2. 海军军医大学(第二军医大学)长征医院消化内科, 上海 200003;
3. 海军军医大学(第二军医大学)长海医院病理科, 上海 200433
2. Department of Gastroenterology, Changzheng Hospital, Naval Medical University(Second Military Medical University), Shanghai 200003, China;
3. Department of Pathology, Changhai Hospital, Naval Medical University(Second Military Medical University), Shanghai 200433, China
Xp11.2易位/转录因子E3(transcription factor E3,TFE3)基因融合相关性肾细胞癌(renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion,Xp11.2/TFE3 RCC)是一种临床罕见的肾脏恶性肿瘤,其发生源于Xp11.2染色体位点上TFE3基因断裂,并与富含脯氨酸的有丝分裂检查点控制因子(proline-rich mitotic checkpoint control factor,PRCC)、富含脯氨酸和谷氨酰胺的剪接因子(splicing factor proline- and glutamine-rich,PSF)、网格蛋白重链(clathrin heavy chain,CLTC)、含非POU结构域的八聚体结合基因(non-POU-domain-containing octamer binding,NonO)等相关基因发生平衡易位形成新的TFE3 融合基因,导致融合型TFE3蛋白的过度表达[1]。2004年,WHO肾脏肿瘤组织将Xp11.2/TFE3 RCC病理学分类列为RCC的一个独立亚型[2]。既往研究指出,Xp11.2/TFE3 RCC主要累及儿童和青少年,约占儿童和青少年肾癌的33.3%[3];在成年人中发病率较低,占成人肾癌的0.5%~1.5%[4]。但近年来不断有成人Xp11.2/TFE3 RCC病例报道。由于该病临床症状及影像学表现缺乏特异性,确诊较为困难,对于此类型肾癌的研究和认识明显落后于其他亚型肾癌。本研究对2014年9月至2019年7月海军军医大学(第二军医大学)长海医院收治的43例成人Xp11.2/TFE3 RCC患者的临床资料进行总结、分析,以期提高对该病的临床诊治水平。
1 资料和方法 1.1 临床资料来源收集2014年9月至2019年7月海军军医大学(第二军医大学)长海医院收治的经成人Xp11.2/TFE3 RCC患者的临床资料,共43例。纳入标准:(1)发病年龄≥18岁;(2)经术后病理确诊。对患者的一般情况、临床症状、影像学资料、治疗方法、病理资料、预后等进行录入、分析和总结。
1.2 影像学检查及图像分析43例患者中,40例行CT平扫+增强检查,1例行MRI平扫检查,8例行MRI平扫+增强检查;6例同时行CT和MRI检查。由2名影像科主治医师分别记录病灶位置、大小、形态、边缘、均匀度、内部特征、强化程度与特点、侵犯或转移情况。病灶形态主要分为类圆形和不规则形;肿瘤均匀度需记录病灶内部囊变或坏死、出血、钙化情况;内部特征包括CT密度和MRI信号高低;强化程度分为明显强化、中度强化和轻度强化,强化特点分为乳头状强化、非乳头状强化;局部侵犯或转移包括肾周侵犯、淋巴结转移、静脉癌栓或远处脏器转移等。
1.3 手术治疗方法20例患者接受根治性肾切除术(15例行腹腔镜根治性肾切除术,5例行开放肾切除术),21例患者接受肾部分切除术(13例行腹腔镜肾部分切除术,7例行智能臂辅助腹腔镜肾部分切除术,1例行开放肾局部切除术),其中2例患者因出现肾静脉、下腔静脉癌栓同时行静脉癌栓取出术。2例患者未行肾脏手术,其中1例行肾穿刺活检术、1例行转移性椎管肿瘤清除术。
