2. 海军军医大学(第二军医大学)长海医院脑血管病中心, 上海 200433
2. Stroke Center, Changhai Hospital, Naval Medical University(Second Military Medical University), Shanghai 200433, China
2016年全球疾病负担(global burden of disease,GBD)研究估计,中国是全球脑卒中终身风险最高的国家,从25岁起脑卒中的终身风险高达39.3%[1]。近年来,脑卒中人群呈现年轻化趋势,流行病学资料显示,55岁以下人群脑卒中的发病率为7/10万~11/10万[2-3]。对危险因素的早期干预和预后的积极评估是防治脑卒中的重要手段。与老年人相比,中青年人脑卒中的发病机制略有不同,除动脉粥样硬化、高血压、吸烟、脂代谢异常、高同型半胱氨酸血症等常见危险因素[4]外,遗传性因素、心源性疾病、烟雾病、血管炎、动脉夹层等非动脉粥样硬化病因也起到非常重要的作用。基因突变是脑血管病的一个重要病因,研究显示亚甲基四氢叶酸还原酶(methylenetetrahydrofolate reductase,MTHFR)与动脉硬化密切相关,染色体9p21基因座和组蛋白去乙酰化酶9(histone deacetylase 9,HDAC9)与大血管病变相关,环指蛋白213(ring finger protein 213,RNF213)与烟雾病血管病变相关,成对样同源结构域转录因子2(paired-liked homodomain transcription factor 2,PITX2)和锌指同源框蛋白3(zinc finger homeobox 3,ZFHX3)与心源性缺血性脑卒中相关[5]。
脑梗死是脑卒中的一个亚型,一项调查研究显示我国青年脑梗死占青年脑卒中的63.6%[6]。本课题组以中青年脑梗死患者为研究对象,收集患者的年龄、性别、既往史、血液学指标等基线资料及HDAC9、RNF213和MTHFR基因多态性特征,并随访1年观察患者的临床结局,同时进行预后影响因素分析、建立预后预测模型,以期为中青年脑梗死患者的早期干预和预后判断提供理论依据和临床指导。
1 资料和方法 1.1 研究对象连续纳入2018年1月1日至2020年1月1日在复旦大学附属中山医院吴淞医院神经内科住院治疗的中青年脑梗死患者。纳入标准:(1)符合《中国急性缺血性脑卒中诊治指南2014》[7]中脑梗死的诊断标准,并经头颅CT或MRI证实;(2)年龄为18~60岁,发病7 d内入院;(3)患者理解研究的目的及要求,能够提供书面知情同意书并同意回院随访。排除标准:(1)任何潜在的可导致心源性栓子的疾病,包括慢性或阵发性心房纤颤、二尖瓣狭窄、人工瓣膜、心内膜炎、心内血栓或赘生物、近3个月内心肌梗死、扩张型心肌病、左心房自发性回声对比、左心室射血分数低于30%;(2)脑卒中发病前1个月内行颅内或颅外动脉介入或外科手术;(3)伴有颅内出血、颅内肿瘤;(4)伴有严重肝脏和/或肾脏疾病、动脉炎、血液系统疾病、甲状腺疾病;(5)神经功能缺损严重,无法配合检查和完成随访;(6)任何可能致预期寿命短于1年的严重或威胁生命的合并症。本研究通过复旦大学附属中山医院吴淞医院伦理委员会审批,所有患者均签署知情同意书。
1.2 资料收集在患者入院后48 h内收集年龄、性别、既往史、吸烟史、饮酒史和基线血压。在入院24 h内,采用美国国立卫生研究院卒中量表(National Institutes of Health stroke scale,NIHSS)对患者进行神经功能缺损程度评分。入院次日清晨空腹抽取肘正中静脉血测定生物化学指标,包括血糖、总胆固醇、甘油三酯、高密度脂蛋白胆固醇、低密度脂蛋白胆固醇、尿素、血肌酐、总胆红素、丙氨酸转氨酶、同型半胱氨酸等。通过颈动脉超声检查评估动脉硬化斑块情况,头颅CT血管造影或磁共振血管造影检查评估血管狭窄情况。根据文献检索数据,确立RNF213(rs112735431)、HDAC9(rs2107595、rs2240419、rs2389995)、MTHFR(C677T)基因位点为本研究中脑梗死影响因素的分析内容。采用PCR进行基因检测,PCR扩增仪为杭州博日科技有限公司产品,试剂盒购自苏州天昊生物医药科技有限公司。
1.3 治疗与随访参照《中国急性缺血性脑卒中诊治指南2014》[7]和《中国急性缺血性脑卒中诊治指南2018》[8],所有患者在入院后均根据病情及适应证选择治疗方案,包括静脉溶栓、抗血小板聚集、降脂、抗凝及肢体康复训练等。同时明确相关危险因素,进行危险因素控制和二级预防管理。
