for coronary heart disease A: Receiver operating characteristic curve analysis of the model discrimination; B: Hosmer-Lemeshow test analysis of the model calibration. AUC: Area under curve. |
2.3 冠心病病变严重程度的影响因素
将冠心病轻度病变组与重度病变组之间比较P<0.2的指标纳入单因素logistic回归分析,结果表明白蛋白、ApoA1是冠心病重度病变的保护因素(OR=0.444、0.543,P=0.001、0.009),年龄、吸烟、糖尿病、白细胞计数、中性粒细胞计数、CRP、α1微球蛋白、β2微球蛋白、胱抑素C、CAR、CRP/ApoA1是冠心病重度病变的危险因素(OR=1.651~7.517,P均<0.05)。将单因素logistic回归分析差异有统计学意义的因素纳入多因素logistic二元回归分析模型,共线性诊断提示CAR、CRP与CRP/ApoA1的方差膨胀因子均>10、容忍度<0.1,考虑本研究主要研究CRP/ApoA1,故去除CAR、CRP,将年龄、吸烟、糖尿病、白细胞计数、中性粒细胞计数、ApoA1、白蛋白、α1微球蛋白、β2微球蛋白、胱抑素C、CRP/ApoA1纳入多因素logistic二元回归分析模型,结果显示年龄(OR=2.988,95% CI 1.708~5.227,P<0.01)、吸烟(OR=2.138,95% CI1.218~3.754,P=0.008)、糖尿病(OR=2.807,95% CI 1.583~4.976,P<0.01)、CRP/ApoA1(OR=6.306,95% CI 3.591~11.073,P<0.01)是冠心病重度病变的独立危险因素,白蛋白(OR=0.546,95% CI0.312~0.995,P=0.034)是冠心病重度病变的独立保护因素。模型区分度检验分析显示,预测模型的ROC AUC值为0.835(95% CI 0.790~0.882,P<0.01),AUC值>0.75,提示该预测模型的区分度较好(图 2A)。通过Hosmer-Lemeshow检验评价预测模型的校准能力,结果显示χ2=6.766、P=0.562,提示模型预测值与实际观测值之间差异无统计学意义,说明预测模型有较好的校准能力(图 2B)。
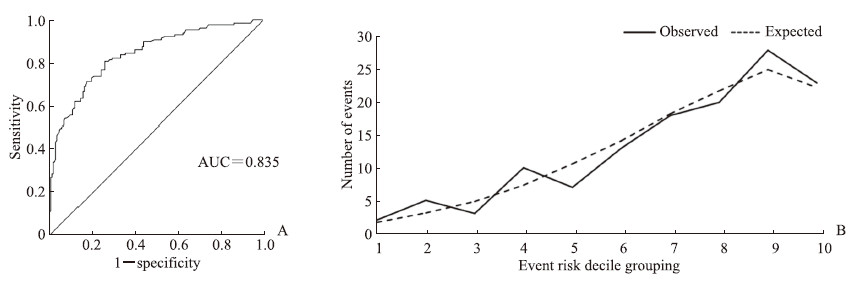
|
图 2 冠心病重度病变多因素logistic二元回归模型的区分度及校准度分析结果 Fig 2 Discrimination and calibration evaluation of multivariate logistic binary regression model for severe coronary heart disease A: Receiver operating characteristic curve analysis of the model discrimination; B: Hosmer-Lemeshow test analysis of the model calibration. AUC: Area under curve. |
2.4 CRP/ApoA1与Gensini积分的相关性
对533例患者的CRP/ApoA1与Gensini积分进行Spearman相关性分析,结果显示两者呈正相关(r=0.419,P<0.01),说明CRP/ApoA1越高,患者的冠状动脉病变越严重。
2.5 CRP/ApoA1、CAR、CRP和ApoA1对冠心病及其严重程度的预测价值采用ROC曲线评估CRP/ApoA1、CAR、ApoA1对冠心病及其严重程度的预测价值,并对AUC值进行比较。结果显示,CRP/ApoA1取临界值1.666时,对冠心病的预测效能最高,灵敏度为0.611,特异度为0.749,其AUC值优于CAR、CRP、ApoA1,差异均有统计学意义(Z=3.801、4.051、2.041,P均<0.05)。CRP/ApoA1取临界值1.993时,对冠心病重度病变的预测效能最高,灵敏度为0.798,特异度为0.740,其AUC值优于CAR、CRP、ApoA1,差异均有统计学意义(Z=2.507、3.358、3.848,P均<0.05)。见图 3。
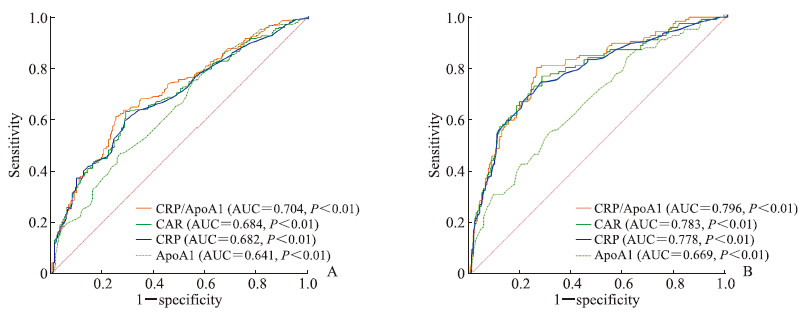
