2. 北部战区总医院第一派驻门诊部, 沈阳 110001
2. The First Outpatient Department, General Hospital of PLA Northern Theater Command, Shenyang 110001, Liaoning, China
前列腺癌(prostate cancer,PCa)严重影响我国老年男性的健康。传统影像学检查对于多发转移性PCa缺乏全局观,在PCa的风险分层、精确分期、低水平前列腺特异性抗原(prostate-specific antigen,PSA)生化复发患者诊断等方面仍存难点。近年来,正电子发射计算机断层显像(positron emission tomography-computed tomography,PET-CT)特异性显像剂68镓标记的前列腺特异性膜抗原(68Gallium-prostate-specific membrane antigen,68Ga-PSMA)开始应用于临床,其灵敏度和特异度较高,尤其在多发转移性PCa的显像和全身评估方面具有明显优势[1]。目前,有关初诊PCa患者68Ga-PSMA PET-CT代谢体积参数与年龄、BMI、PSA、格里森评分(Gleason score,GS)等临床指标相关性的报道较少,主要为欧美人群小样本、单中心的诊断效能研究。基于PET-CT的代谢体积参数是反映患者全身肿瘤负荷的重要定量指标。本研究通过收集初诊PCa患者68Ga-PSMA-11 PET-CT的代谢体积参数等影像资料和多项临床资料,分析代谢体积参数与临床指标的相关性,为PCa核素治疗的疗效监测和预后评估提供参考。
1 资料和方法 1.1 资料来源收集2019年1月至12月首次经海军军医大学(第二军医大学)长海医院前列腺活体组织穿刺病理学确诊或经外院穿刺病理学确诊并经长海医院病理科会诊,后于长海医院行68Ga-PSMA-11 PET-CT检查的PCa患者的临床资料。纳入标准:(1)临床资料完整,经前列腺活体组织穿刺病理学确诊并行68Ga-PSMA-11 PET-CT检查的PCa患者;(2)68Ga-PSMA-11 PET-CT检查前未接受任何临床治疗(包括手术、内分泌治疗、放疗和化疗等);(3)68Ga PSMA-11 PET-CT检查前2周内检测血清PSA(正常参考值范围0~4 ng/mL);(4)前列腺活体组织穿刺与68Ga-PSMA-11 PET-CT检查时间间隔不超过1个月。排除标准:(1)临床资料不完整者;(2)经过治疗的PCa患者;(3)同时罹患其他肿瘤的患者;(4)医学数字成像和通信标准(digital imaging and communications in medicine,DICOM)格式的图像上无法明确病灶性质的患者。根据纳入和排除标准,本研究共纳入85例初诊PCa患者。本研究经海军军医大学(第二军医大学)长海医院伦理委员会批准并在研究数据备案(research data deposit,RDD)平台备案(伦理批件编号:CHEC2019-0905;方案受理编号:2019-029),患者均知情同意并签署知情同意书。
1.2 68Ga-PSMA-11 PET-CT显像利用锗镓发生器(740 MBq 68Ge/68Ga发生器,德国ITG公司,放化纯度大于95%)自动化淋洗前列腺特异性膜抗原(prostate-specific membrance antigen,PSMA)-11前体(上海嘉标生物科技有限公司),合成68Ga-PSMA-11。患者注射示踪剂前静息15 min,按体重静脉给药(2.00~2.33 MBq/kg)后静息50 min,饮水及排空尿液后行PET-CT(Biograph 64型,德国Siemens公司)检查。扫描时患者双手置于身体两侧,范围从头部至股骨中段;先行体部CT显像,电流170 mA、电压120 kV、重建层厚3.0 mm;然后行体部PET显像,共6~7个床位,每床位约2.0~3.0 min,扫描时管电流依厚度及密度自动调节强度。数据衰减校正后行迭代重建,利用麦迪克斯工作站系统(北京麦迪克斯科技有限公司)进行图像自动对位融合显示,得到横断位、冠状位、矢状位断层图像及最大密度投影(maximum intensity projection,MIP)图像。