由经过统一培训的神经内科医师对患者进行为期1年的随访,随访方式包括门诊复查、家访和电话随访。根据患者随访1年时的临床结局,将患者分为病情发展组和病情未发展组。病情未发展定义为满足以下3项:无脑卒中复发、无新发头颈动脉硬化斑块或狭窄程度无进展、改良Rankin量表(modified Rankin scale,mRS)评分<3分;病情发展定义为满足以下4项中的任意1项:随访期间出现脑卒中复发、有新发动脉硬化斑块或狭窄程度进展、mRS评分≥3分、死亡。
1.4 统计学处理应用SAS 9.4和R 3.6软件进行统计学分析。呈正态分布的计量资料以x±s表示,组间比较采用独立样本t检验;非正态分布的计量资料以中位数(下四分位数,上四分位数)表示,组间比较采用Mann-Whitney U检验;计数资料以例数和百分数表示,组间比较采用χ2检验或Fisher确切概率法。所有检验均采用双侧检验,检验水准(α)为0.05。
以人口学资料、实验室相关指标等基线资料及RNF213(rs112735431)、HDAC9(rs2107595、rs2240419、rs2389995)和MTHFR(C677T)基因多态性特征为自变量,随访1年时的临床结局为因变量,建立病情发展的预测模型。将数据集按7∶3分为训练集和测试集,利用训练集数据采用逐步回归法建立logistic回归预后模型;利用训练集和测试集数据采用ROC曲线对预后模型进行评价。
2 结果 2.1 基线数据分析2018年1月1日至2020年1月1日在复旦大学附属中山医院吴淞医院神经内科住院治疗的中青年脑梗死患者共372例,其中298例符合纳入和排除标准。298例患者中失访4例,最终294例患者的数据纳入分析。294例患者中男187例、女107例,年龄为36~60岁,平均(55.03±4.81)岁;病情未发展组177例(60.20%),病情发展组117例(39.80%)。病情发展组NIHSS评分及血肌酐、总胆红素、同型半胱氨酸水平均高于病情未发展组,差异均有统计学意义(P均<0.05);病情发展组与病情未发展组的MTHFR(C677T)基因多态性分布不同,差异有统计学意义(P<0.01)。见表 1。
|
|
表 1 294例中青年脑梗死患者的基线资料 Tab 1 Baseline data of 294 young and middle-aged patients with cerebral infarction |
2.2 预后预测模型建立
将数据样本按7∶3的比例划分为训练集和测试集,其中训练集206例,测试集88例。均衡性分析结果显示,训练集和测试集之间各变量的差异均无统计学意义(P均>0.05)。利用训练集数据建立预测模型。将患者分为病情发展组和病情未发展组进行差异性分析,结果(表 2)显示,病情发展组NIHSS评分、同型半胱氨酸水平高于病情未发展组,差异均有统计学意义(P均<0.05);病情发展组与病情未发展组的MTHFR(C677T)基因多态性分布不同,差异有统计学意义(P=0.001)。为避免重要变量的遗漏,将差异性分析中P<0.1的变量纳入多因素logistic回归模型,采用逐步回归法进行分析。结果(表 3)表明,NIHSS评分每增加1分,病情发展的风险增加76.8%;MTHFR(C677T)基因TT型患者病情发展的风险是CC型的4.128倍;患有高血压的患者病情发展的风险是未患高血压患者的3.421倍。训练集拟合优度检验提示该预后模型整体拟合良好(χ2=5.255,P=0.730),预测值与真实值之间平均绝对误差为0.031。
|
|
表 2 训练集206例中青年脑梗死患者的基线资料 Tab 2 Baseline data of 206 young and middle-aged patients with cerebral infarction in training set |
|
|
表 3 中青年脑梗死患者预后预测的多因素logistic回归模型 Tab 3 Multivariate logistic regression model of prognosis prediction in young and middle-aged patients with cerebral infarction |
2.3 预后预测模型评估
利用训练集和测试集数据考察预后预测模型对中青年脑梗死患者病情发展的预测效果,训练集ROC曲线的AUC值为0.856(95% CI 0.806~0.906),测试集ROC曲线的AUC值为0.847(95% CI 0.756~0.937),表明模型的预测能力良好。见图 1。
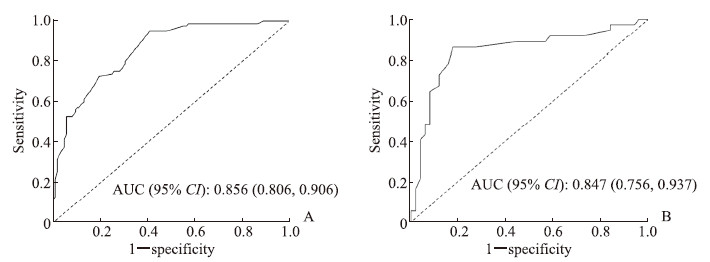
|
图 1 ROC曲线评价预后预测模型对中青年脑梗死患者病情发展的预测效果 Fig 1 ROC curve evaluating prognosis prediction model for progression of young and middle-aged patients with cerebral infarction A: Training set; B: Test set. ROC: Receiver operating characteristic; AUC: Area under curve; CI: Confidence interval. |