|
图 3 CRP/ApoA1、CAR、CRP和ApoA1预测冠心病的ROC曲线分析 Fig 3 ROC curve analyses of CRP/ApoA1, CAR, CRP and ApoA1 in predicting coronary heart disease A: ROC curves of CRP/ApoA1, CAR, CRP and ApoA1 in predicting coronary heart disease; B: ROC curves of CRP/ApoA1, CAR, CRP and ApoA1 in predicting severe coronary heart disease. CRP: C reactive protein; ApoA1: Apolipoprotein A1; CAR: C reactive protein to albumin ratio; ROC: Receiver operating characteristic; AUC: Area under curve. |
2.6 CRP/ApoA1与病变血管支数的关系
对533例患者的CRP/ApoA1与冠状动脉病变血管支数进行Spearman相关性分析,结果显示两者呈正相关(r=0.431,P<0.05),说明患者的CRP/ApoA1越高,冠状动脉病变血管支数越多。冠状动脉正常组、冠状动脉单支血管病变组、冠状动脉双支血管病变组、冠状动脉3支及以上血管病变组患者的CRP/ApoA1分别为0.986(0.681,2.054)、1.414(0.841,2.477)、2.262(0.920,6.082)、5.857(2.596,12.936),差异有统计学意义(P<0.05)。采用ROC曲线评估CRP/ApoA1、CAR、CRP、ApoA1对冠状动脉病变血管支数的预测价值,结果(图 4)显示,CRP/ApoA1取临界值2.545时,对冠状动脉3支及以上血管病变的预测效能最高,灵敏度为0.768,特异度为0.769,其AUC值优于CAR、CRP、ApoA1,差异均有统计学意义(Z=2.725、3.071、3.234,P均<0.01)。
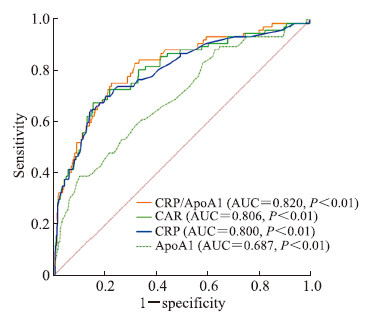
|
图 4 CRP/ApoA1、CAR、CRP和ApoA1预测冠状动脉3支及以上血管病变的ROC曲线分析 Fig 4 ROC curve analyses of CRP/ApoA1, CAR, CRP and ApoA1 in predicting 3 or more vessel coronary artery disease CRP: C reactive protein; ApoA1: Apolipoprotein A1; CAR: C reactive protein to albumin ratio; ROC: Receiver operating characteristic; AUC: Area under curve. |
3 讨论
炎症和血脂异常是冠心病发生和发展的重要危险因素,本研究将血脂与炎症联合,探讨CRP/ApoA1与冠状动脉病变的相关性。通过比较CRP/ApoA1与其他传统炎症、血脂参数预测冠心病及冠心病重度病变的AUC值,观察到其预测冠心病重度病变的优越性。Spearman相关性分析表明CRP/ApoA1越高冠状动脉病变越严重。CRP/ApoA1与病变血管支数也呈正相关,3支及以上血管病变组患者的CRP/ApoA1高于其他分组。多因素logistic二元回归分析结果也支持CRP/ApoA1是冠心病及冠心病重度病变的独立危险因素,且该模型具有较好的区分度及校准度。ROC曲线分析结果表明CRP/ApoA1对冠状动脉的病变情况具有较高的判断价值,其对冠心病、冠心病重度病变和冠状动脉3支及以上血管病变的诊断效能均优于CAR、CRP、ApoA1。目前已有文献报道了CAR与其他炎症标志物在稳定型心绞痛患者中的诊断价值,并发现CAR检测冠心病(有意义病变)的诊断价值最显著[1];在血脂参数中,ApoA1与冠心病的相关性显著[16],而CRP/ApoA1对冠心病、冠心病重度病变和冠状动脉3支及以上血管病变的诊断效能均优于CAR、ApoA1,这表明CRP/ApoA1相较单一的炎症及血脂参数能够更灵敏地预测冠心病的病变情况。