1.3 图像分析68Ga-PSMA-11 PET-CT图像由2位经验丰富的核医学高级职称医师双盲阅片分析。前列腺、唾液腺、泪腺、下颌下腺、肝脏、小肠、胰腺可见正常生理性摄取,胆囊、肾脏、输尿管、膀胱可见生理性浓聚。生理性摄取和浓聚灶以外的局部异常摄取灶则诊断为肿瘤病灶。前列腺内的肿瘤病灶为PCa原发灶,前列腺毗邻组织肿瘤病灶为直接侵犯灶,淋巴结和骨骼的肿瘤病灶为转移灶。采用三维勾画法在PET-CT融合图像上自动测量和勾画肿瘤病灶感兴趣区(region of interest,ROI),系统自动计算病灶最大标准摄取值(maximum standardized uptake value,SUVmax)、平均标准摄取值(mean standardized uptake value,SUVmean)、肝脏摄取本底SUVmax(liver background SUVmax,bSUVmax)等参数。以SUVmax的40%为界值[2-3],自动勾画ROI内SUVmax大于上述界值部分的体积,记为病灶PSMA体积(tumor volume of prostate-specific membrane antigen,PSMA-TV)。病灶半定量值SUVmean与其PSMA-TV的乘积记为病灶PSMA总量(total lesion of prostate-specific membrane antigen,TL-PSMA)。TL-PSMA兼顾了肿瘤体积及代谢双重因素,可反映该病灶的肿瘤负荷。基于患者全身视野内所有病灶TL-PSMA的加和定义为全身肿瘤总量,记为TL-PSMA全身,反映患者的全身肿瘤负荷。相应地,PCa原发灶及直接侵犯灶TL-PSMA和记为TL-PSMA原发,转移灶TL-PSMA记为TL-PSMA转移。TL-PSMA全身=TL-PSMA原发+TL-PSMA转移。
1.4 统计学处理应用SPSS 21.0软件进行统计学分析。符合正态分布的计量资料以x±s表示,不符合正态分布的计量资料以中位数(最小值~最大值)表示,计数资料以例数(百分数)表示。采用Spearman秩相关检验分别分析无转移组、转移组和全体患者bSUVmax、原发灶SUVmax、原发灶SUVmean及TL-PSMA原发、TL-PSMA转移、TL-PSMA全身等多个代谢体积参数与年龄、BMI、GS、PSA等临床指标的相关性。采用双侧检验,检验水准(α)为0.05。
2 结果 2.1 患者基本情况纳入研究的85例初诊PCa患者中无转移组46例、转移组39例,年龄49~88岁,平均年龄(69.1±7.7)岁。患者的临床特征及影像参数见表 1,典型患者的影像图见图 1。
|
|
表 1 初诊PCa患者临床特征和影像参数描述性统计 Tab 1 Descriptive statistics of clinical characteristics and imaging parameters of newly diagnosed PCa patients |
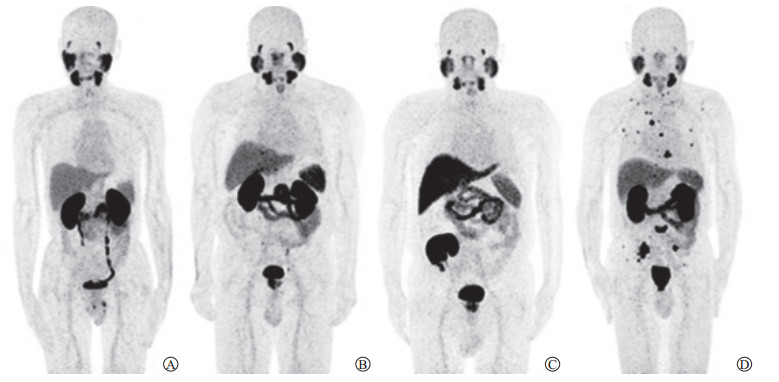
|
图 1 4例典型初诊PCa患者MIP图 Fig 1 MIP images of four typical newly diagnosed PCa patients A: 66-year-old, GS 6, PSA 7.42 ng/mL, primary tumor SUVmax 4.5, TL-PSMAwhole-body 3.2 mL; B: 65-year-old, GS 7, PSA 45.99 ng/mL, primary tumor SUVmax 15.2, TL-PSMAwhole-body 154.0 mL; C: 65-year-old, GS 7, PSA 62.00 ng/mL, primary tumor SUVmax 11.9, TL-PSMAwhole-body 361.5 mL, radionuclide concentrated in the transplanted kidney in right pelvic cavity but not in the atrophied kidneys; D: 65-year-old, GS 7, PSA 411.31 ng/mL, primary tumor SUVmax 30.8, TL-PSMAwhole-body 4 510.4 mL. MIP: Maximum intensity projection; PCa: Prostate cancer; GS: Gleason score; PSA: Prostate-specific antigen; SUVmax: Maximum standardized uptake value; TL-PSMA: Total lesion of prostate-specific membrane antigen. |