1.4 组织病理H-E染色、免疫组织化学染色及荧光原位杂交(fluorescence in situ hybridization,FISH)检测43例患者术后标本行常规组织病理H-E染色及免疫组织化学染色,其中3例同时行FISH检测。免疫组织化学染色检测指标包括TFE3、碳酸酐酶Ⅸ(carbonic anhydrase Ⅸ,CAⅨ)、Ki-67、转化相关蛋白53(transformation-related protein 53,P53)、亲和素-生物素-过氧化物酶复合物(avidin-biotin-peroxidase complex,ABC)、CD10、配对盒基因8(paired box gene 8,Pax-8)、α-甲酰基辅酶A消旋酶(α-methylacyl-coenzyme A racemase,P504s)、希佩尔林道蛋白(von Hippel-Lindau,VHL)、CD147、低分子量细胞角蛋白(low-molecular-weight cytokeratin,CAM5.2)、钙黏蛋白6。FISH检测使用TFE3基因断裂重组检测试剂盒(美国Empire Genomics公司)。
1.5 术后随访术后随访截至2020年5月,随访内容包括患者存活情况、肿瘤复发和转移、靶向药物使用情况等。
1.6 统计学处理应用SPSS 18.0软件对数据进行统计分析。计量资料以x±s表示,组间比较采用独立样本t检验。计数资料以例数和百分数表示。检验水准(α)为0.05。
2 结果 2.1 患者基本情况本组43例患者中男24例、女19例,年龄25~77岁,平均年龄为(47.5±15.2)岁。男性患者平均年龄为(48.0±14.7)岁,女性为(46.9±16.2)岁,两组患者年龄差异无统计学意义(P>0.05)。首发症状为肉眼血尿5例,腰腹部不适6例,转移部位症状(骨痛)1例;健康体检时发现、无明显症状者31例。有症状患者平均年龄为(49.9±16.4)岁,无症状患者平均年龄为(46.6±14.9)岁,两组患者年龄差异无统计学意义(P>0.05)。术后病理TNM分期:T1N0M0期1例,T2N0M0期22例,T3N0M0期11例,T3N2M0期2例,T4N2M0期1例,T4N0M1期1例,T4N2M1期4例,T4N3M1期1例。
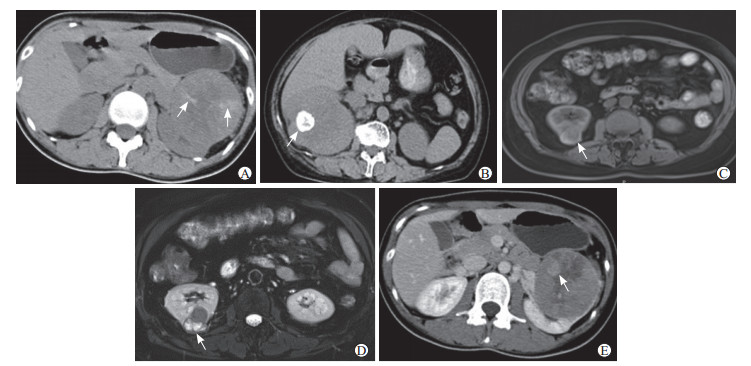
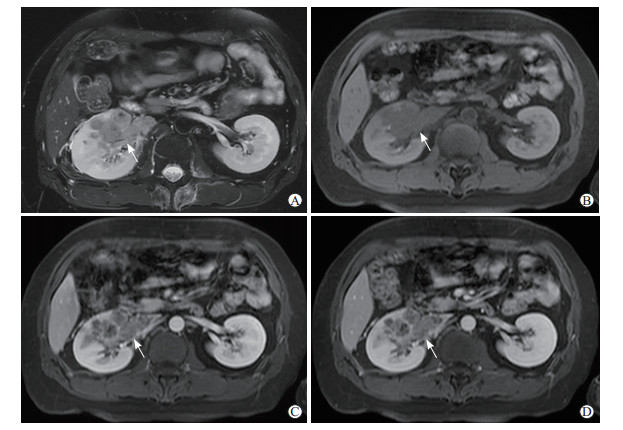
2.2 影像学表现病灶位于右肾28例,其中右肾上极8例、右肾中部5例、右肾下极14例、累及右肾全肾1例;左肾15例,其中左肾上极7例、左肾中部2例、左肾下极6例。病灶最大径1.1~9.5 cm,平均为(5.1±2.7)cm。类圆形29例,不规则形14例。边界清楚、有假包膜37例,边界不清晰6例。密度或信号不均29例,其中出血21例(图 1A)、囊变或坏死24例、钙化11例(图 1B)。CT平扫中,高或稍高密度20例、等密度2例、低密度5例、高低混杂密度13例。MRI平扫中,T1加权像稍高或等高信号7例、等低信号1例、高低混杂信号1例(图 1C);T2加权像等高信号1例、高低混杂信号5例(图 1D)、稍低信号3例。CT和MRI增强扫描中,明显强化22例、中度强化8例、轻度强化13例;乳头状强化23例(图 1E)、非乳头状强化20例。出现肾周侵犯10例(图 2A、2B);远处转移8例,包括腹膜后淋巴结转移2例、腹腔转移1例、肺转移2例、肾静脉及下腔静脉转移2例(图 2C、2D)、骨转移1例。影像学报告误诊4例,分别为畸胎瘤1例,嫌色细胞癌1例,乏脂肪血管平滑肌脂肪瘤1例,骨髓瘤浸润1例。
|
图 1 成人Xp11.2/TFE3 RCC患者影像学表现 Fig 1 Image findings of adult Xp11.2/TFE3 RCC patients A: Female, 28 years old, Xp11.2/TFE3 RCC in the left upper kidney pole. Plain CT showed flocculent hemorrhagic foci (arrows). B: Female, 70 years old, Xp11.2/TFE3 RCC in right kidney. Plain CT showed quasi-circular calcification (arrow). C: Female, 57 years old, Xp11.2/TFE3 RCC in the right lower kidney pole. MR T1-weighted imaging showed mixed signal of the tumor (arrow). D: Female, 57 years old, Xp11.2/TFE3 RCC in the right lower kidney pole. MR T2-weighted imaging showed mixed signal of the tumor (arrow). E: Female, 28 years old, Xp11.2/TFE3 RCC in the left upper kidney pole. Enhanced CT showed papillary enhancement nodule of the tumor (arrow). Xp11.2/TFE3 RCC: Renal cell carcinoma associated with Xp11.2 translocation/transcription factor E3 gene fusion; CT: Computed tomography; MR: Magnetic resonance. |
|
图 2 1例成人Xp11.2/TFE3 RCC患者伴随肾外影像学表现 Fig 2 Extrarenal image findings of an adult Xp11.2/TFE3 RCC patient Male, 35 years old, Xp11.2/TFE3 RCC in the right upper kidney pole. A: MR T2-weighted imaging showed inhomogeneous low signal of the tumor with perirenal invasion (arrow); B: MR T1-weighted imaging showed low signal of the tumor with perirenal invasion (arrow); C, D: Cortex medullary junction phase (C) and parenchymal phase (D) enhanced MR T1-weighted imaging showed heterogeneous enhancement of the tumor with tumor thrombus in renal vein and inferior vena cava (arrows). Xp11.2/TFE3 RCC: Renal cell carcinoma associated with Xp11.2 translocation/transcription factor E3 gene fusion; MR: Magnetic resonance. |
2.3 病理表现 2.3.1 大体标本
大体标本表现为实性或囊实性,切片多呈灰色、淡黄色或暗红色,可伴有囊变、坏死、出血、钙化等。
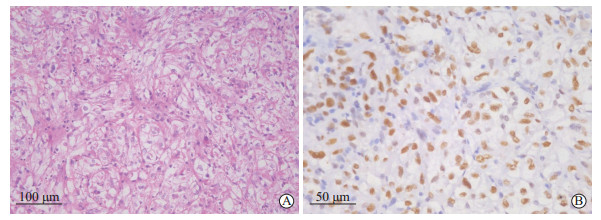
2.3.2 H-E染色结果切片经H-E染色后,显微镜下观察可见肿瘤组织主要表现为乳头状、巢状或腺泡状结构混合生长。肿瘤细胞呈圆形或椭圆形,细胞核大、深染、异型明显,胞质透亮,部分嗜酸性染色丰富(图 3A),部分病例可伴灶状出血、坏死。
|
图 3 1例成人Xp11.2/TFE3 RCC患者肿瘤组织H-E染色和免疫组织化学染色结果 Fig 3 H-E and immunohistochemistry staining results of the tumor tissues of an adult Xp11.2/TFE3 RCC patient Female, 60 years old. A: H-E staining showed the tumor tissues were arranged in irregular glandular and lenticular