ApoA1作为高密度脂蛋白颗粒的主要蛋白质成分,在抗动脉粥样硬化中起着重要作用[17]。研究表明ApoA1可通过降低凝血酶原片段F1+2和D-二聚体抑制促炎细胞因子IL-6释放,而IL-6能诱导肝细胞合成CRP[18],提示ApoA1与炎症因子的产生密不可分。研究发现ApoA1能够通过介导胆固醇流出的调节脂筏来抑制应激[19],也可通过抑制脂多糖诱导的p38 MAPK信号转导通路激活进一步促进泡沫细胞锌指蛋白36生成[20],从而抑制相关炎症因子的释放。ApoA1还可以阻断T淋巴细胞对单核细胞的接触激活,从而抑制IL-6、TNF-α等炎症因子产生[21],这些均是CRP产生过程中重要的炎症因子,说明ApoA1从病理机制上可以抑制CRP产生。
ApoA1具有潜在的抗炎作用,被认为是直接抑制细胞炎症因子生成的蛋白质[22],但全身的炎症状态同样会对ApoA1的生成造成负性影响。文献报道,感染会改变内皮功能、抑制ApoA1水平并提高载脂蛋白B与ApoA1的比值[23];血管炎症还可以通过调节髓过氧化物酶对ApoA1的氧化修饰引起ApoA1发生功能障碍,从而失去对冠状动脉的保护作用[24]。Kaysen等[25]研究发现血液透析的患者开始血液透析后血清炎症标志物CRP的改变与ApoA1改变密切相关;一项关于全身炎症反应与大肠癌患者生存率相关性的研究也发现ApoA1水平与血清CRP水平呈负相关[26],与本研究结果一致。这为本研究探讨CRP/ApoA1与冠状动脉病变的关系提供了一定的理论基础。
本研究将CRP与ApoA1联合,从冠心病发病机制中的炎症及血脂2个方面进行探索,具有一定的科学性、合理性、创新性。采用Gensini积分、病变血管支数评价冠状动脉病变情况,以确保冠状动脉病变程度描述的客观性,使研究结果更加准确。CRP/ApoA1结合血脂及炎症标志物预测冠状动脉病变程度,其预测结果更具指导意义。特别是对没有能力行冠状动脉造影检查的基层医院,采用CRP/ApoA1预测冠状动脉病变情况,有助于早期识别冠心病高危患者,从而强化药物治疗或转上级医院进一步诊治,这对降低冠心病的发病率及病死率十分重要。
本研究有以下不足之处:(1)本研究使用Gensini积分评价冠状动脉病变情况,评分系统未包括分支病变、扭曲病变及钙化病变等特征,具有一定的局限性。(2)CRP和ApoA1等均为术前单次血样检测结果,未进行动态检测。患者进行血运重建(如经皮冠状动脉介入治疗或冠状动脉旁路移植术)后,CAR和同型半胱氨酸与HDL-C比值是否会发生相应变化,值得关注。(3)本研究仅讨论了CRP/ApoA1与冠状动脉病变情况的关系,其与冠心病的长期预后、不良事件发生的关系仍有待进一步研究。
| [1] |
TANRIVERDI Z, GUNGOREN F, TASCANOV M B, BESLI F, ALTIPARMAK I H. Comparing the diagnostic value of the C-reactive protein to albumin ratio with other inflammatory markers in patients with stable angina pectoris[J]. Angiology, 2020, 71: 360-365. DOI:10.1177/0003319719897490 |
| [2] |
ÇADA M, RENCÜZOULLARI I, KARAKOYUN S, KARABA Y, YESIN M, ARTAÇ I, et al. Assessment of relationship between C-reactive protein to albumin ratio and coronary artery disease severity in patients with acute coronary syndrome[J]. Angiology, 2019, 70: 361-368. DOI:10.1177/0003319717743325 |
| [3] |
FANG Z, LV J, WANG J, QIN Q, HE J, WANG M, et al. C-reactive protein promotes the activation of fibroblast-like synoviocytes from patients with rheumatoid arthritis[J/OL]. Front Immunol, 2020, 11: 958. DOI: 10.3389/fimmu.2020.00958.