2.2 无转移组初诊PCa患者代谢体积参数与临床指标的相关性
无转移组初诊PCa患者TL-PSMA原发中位数为37.2(3.2~399.3)mL,Spearman秩相关分析结果(表 2)显示,PSA与原发灶SUVmax、TL-PSMA原发呈中度正相关(rs=0.409,P=0.005;rs=0.587,P<0.001);而GS与原发灶SUVmax、TL-PSMA原发不相关(rs=0.181,P=0.229;rs=0.101,P=0.505)。
|
|
表 2 无转移组46例初诊PCa患者代谢体积参数和临床指标Spearman秩相关分析结果 Tab 2 Results of Spearman correlation analysis between metabolic volume parameters and clinical indicators of 46 newly diagnosed PCa patients in non-metastasis group |
2.3 转移组初诊PCa患者代谢体积参数与临床指标的相关性
转移组初诊PCa患者TL-PSMA原发、TL-PSMA转移、TL-PSMA全身的中位数分别为318.4(5.1~3 822.0)、125.0(0.9~824.1)、628.0(14.7~4 510.4)mL,Spearman秩相关分析结果(表 3)显示,PSA与TL-PSMA原发、TL-PSMA转移、TL-PSMA全身呈中度正相关(rs=0.439,P=0.005;rs=0.588,P<0.001;rs=0.569,P<0.001),与原发灶SUVmax不相关(rs=0.255,P=0.117);而GS与原发灶SUVmax、TL-PSMA原发、TL-PSMA转移、TL-PSMA全身均不相关(rs=0.069,P=0.675;rs=0.194,P=0.237;rs=0.299,P=0.064;rs=0.300,P=0.063)。
|
|
表 3 转移组39例初诊PCa患者代谢体积参数与临床指标Spearman秩相关分析结果 Tab 3 Results of Spearman correlation analysis between metabolic volume parameters and clinical indicators of 39 newly diagnosed patients in metastasis group |
2.4 全体85例患者代谢体积参数与临床指标的相关性
Spearman秩相关性分析结果(表 4)显示,PSA与原发灶SUVmax、TL-PSMA原发、TL-PSMA转移、TL-PSMA全身呈中度正相关(rs=0.418,P<0.001;rs=0.629,P<0.001;rs=0.676,P<0.001;rs=0.763,P<0.001),而与GS不相关(rs=0.204,P=0.061);GS与TL-PSMA原发、TL-PSMA转移、TL-PSMA全身的呈弱正相关(rs=0.234,P=0.031;rs=0.277,P=0.010;rs=0.311,P=0.004),而与SUVmax不相关(rs=0.205,P=0.059);TL-PSMA原发与TL-PSMA转移呈中度正相关(rs=0.472,P<0.001)。
|
|
表 4 全体85例初诊PCa患者代谢体积参数与临床指标Spearman秩相关分析结果 Tab 4 Results of Spearman correlation analysis between metabolic volume parameters and clinical indicators of total 85 newly diagnosed patients |
3 讨论
由于丧失了根治性手术的时机,多发转移性PCa患者的治疗以化疗、内分泌治疗、体内外照射治疗等系统性治疗为主。瘤灶形态及活性组织分布不规则,因此常规影像学测得的肿瘤体积难以精准地反映肿瘤活性成分的多寡。精准评估全身肿瘤负荷对临床治疗及预后监测有指导意义,但难度较大。