structures. The tumor cells were round and oval, with large nuclei, visible nucleoli and red cytoplasm. B: Immunohistochemistry staining showed strongly positive result of TFE3. Xp11.2/TFE3 RCC: Renal cell carcinoma associated with Xp11.2 translocation/transcription factor E3 gene fusion; H-E: Hematoxylin-eosin. |
2.3.3 免疫组织化学染色及FISH检测结果
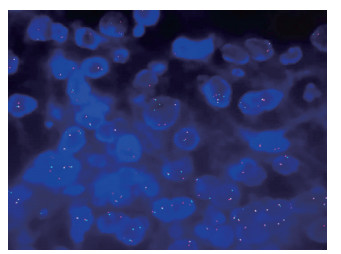
免疫组织化学染色检测中TFE3表达阳性是诊断Xp11.2/TFE3 RCC的重要基础,本组43例患者TFE3免疫组织化学染色均为阳性表达(图 3B)。其他免疫组织化学染色标志物阳性表达情况分别为:CAⅨ(74.4%,32/43)、P53(7.0%,3/43)、ABC(93.0%,40/43)、CD10(93.0%,40/43)、Pax-8(11.6%,5/43)、P504s(9.3%,4/43)、VHL(72.1%,31/43)、CD147(41.9%,18/43)、CAM5.2(83.7%,36/43)、钙黏蛋白6(41.9%,18/43)。Ki-67阳性率为1%~60%,平均为9.7%。3例患者进行TFE3基因断裂重组FISH检测,均为阳性(图 4),发生细胞分离比例分别为63.0%(63/100)、17.0%(17/100)、15.0%(15/100)。
|
图 4 1例成人Xp11.2/TFE3 RCC患者FISH检测结果(1 000×) Fig 4 FISH result of an adult Xp11.2/TFE3 RCC patient (1 000×) Male, 41 years old, FISH result showed 63 cells isolated out of 100 cells indicating a positive result of the detection for TFE3 gene disruption and recombination. Xp11.2/TFE3 RCC: Renal cell carcinoma associated with Xp11.2 translocation/transcription factor E3 gene fusion; FISH: Fluorescence in situ hybridization. |
2.4 术后随访
术后对患者存活情况、肿瘤复发和转移、靶向药物使用情况进行随访。除6例失访外,其余37例患者随访11~68个月,平均(47.4±17.5)个月。1例患者术后出现肿瘤复发,5例患者术后发生全身多发转移。5例术后全身多发转移患者中,3例患者于术后23、31、54个月死亡;另2例患者使用靶向药物治疗,分别随访50个月和57个月,病情无明显进展,患者仍存活。
3 讨论Xp11.2/TFE3 RCC是一种特殊病理学分类的肾细胞癌独立亚型,该类型肾癌的发病率、疾病进展和预后在儿童和成人中均相差很大[5]。目前普遍认为Xp11.2/TFE3 RCC好发于儿童及青少年,在成人中发病率整体较低,女性发病率高于男性[3-4, 6-7]。但不同文献报道的发病率差异较大,可能与诊断策略及病例选择有关。亦有学者认为成人Xp11.2/TFE3 RCC发病率被严重低估[8]。本研究回顾分析了43例成人Xp11.2/TFE3 RCC患者资料,男性患者多于女性(24例vs 19例),与既往研究[6]不同。但成人不同性别发病率的差异需要大样本研究才能准确判定。
Xp11.2/TFE3 RCC病因仍不明确,部分儿童及青少年患者有化疗史,因此化疗可能是其危险因素[9]。成人Xp11.2/TFE3 RCC患者在幼年时可能已发生基因突变,直到成年后因疾病进展出现相关症状才被发现[10]。总体上,Xp11.2/TFE3 RCC与常见肾癌相比预后差。但在儿童及青少年患者中Xp11.2/TFE3 RCC大多表现为惰性,进展较为缓慢,经治疗后预后较好[11]。在成人患者中,其常表现为侵袭性强、疾病进展快、预后差的特点[12]。