|
| [4] |
DEVARAJ S, DU CLOS T W, JIALAL I. Binding and internalization of C-reactive protein by Fcgamma receptors on human aortic endothelial cells mediates biological effects[J]. Arterioscler Thromb Vasc Biol, 2005, 25: 1359-1363. DOI:10.1161/01.ATV.0000168573.10844.ae |
| [5] |
KINGWELL B A, CHAPMAN M J, KONTUSH A, MILLER N E. HDL-targeted therapies: progress, failures and future[J]. Nat Rev Drug Discov, 2014, 13: 445-464. DOI:10.1038/nrd4279 |
| [6] |
BIAN F, YANG X Y, XU G, ZHENG T, JIN S. CRP-induced NLRP3 inflammasome activation increases LDL transcytosis across endothelial cells[J/OL]. Front Pharmacol, 2019, 10: 40. DOI: 10.3389/fphar.2019.00040.
|
| [7] |
LIU M, MEI X, HERSCOVITZ H, ATKINSON D. N-terminal mutation of ApoA-Ⅰ and interaction with ABCA1 reveal mechanisms of nascent HDL biogenesis[J]. J Lipid Res, 2019, 60: 44-57. DOI:10.1194/jlr.M084376 |
| [8] |
BARRETT T J, DISTEL E, MURPHY A J, HU J, GARSHICK M S, OGANDO Y, et al. Apolipoprotein A-Ⅰ) promotes atherosclerosis regression in diabetic mice by suppressing myelopoiesis and plaque inflammation[J]. Circulation, 2019, 140: 1170-1184. DOI:10.1161/CIRCULATIONAHA.119.039476 |
| [9] |
MCQUEEN M J, HAWKEN S, WANG X, OUNPUU S, SNIDERMAN A, PROBSTFIELD J, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study[J]. Lancet, 2008, 372: 224-233. DOI:10.1016/S0140-6736(08)61076-4 |
| [10] |
ZHANG M, LI L, XIE W, WU J F, YAO F, TAN Y L, et al. Apolipoprotein A-1 binding protein promotes macrophage cholesterol efflux by facilitating apolipoprotein A-1 binding to ABCA1 and preventing ABCA1 degradation[J]. Atherosclerosis, 2016, 248: 149-159. DOI:10.1016/j.atherosclerosis.2016.03.008 |
| [11] |
LI C, ZHANG Z, PENG Y, GAO H, WANG Y, ZHAO J, et al. Plasma neutrophil gelatinase-associated lipocalin levels are associated with the presence and severity of coronary heart disease[J/OL]. PLoS One, 2019, 14: e0220841. DOI: 10.1371/journal.pone.0220841.
|
| [12] |
TANI S, NAGAO K, HIRAYAMA A. Association of systemic inflammation with the serum apolipoprotein A-1 level: a cross-sectional pilot study[J]. J Cardiol, 2016, 68: 168-177. DOI:10.1016/j.jjcc.2015.08.016 |
| [13] |
RYAN T J, BAUMAN W B, KENNEDY J W, KEREIAKES D J, KING S B 3rd, MCCALLISTER B D, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American Heart Association/American College of Cardiology task force on assessment of diagnostic and therapeutic cardiovascular procedures (Committee on Percutaneous Transluminal Coronary Angioplasty)[J]. Circulation, 1993, 88: 2987-3007.