PSMA在PCa细胞中高表达,尤其在激素难治性PCa和转移性组织中强烈表达,成为PCa诊断和治疗的重要靶点[4]。靶向探针68Ga-PSMA-11可以从分子水平反映PCa细胞中PSMA的表达。一项meta分析结果表明,68Ga-PSMA-11 PET-CT检测初诊PCa患者的灵敏度和特异度分别为0.74(95% CI 0.51~0.89)、0.96(95% CI 0.85~0.99),检测生化复发PCa患者的阳性预测值为0.99(95% CI 0.96~1.00),PSA≤2.0 ng/mL及>2.0 ng/mL时检出率分别为0.63(95% CI 0.55~0.70)、0.94(95% CI 0.91~0.96)[5]。研究表明,68Ga-PSMA PET-CT与多参数MRI相比在PCa原发病灶检出率及代谢体积评估方面更具优势[6]。68Ga-PSMA-11 PET-CT诊断效能优异,已成为评估PCa的重要手段[7]。
初诊PCa患者68Ga-PSMA-11 PET-CT摄取参数与PSA、GS等临床指标的相关性研究较少,且多为诊断效能研究的附加研究。Onal等[8]回顾201例PCa患者的68Ga-PSMA-11 PET-CT图像发现,在PSA>10 ng/mL亚组、GS>7分亚组、D’Amico高危亚组及盆腔淋巴结转移亚组的原发瘤灶摄取明显高于相对应的其他亚组;PSA>10 ng/mL亚组及GS>7分亚组中PSMA PET-CT的检出率明显高于相对应的其他亚组;原发灶和转移淋巴结的SUVmax中位数分别为13.2和11.4;PSA与原发灶SUVmax呈中度正相关(rs= 0.425,P<0.001)。本研究中,85例患者PSA与原发灶SUVmax亦呈中度正相关(rs=0.418,P<0.001),与上述研究结果一致。Ergül等[9]回顾78例新诊断PCa患者的68Ga-PSMA-11 PET-CT图像发现,GS 6~7分和8~10分亚组间、无转移亚组和转移亚组间、不同PSA水平(<10 ng/mL、10~<20 ng/mL、≥20 ng/mL)的3个亚组间原发灶SUVmax差异均有统计学意义。Uprimny等[10]探讨了90例PCa患者的68Ga-PSMA-11摄取参数与GS、PSA的关系,结果发现GS为6、7a(3+4)、7b(4+3)、8~10的4个亚组间原发灶SUVmax差异有统计学意义(中位数分别为5.9、8.3、8.2、21.2),PSA≥10.0 ng/mL与PSA<10.0 ng/mL亚组间原发灶SUVmax差异亦有统计学意义(中位数分别为17.6、7.7)。Sachpekidis等[11]的研究也得到类似结果。
近年来,若干代谢体积参数的研究表明,血清PSA水平与TL-PSMA全身显著相关[12]。由于骨转移灶较软组织转移灶负荷的界定相对容易,Bieth等[13]及Hammes等[14]尝试利用68Ga-PSMA PET-CT定量评价骨转移负荷,但骨转移瘤负荷并不能完全反映全身负荷水平。Schmuck等[15]对101例根治术后生化复发PCa患者的研究发现,PSA水平与全身病灶PSMA-TV(r=0.480,P<0.000 1)和TL-PSMA全身(r=0.520,P<0.000 1)呈正相关,而与原发灶SUVmax和SUVmean不相关;全身病灶PSMA-TV和TL-PSMA全身较原发灶SUVmax能更好地评估根治性前列腺切除术后复发及转移患者的全身肿瘤负荷。本研究全体85例患者的相关分析显示PSA与TL-PSMA全身的相关性为rs=0.763(P<0.001),强于Schmuck等[15]的报道(r=0.520,P<0.000 1),这可能受种族差异、阈值选择、患者纳入与排除标准等多种因素影响。Acar等[16]对19例接受177Lu-PSMA-I & T治疗的PCa患者的研究发现,治疗后PSMA-TV、TL-PSMA、SUVmax最高病灶的摄取程度随着PSA水平的下降而降低,PSMA-TV和TL-PSMA降低患者的生存率较高(P<0.001)。Michalski等[17]的研究也支持上述结论。Gadot等[18]发现,在52例接受177Lu-PSMA- 617靶向治疗的患者中,28例(54%)患者的PSA水平降低,其中26例(50%)下降超过20%,18例(35%)下降超过50%,治疗后PSA水平的下降程度与存活率显著相关。上述研究表明,PSA水平随PSMA-TV和TL-PSMA的降低而下降,三者呈显著相关,PSMA-TV和TL-PSMA有助于评价治疗反应和预测生存率。虽然上述研究人群与本研究不同,但仍有一定借鉴意义。