3 讨论
由于中青年人的预期寿命较长,脑卒中的高致残和致死率对社会和家庭造成了极大负担,因此,中青年脑梗死引起了国内外学者的广泛关注,成为目前研究的热点。本研究对包括基因在内的影响中青年脑梗死患者预后的多种危险因素进行了分析,并首次建立了预测中青年脑梗死患者病情发展的预测模型,以期早期识别预后不良的高危患者,从而给予针对性的干预措施,改善患者的预后。本研究根据随访1年的临床结局,将294例中青年脑梗死患者分为病情发展组与未发展组,病情发展组患者的NIHSS评分及血肌酐、总胆红素、同型半胱氨酸水平均高于病情未发展组且两组的MTHFR(C677T)基因多态性分布不同,差异均有统计学意义(P均<0.05)。说明NIHSS评分、血肌酐、总胆红素、同型半胱氨酸、MTHFR(C677T)基因多态性可能是中青年脑梗死患者病情发展的危险因素。
多项研究表明,若早期识别青年脑卒中患者的基因突变位点并早期进行有效干预,可以极大地降低青年脑卒中的发病率和死亡率,改善患者预后[9-10]。RNF213基因编码一种呈环指形态结构的承载蛋白,其功能类似于E3泛素连接酶。在斑马鱼中,沉默RNF213基因表达后颅内动脉可形成不规则的管壁形态并出现很多直径不规则的芽生小血管,提示RNF213基因可能在颅内血管生成中发挥重要作用[11]。英国、德国等欧洲国家开展的一项全基因组关联研究(3 548例脑卒中患者及5 972名对照者)确定了HDAC9(7p21.1)基因与大动脉相关脑卒中的关系[12],其后多项国际多中心全基因组关联研究均证明HDAC9基因多态性与大动脉粥样硬化性脑卒中相关[13-14]。HDAC9基因座上的rs2107595位点与欧洲人口的大动脉粥样硬化相关,但是在中国南方汉族人群中的一项涉及816例脑卒中患者及816名正常对照的研究发现,HDAC9(rs2107595)基因多态性可能并不是缺血性脑卒中的危险因素,推测可能与种族异质性有关[15]。一项在中国进行的涉及279例脑卒中患者和984名对照者的研究提示,HDAC9基因的rs11984041位点与大血管病风险无关,而rs2389995和rs2240419位点与大血管病风险明显相关[16]。但另一项在中国进行的相对较大样本的研究(2 317例冠心病患者和2 404名对照者)提示HDAC9基因的rs2107595位点与冠状动脉硬化相关[17]。本研究将中青年脑梗死患者分为病情发展组与未发展组,两组患者RNF213(rs112735431)、HDAC9(rs2107595、rs2240419、rs2389995)基因多态性分布差异均无统计学意义(P均>0.05)。不同研究中所得结果并不完全一致,考虑可能与研究人群、样本量和研究方法不同有关。
研究证实同型半胱氨酸与脑血管疾病的发生和发展密切相关,其代谢过程涉及多个关键酶,其中MTHFR是同型半胱氨酸再甲基化代谢途径的关键酶。Araji等[18]对35例青年脑卒中患者的基因突变情况进行研究,结果显示MTHFR基因的独立突变或与其他基因的联合突变都是青年脑卒中的重要危险因素。克罗地亚学者开展了一项包括155例55岁以下中青年脑梗死患者和150名对照者的基因学研究,发现两组在高血压、吸烟、降低的高密度脂蛋白、较高的低密度脂蛋白、MTHFR(C677T)基因多态性和基因多态性总数等方面差异均有统计学意义(P均<0.05)[19]。我国学者研究发现MTHFR(C677T)位点突变与脑梗死患者颈动脉狭窄及其程度相关[20]。本研究中病情发展组和病情未发展组中青年脑梗死患者的同型半胱氨酸和MTHFR(C677T)基因多态性分布差异均有统计学意义,说明其可能与中青年脑梗死患者的病情发展有关。
本研究将294例患者的数据样本按7∶3的比例划分为训练集和测试集,差异性分析表明,训练集病情发展组NIHSS评分、同型半胱氨酸水平高于病情未发展组,且两组MTHFR(C677T)基因多态性分布不同。将差异性分析中P<0.1的变量纳入多因素logistic回归模型,采用逐步回归法进行分析,结果显示NIHSS评分、MTHFR(C677T)基因TT型、患有高血压是中青年脑梗死患者病情发展的危险因素。利用ROC曲线评估该预后预测模型的效能,结果表明模型的预测能力良好。
本研究尚存在一些不足。首先,本研究为单中心研究,样本量较小,患者年龄范围较大(36~60岁)且缺少18~35岁人群,期待今后开展大规模多中心研究,进一步以45岁为界将研究人群进行年龄分层分析,使青年脑卒中的研究更具代表性。其次,中青年脑梗死的病因很多,本研究主要针对动脉粥样硬化的病因进行研究,且考虑到随访依从性和预后因素检测的敏感性,排除了神经功能缺损较严重的患者,今后的研究会增加不同病因和严重程度的样本数据并进行亚组分析,以深化研究结果的理论价值。另外,目前还没有针对中青年脑梗死患者的预后预测模型进行参考,该模型的准确性需要后续的研究加以验证。