Xp11.2/TFE3 RCC临床表现与其他类型肾癌相比无明显特异性,但较少出现典型的“血尿、腰痛、腹部肿块”肾癌三联征,有较大比例患者发现时无明显症状,同时也存在患者因出现转移部位症状就诊[13]。本组43例患者中,11例主要临床表现为单独发生的肉眼血尿或腰腹部不适,1例出现转移部位症状(骨痛),31例患者在健康体检时发现、无明显临床症状。无症状患者平均年龄略低于有症状患者,但差异无统计学意义(P>0.05)。
通过影像学检查发现,本组患者中病灶累及右肾多于左肾(28:15),右肾尤以右肾下极为著。病灶多表现为类圆形或不规则形,大多肿瘤边界清楚、有假包膜(86.0%,37/43)。CT或MRI影像上,病变多出现密度或信号不均质改变,可表现为出血(48.8%,21/43)、囊变或坏死(55.8%,24/43)、钙化(25.6%,11/43)。在增强扫描中,本组所有病例均出现强化,强化程度不尽相同,有23例出现乳头状强化。以上影像学表现和既往研究[14]较为一致。常规CT或MRI检查缺乏特异性征象,对鉴别诊断价值有限。如临床常见的肾透明细胞癌,其CT表现亦可出现低密度或混杂密度,同时可伴有囊变、坏死,但其钙化少见,增强扫描可表现为“快进快出”特点。但是不能否认影像学技术在术前评估及肿瘤分期中的重要价值。通过回顾分析我们还发现,本组43例Xp11.2 RCC影像学报告出现4例误诊情况,分别误诊为畸胎瘤1例,嫌色细胞癌1例,乏脂肪血管平滑肌脂肪瘤1例,骨髓瘤浸润1例。有学者认为,平均CT值增强量对比及功能MRI可能对判断肾癌的病理亚型有一定帮助[15]。
Xp11.2 RCC在H-E染色中无特异性表现,确诊较为困难。TFE3免疫组织化学染色阳性是诊断Xp11.2 RCC的重要依据,具有较高的灵敏度和特异度,但仍存在一定的假阳性和假阴性。FISH可在基因水平检测到TFE3易位,是确诊Xp11.2 RCC的金标准[16]。但受限于技术难度大、费用昂贵等,该方法难以广泛开展。故目前免疫组织化学染色仍然是诊断Xp11.2/TFE3 RCC的重要方法。免疫组织化学染色除检测标志性TFE3蛋白外,还可检测其他标志物(如CAⅨ、ABC、CD10、VHL、CD147、CAM5.2、钙黏蛋白6等)协助诊断。本组病例中,43例患者全部完成免疫组织化学染色检测,其中3例同时完成FISH检测,TFE3结果均为阳性,免疫组织化学染色检测中CAⅨ(74.4%,32/43)、ABC(93.0%,40/43)、CD10(93.0%,40/43)、VHL(72.1%,31/43)、CAM5.2(83.7%,36/43)阳性表达率较高。
Xp11.2/TFE3 RCC对免疫治疗、放疗、化疗等均不敏感,手术治疗是Xp11.2/TFE3 RCC首选方法。成人患者根据肿瘤大小、部位及肿瘤分期等,可采用保留肾单位手术或根治性肾切除术。本组43例患者中除2例未行肾脏手术(1例行肾穿刺活检术,1例行转移性椎管肿瘤清除术)外,20例患者接受根治性肾切除术,21例患者接受肾部分切除术,其中2例患者因出现肾静脉、下腔静脉癌栓同时行静脉癌栓取出术。本研究大部分患者预后情况良好,少数患者术后出现肿瘤复发及全身转移。近年研究发现,对于失去手术机会的Xp11.2/TFE3 RCC患者,使用索拉非尼、舒尼替尼等靶向药物有望延长其无疾病进展生存期[17-18]。本研究中2例患者因术后转移使用靶向药物治疗,经随访,病情无明显进展,患者仍存活。
虽然成人Xp11.2/TFE3 RCC发病率并不高,但其起病隐匿,多数人发病时无明显症状,且进展迅速,需引起临床、影像和病理医师的重视。目前对该类肿瘤的发病机制、临床特点、生物学特性等方面的认识仍不全面,且临床中还存在一定误诊率,需要进一步研究以加深对该疾病的认识,提高诊治水平。
| [1] |
陈显成, 甘卫东, 郭宏骞.Xp11.2易位/TFE3基因融合相关性肾癌: 一种需要更多认识的肾癌亚型[J/CD].中华临床医师杂志(电子版), 2014, 8: 3749-3752.