本研究采用分层分析,发现无转移组GS与TL-PSMA全身不相关(rs=0.101,P=0.505),转移组GS与TL-PSMA全身亦不相关(rs=0.300,P=0.063),但转移组相关系数高于无转移组。可能的原因是:转移未发生时,无论GS的高低,前列腺床内原发灶TL-PSMA都因受到前列腺包膜的限制而处于较低水平,因而两者无明显相关性;随着原发灶癌细胞不断去分化和GS的增加,原发灶癌细胞最终突破前列腺包膜形成转移灶,但TL-PSMA与GS的增加并不平行,因而造成虽然相关系数较无转移组增高,但依然未达到有统计学意义的相关性。
本研究用临床统计学的方法证实反映肿瘤负荷的代谢体积参数与多个临床指标的相关性,结果显示血清PSA与TL-PSMA全身呈正相关;部分参数间相关性的特征与其他中心的研究[19-20]基本一致,符合临床经验和逻辑预期[21]。本研究将85例患者分别纳入转移组和无转移组进行相关性的分层分析,尽可能减少TL-PSMA全身构成比不同而对相关系数的干扰,对全体患者分析的相关系数仅做参考。本研究的优势还在于入组的都是自然到访且没有经过任何治疗的初诊患者。对PCa患者进行筛选可能会隐藏患者部分临床特征,内分泌治疗也会改变肿瘤细胞PSMA的表达和血清PSA水平[22],从而影响SUVmax阈值和代谢体积参数的原始特征,因此初诊患者代谢体积参数与临床指标相关系数更具说服力。值得注意的是,本研究中bSUVmax与原发灶SUVmax的Spearman相关系数为负值(无转移组rs=-0.070,P=0.646;转移组rs=-0.080,P=0.629;全体患者rs=-0.025,P=0.819),虽然未达到有统计学意义的负相关,但笔者认为可能是生理性肝脏摄取和病理性肿瘤摄取之间的竞争关系导致负相关系数的出现。
目前尚无研究明确活体组织穿刺对PSMA的表达是否存在干扰,本研究入组的患者均为穿刺确诊后再行68Ga-PSMA-11 PET-CT检查,以尽可能减少这种潜在干扰的个体间差异。本研究为单中心、小样本的回顾性研究。由于入组标准严苛,导致患者数量较少,增加入组患者数量可能会得到更加准确、稳定的相关系数。本研究的GS均由前列腺活体组织穿刺所得,增加患者手术病理资料可能会进一步丰富本研究的结果。本研究在参考多项研究后,采用SUVmax的40%[2-3]作为自动测量全身病灶PSMA-TV的阈值,且全身病灶PSMA-TV和TL-PSMA的自动测量和计算均由同一位医师完成,因此在进行相关性分析时,测量误差为系统性误差,即对各参数的影响呈系统一致性。对阈值的选择尚无统一标准,一部分研究选择各病灶SUVmax的40%左右阈值,亦有研究采用固定SUV作为阈值[16]。限于篇幅,本研究尚未对其他阈值进行探索,这是本研究的不足之一。人工神经网络可能在探索全身肿瘤负荷自动量化方法上更具优势。
总之,本研究结果表明反映初诊PCa患者全身肿瘤负荷的代谢体积参数TL-PSMA与血清PSA呈正相关,而与反映原发灶分化程度的GS不相关。
| [1] |
EISSA A, ELSHERBINY A, COELHO R F, RASSWEILER J, DAVIS J W, PORPIGLIA F, et al. The role of 68Ga-PSMA PET/CT scan in biochemical recurrence after primary treatment for prostate cancer: a systematic review of the literature[J]. Minerva Urol Nefrol, 2018, 70: 462-478. |
| [2] |
JIANG C, TENG Y, CHEN J Y, WANG Z, ZHOU Z, DING C, et al. Baseline total metabolic tumor volume combined with international peripheral T-cell lymphoma project may improve prognostic stratification for patients with peripheral T-cell lymphoma (PTCL)[J/OL]. EJNMMI Res, 2020, 10: 110. DOI: 10.1186/s13550-020-00698-y.