综上所述,NIHSS评分、MTHFR(C677T)基因多态性、高血压是中青年脑梗死患者病情发展的危险因素,所建立的含有NIHSS评分、MTHFR(C677T)基因多态性、高血压3个自变量的预后预测模型有助于判断中青年脑梗死患者的预后,能为中青年脑梗死患者的预后判断和个体化干预提供临床依据。
| [1] |
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016:a systematic analysis for the global burden of disease study 2016[J]. Lancet, 2017, 390: 1211-1259. DOI:10.1016/S0140-6736(17)32154-2 |
| [2] |
FEIGIN V L, LAWES C M, BENNETT D A, BARKER-COLLO S L, PARAG V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review[J]. Lancet Neurol, 2009, 8: 355-369. DOI:10.1016/S1474-4422(09)70025-0 |
| [3] |
European Registers of Stroke (EROS) Investigators, HEUSCHMANN P U, DI CARLO A, BEJOT Y, RASTENYTE D, RYGLEWICZ D, et al. Incidence of stroke in Europe at the beginning of the 21st century[J]. Stroke, 2009, 40: 1557-1563. DOI:10.1161/STROKEAHA.108.535088 |
| [4] |
SVEINSSON O A, KJARTANSSON O, VALDIMARSSON E M. Cerebral ischemia/infarction-epidemiology, causes and symptoms[J]. Laeknabladid, 2014, 100: 271-279. |
| [5] |
TRAYLOR M, FARRALL M, HOLLIDAY E G, SUDLOW C, HOPEWELL J C, CHENG Y C, et al. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE collaboration): a meta-analysis of genome-wide association studies[J]. Lancet Neurol, 2012, 11: 951-962. DOI:10.1016/S1474-4422(12)70234-X |
| [6] |
毕齐, 张茁, 张微微, 贾妍娜. 2359例青年脑卒中患者危险因素研究[J]. 中华流行病学杂志, 2003, 24: 106-108. |
| [7] |
中华医学会神经病学分会, 中华医学会神经病学分会脑血管病学组. 中国急性缺血性脑卒中诊治指南2014[J]. 中华神经科杂志, 2015, 48: 246-257. |
| [8] |
中华医学会神经病学分会, 中华医学会神经病学分会脑血管病学组. 中国急性缺血性脑卒中诊治指南2018[J]. 中华神经科杂志, 2018, 51: 666-682. DOI:10.3760/cma.j.issn.1006-7876.2018.09.004 |
| [9] |
刘畅, 钱丹, 田野, 刘飞. 急性脑血管病患者MTHFR基因多态性分布及血清同型半胱氨酸、脂蛋白相关磷脂酶A2水平的临床研究[J]. 国际检验医学杂志, 2020, 41: 2962-2965, 2969. DOI:10.3969/j.issn.1673-4130.2020.24.005 |
| [10] |
王燕, 陈思, 袁慧, 曾小莉, 崔颖. 北京地区汉族人群溶质载体有机阴离子转运蛋白家族1B1与载脂蛋白E基因多态性分布及其意义[J]. 中国医药, 2020, 15: 1947-1951. |
| [11] |
LIU W, MORITO D, TAKASHIMA S, MINEHARU Y, KOBAYASHI H, HITOMI T, et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development[J/OL]. PLoS One, 2011, 6: e22542. DOI: 10.1371/journal.pone.0022542.