|
| [14] |
UYSAL H B, DALI B, AKGÜLLÜ C, AVCIL M, ZENCIR C, AYHAN M, et al. Blood count parameters can predict the severity of coronary artery disease[J]. Korean J Intern Med, 2016, 31: 1093-1100. DOI:10.3904/kjim.2015.199 |
| [15] |
ALAN B, AKPOLAT V, AKTAN A, ALAN S. Relationship between osteopenic syndrome and severity of coronary artery disease detected with coronary angiography and Gensini score in men[J]. Clin Interv Aging, 2016, 11: 377-382. |
| [16] |
吴祖飞, 陈诗, 刘叶红, 苏文韬, 宗刚军, 吴刚勇. 不同血脂成分与冠状动脉病变相关性的初步探讨[J]. 临床心血管病杂志, 2021, 37: 816-824. |
| [17] |
CHRONI A, KARDASSIS D. HDL dysfunction caused by mutations in ApoA-Ⅰ and other genes that are critical for HDL biogenesis and remodeling[J]. Curr Med Chem, 2019, 26: 1544-1575. DOI:10.2174/0929867325666180313114950 |
| [18] |
VAN LEUVEN S I, BIRJMOHUN R S, FRANSSEN R, BISOENDIAL R J, DE KORT H, LEVELS J H, et al. ApoAⅠ-phosphatidylcholine infusion neutralizes the atherothrombotic effects of C-reactive protein in humans[J]. J Thromb Haemost, 2009, 7: 347-354. DOI:10.1111/j.1538-7836.2008.03175.x |
| [19] |
TANG C, HOUSTON B A, STOREY C, LEBOEUF R C. Both STAT3 activation and cholesterol efflux contribute to the anti-inflammatory effect of ApoA-Ⅰ/ABCA1 interaction in macrophages[J]. J Lipid Res, 2016, 57: 848-857. DOI:10.1194/jlr.M065797 |
| [20] |
ZHANG M, ZHAO G J, YIN K, XIA X D, GONG D, ZHAO Z W, et al. Apolipoprotein A-1 binding protein inhibits inflammatory signaling pathways by binding to apolipoprotein A-1 in THP-1 macrophages[J]. Circ J, 2018, 82: 1396-1404. DOI:10.1253/circj.CJ-17-0877 |
| [21] |
HYKA N, DAYER J M, MODOUX C, KOHNO T, EDWARDS C K, ROUX-LOMBARD P, et al. Apolipoprotein A-Ⅰ inhibits the production of interleukin-1β and tumor necrosis factor-α by blocking contact-mediated activation of monocytes by T lymphocytes[J]. Blood, 2001, 97: 2381-2389. DOI:10.1182/blood.V97.8.2381 |
| [22] |
WANG J L, GONG D, HU X Y, WU S, ZHENG X L, WU J, et al. ApoA-1 mimetic peptide ELK-2A2K2E decreases inflammatory factor levels through the ABCA1-JAK2-STAT3-TTP axis in THP-1-derived macrophages[J]. J Cardiovasc Pharmacol, 2018, 72: 60-67. DOI:10.1097/FJC.0000000000000594 |
| [23] |
KILICKAP M, GOKSULUK H, CANDEMIR B, KAYA C T, OZCAN O U, TURHAN S, et al. Evaluation of acute infection-induced endothelial dysfunction and its potential mediators[J]. Acta Cardiol, 2011, 66: 581-587. DOI:10.1080/AC.66.5.2131082 |
| [24] |
CHAN G K, WITKOWSKI A, GANTZ D L, ZHANG T O, ZANNI M T, JAYARAMAN S, et al. Myeloperoxidase-mediated methionine oxidation promotes an amyloidogenic outcome for apolipoprotein A-Ⅰ[J]. J Biol Chem, 2015, 290: 10958-10971. DOI:10.1074/jbc.M114.630442 |
| [25] |
KAYSEN G A, DALRYMPLE L S, GRIMES B, CHERTOW G M, KORNAK J, JOHANSEN K L. Changes in serum inflammatory markers are associated with changes in apolipoprotein A1 but not B after the initiation of dialysis[J]. Nephrol Dial Transplant, 2014, 29: 430-437. DOI:10.1093/ndt/gft370 |
| [26] |
SIRNIÖ P, VÄYRYNEN J P, KLINTRUP K, MÄKELÄ J, MÄKINEN M J, KARTTUNEN T J, et al. Decreased serum apolipoprotein A1 levels are associated with poor survival and systemic inflammatory response in colorectal cancer[J/OL]. Sci Rep, 2017, 7: 5374. DOI: 10.1038/s41598-017-05415-9.
|
 2021, Vol. 42
2021, Vol. 42