|
| [3] |
DUNET V, HALKIC N, SEMPOUX C, DEMARTINES N, MONTEMURRO M, PRIOR J O, et al. Prediction of tumour grade and survival outcome using pre-treatment PET- and MRI-derived imaging features in patients with resectable pancreatic ductal adenocarcinoma[J/OL]. Eur Radiol, 2020. DOI: 10.1007/s00330-020-07191-z.
|
| [4] |
PASTORINO S, RIONDATO M, UCCELLI L, GIOVACCHINI G, GIOVANNINI E, DUCE V, et al. Toward the discovery and development of PSMA targeted inhibitors for nuclear medicine applications[J]. Curr Radiopharm, 2020, 13: 63-67. DOI:10.2174/1874471012666190729151540 |
| [5] |
HOPE T A, GOODMAN J Z, ALLEN I E, CALAIS J, FENDLER W P, CARROLL P R. Meta analysis of 68Ga-PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology[J]. J Nucl Med, 2019, 60: 786-793. DOI:10.2967/jnumed.118.219501 |
| [6] |
SPOHN S, JAEGLE C, FASSBENDER T F, SPRAVE T, GKIKA E, NICOLAY N H, et al. Intraindividual comparison between 68Ga-PSMA-PET/CT and mpMRI for intraprostatic tumor delineation in patients with primary prostate cancer: a retrospective analysis in 101 patients[J]. Eur J Nucl Med Mol Imaging, 2020, 47: 2796-2803. DOI:10.1007/s00259-020-04827-6 |
| [7] |
WEBER M, KUREK C E, BARBATO F, EIBER M, MAURER T, NADER M, et al. PSMA-ligand PET for early castration-resistant prostate cancer: a retrospective single-center study[J/OL]. J Nucl Med, 2020. DOI: 10.2967/jnumed.120.245456.
|
| [8] |
ONAL C, TORUN N, OYMAK E, GULER O C, REYHAN M, YAPAR A F. Retrospective correlation of 68GA-PSMA uptake with clinical parameters in prostate cancer patients undergoing definitive radiotherapy[J]. Ann Nucl Med, 2020, 34: 388-396. DOI:10.1007/s12149-020-01462-x |
| [9] |
ERGÜL N, YILMAZ GÜNEŞ B, YÜCETAŞ U, TOKTAŞ M G, ÇERMIK T F. 68Ga-PSMA-11 PET/CT in newly diagnosed prostate adenocarcinoma[J/OL]. Clin Nucl Med, 2018, 43: e422-e427. DOI: 10.1097/RLU.0000000000002289.
|
| [10] |
UPRIMNY C, KROISS A S, DECRISTOFORO C, FRITZ J, VON GUGGENBERG E, KENDLER D, et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour[J]. Eur J Nucl Med Mol Imaging, 2017, 44: 941-949. DOI:10.1007/s00259-017-3631-6 |
| [11] |
SACHPEKIDIS C, KOPKA K, EDER M, HADASCHIK B A, FREITAG M T, PAN L, et al. 68Ga-PSMA-11 dynamic PET/CT imaging in primary prostate cancer[J/OL]. Clin Nucl Med, 2016, 41: e473-e479. DOI: 10.1097/RLU.0000000000001349.