|
| [12] |
International Stroke Genetics Consortium (ISGC); Wellcome Trust Case Control Consortium 2(WTCCC2), BELLENGUEZ C, BEVAN S, GSCHWENDTNER A, SPENCER C C, BURGESS A I, et al. Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke[J]. Nat Genet, 2012, 44: 328-333. DOI:10.1038/ng.1081 |
| [13] |
CHAUHAN G, DEBETTE S. Genetic risk factors for ischemic and hemorrhagic stroke[J/OL]. Curr Cardiol Rep, 2016, 18: 124. DOI: 10.1007/s11886-016-0804-z.
|
| [14] |
MALIK R, TRAYLOR M, PULIT S L, BEVAN S, HOPEWELL J C, HOLLIDAY E G, et al. Low-frequency and common genetic variation in ischemic stroke: the METASTROKE collaboration[J]. Neurology, 2016, 86: 1217-1226. DOI:10.1212/WNL.0000000000002528 |
| [15] |
SU L, SHEN T T, LIANG B Y, XIE J J, TAN J J, CHEN Q, et al. Association of GWAS-supported loci rs2107595 in HDAC9 gene with ischemic stroke in southern Han Chinese[J]. Gene, 2015, 570: 282-287. |
| [16] |
HAN Y, SUN W Z, WANG L, TAO S, TIAN L, HAO Y, et al. HDAC9 gene is associated with stroke risk in a Chinese population[J]. Exp Biol Med (Maywood), 2013, 238: 842-847. DOI:10.1177/1535370213494650 |
| [17] |
WANG X B, HAN Y D, SABINA S, CUI N H, ZHANG S, LIU Z J, et al. HDAC9 variant rs2107595 modifies susceptibility to coronary artery disease and the severity of coronary atherosclerosis in a Chinese Han population[J/OL]. PLoS One, 2016, 11: e0160449. DOI: 10.1371/journal.pone.0160449.
|
| [18] |
ARAJI A A, SAWAYA H R, SAWAYA R A. Gene mutations and stroke in the young adult[J]. J Stroke Cerebrovasc Dis, 2014, 23: 2554-2558. |
| [19] |
SUPANC V, SONICKI Z, VUKASOVIC I, SOLTER V V, ZAVOREO I, KES V B. The role of classic risk factors and prothrombotic factor gene mutations in ischemic stroke risk development in young and middle-aged individuals[J/OL]. J Stroke Cerebrovasc Dis, 2014, 23: e171-e176. DOI: 10.1016/j.jstrokecerebrovasdis.2013.09.025.
|
| [20] |
刘斌, 田冉, 高端, 郭志义. 同型半胱氨酸代谢酶MTHFR、CBS、MS基因多态性与脑梗死患者颈动脉狭窄的相关性[J]. 中国现代医学杂志, 2011, 21: 4103-4108. |
 2021, Vol. 42
2021, Vol. 42