|
| [12] |
YILMAZ U, KOMEK H, CAN C, ALTINDAG S. The role of (68Ga)PSMA I & T in biochemical recurrence after radical prostatectomy: detection rate and the correlation between the level of PSA, Gleason score, and the SUVmax[J]. Ann Nucl Med, 2019, 33: 545-553. DOI:10.1007/s12149-019-01360-x |
| [13] |
BIETH M, KRÖNKE M, TAUBER R, DAHLBENDER M, RETZ M, NEKOLLA S G, et al. Exploring new multimodal quantitative imaging indices for the assessment of osseous tumor burden in prostate cancer using 68Ga-PSMA PET/CT[J]. J Nucl Med, 2017, 58: 1632-1637. DOI:10.2967/jnumed.116.189050 |
| [14] |
HAMMES J, TÄGER P, DRZEZGA A. EBONI: a tool for automated quantification of bone metastasis load in PSMA PET/CT[J]. J Nucl Med, 2018, 59: 1070-1075. DOI:10.2967/jnumed.117.203265 |
| [15] |
SCHMUCK S, VON KLOT C A, HENKENBERENS C, SOHNS J M, CHRISTIANSEN H, WESTER H J, et al. Initial experience with volumetric 68Ga-PSMA I & T PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with prostate cancer[J]. J Nucl Med, 2017, 58: 1962-1968. DOI:10.2967/jnumed.117.193581 |
| [16] |
ACAR E, ÖZDOĞAN Ö, AKSU A, DEREBEK E, BEKIŞ R, ÇAPA KAYA G, et al. The use of molecular volumetric parameters for the evaluation of Lu-177 PSMA I & T therapy response and survival[J]. Ann Nucl Med, 2019, 33: 681-688. DOI:10.1007/s12149-019-01376-3 |
| [17] |
MICHALSKI K, MIX M, MEYER P T, RUF J. Determination of whole-body tumour burden on 68Ga PSMA-11 PET/CT for response assessment of 177Lu PSMA-617 radioligand therapy: a retrospective analysis of serum PSA level and imaging derived parameters before and after two cycles of therapy[J]. Nuklearmedizin, 2019, 58: 443-450. DOI:10.1055/a-1035-9052 |
| [18] |
GADOT M, DAVIDSON T, AHARON M, ATENAFU E G, MALKI A, LEVARTOVSKY M, et al. Clinical variables associated with PSA response to lutetium-177-PSMA (177Lu-PSMA-617) radionuclide treatment in men with metastatic castration-resistant prostate cancer[J/OL]. Cancers (Basel), 2020, 12: 1078. DOI: 10.3390/cancers12051078.
|
| [19] |
SCHMUCK S, VON KLOT C A, HENKENBERENS C, SOHNS J M, CHRISTIANSEN H, WESTER H J, et al. Initial experience with volumetric 68Ga-PSMA I & T PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with prostate cancer[J]. J Nucl Med, 2017, 58: 1962-1968. DOI:10.2967/jnumed.117.193581 |
| [20] |
KOERBER S A, UTZINGER M T, KRATOCHWIL C, KESCH C, HAEFNER M F, KATAYAMA S, et al. 68Ga-PSMA-11 PET/CT in newly diagnosed carcinoma of the prostate: correlation of intraprostatic PSMA uptake with several clinical parameters[J]. J Nucl Med, 2017, 58: 1943-1948. DOI:10.2967/jnumed.117.190314 |
| [21] |
RAMÍREZ-BACKHAUS M, MIR MARESMA M C, MASCARÓS J M, BERTOLO R, HERNÁNDEZ J, GÓMEZ FERRER A, et al.[Undetectable PSA after radical prostatectomy is more likely in low burden N+ prostate cancer patients when an extended lymph node dissection is performed][J]. Actas Urol Esp, 2019, 43: 480-487.
|
| [22] |
BATRA J S, PIENTA K J, POMPER M G, GORIN M A, ROWE S P. Can the interplay between androgen signaling and PSMA expression be leveraged for theranostic applications?[J]. Transl Androl Urol, 2019, 8(Suppl 3): S263-S264. |
 2021, Vol. 42
2021, Vol. 42