|
| [2] |
LOPEZ-BELTRAN A, SCARPELLI M, MONTIRONI R, KIRKALI Z. 2004 WHO classification of the renal tumors of the adults[J]. Eur Urol, 2006, 49: 798-805. DOI:10.1016/j.eururo.2005.11.035 |
| [3] |
ARGANI P, REUTER V E, ZHANG L, SUNG Y S, NING Y, EPSTEIN J I, et al. TREB-amplified renal cell carcinomas: an aggressive molecular subset demonstrating variable melanocytic marker expression and morphologic heterogeneity[J]. Am J Surg Pathol, 2016, 40: 1484-1495. DOI:10.1097/PAS.0000000000000720 |
| [4] |
KOMAI Y, FUJIWARA M, FUJⅡ Y, MUKAI H, YONESE J, KAWAKAMI S, et al. Adult Xp11 translocation renal cell carcinoma diagnosed by cytogenetics and immunohistochemistry[J]. Clin Cancer Res, 2009, 15: 1170-1176. DOI:10.1158/1078-0432.CCR-08-1183 |
| [5] |
CLASSE M, MALOUF G G, SU X, YAO H, THOMPSON E J, DOSS D J, et al. Incidence, clinicopathological features and fusion transcript landscape of translocation renal cell carcinomas[J]. Histopathology, 2017, 70: 1089-1097. DOI:10.1111/his.13167 |
| [6] |
曹松强, 张雪培, 董彪, 朱照伟, 王声政, 付天龙, 等. 成人Xp11.2易位/TFE3基因融合相关性肾癌26例报告[J]. 临床泌尿外科杂志, 2019, 34: 394-397. |
| [7] |
黄剑华, 周芳坚. Xp11.2易位性肾癌的研究进展[J]. 癌症, 2008, 27: 1006-1008. DOI:10.3321/j.issn:1000-467X.2008.09.022 |
| [8] |
ARGANI P, OLGAC S, TICKOO S K, GOLDFISCHER M, MOCH H, CHAN D Y, et al. Xp11 translocation renal cell carcinoma in adults: expanded clinical, pathologic, and genetic spectrum[J]. Am J Surg Pathol, 2007, 31: 1149-1160. DOI:10.1097/PAS.0b013e318031ffff |
| [9] |
苏明昌, 付伟金.Xp11.2易位/TFE3基因融合相关性肾癌研究进展[J/CD].泌尿外科杂志(电子版), 2018, 10: 1-4.
|
| [10] |
MEYER P N, CLARK J I, FLANIGAN R C, PICKEN M M. Xp11.2 translocation renal cell carcinoma with very aggressive course in five adults[J]. Am J Clin Pathol, 2007, 128: 70-79. DOI:10.1309/LR5G1VMXPY3G0CUK |
| [11] |
ARMAH H B, PARWANI A V. Xp11.2 translocation renal cell carcinoma[J]. Arch Pathol Lab Med, 2010, 134: 124-129. DOI:10.5858/2008-0391-RSR.1 |
| [12] |
LIU N, WANG Z, GAN W, XIONG L, MIAO B, CHEN X, et al. Renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusions: clinical features, treatments and prognosis[J/OL]. PLoS One, 2016, 11: e0166897. DOI: 10.1371/journal.pone.0166897.
|
| [13] |
DANG T T, ZIV E, WEINSTEIN S, MENG M V, WANG Z, COAKLEY F V. Computed tomography and magnetic resonance imaging of adult renal cell carcinoma associated with Xp11.2 translocation[J]. Comput Assist Tomogr, 2012, 36: 669-674. DOI:10.1097/RCT.0b013e3182680182 |
| [14] |
程瑾, 陈皓, 史景丽, 张银丽, 洪楠. Xp11.2易位/TFE3基因融合相关性肾癌的CT和MRI表现[J]. 放射学实践, 2018, 33: 811-815. |
| [15] |
徐晓晨, 甘卫东, 李笑弓, 张古田, 刘铁石, 姚林方, 等. Xp11.2易位/TFE3基因融合相关性肾癌与肾透明细胞癌的螺旋CT诊断鉴别[J]. 现代泌尿外科杂志, 2012, 17: 122-124. DOI:10.3969/j.issn.1009-8291.2012.02.004 |
| [16] |
GREEN W M, YONESCU R, MORSBERGER L, MORRIS K, NETTO G J, EPSTEIN J I, et al. Utilization of a TFE3 break apart FISH assay in a renal tumor consultation service[J]. Am J Srug Pathol, 2013, 37: 1150-1163. DOI:10.1097/PAS.0b013e31828a69ae |
| [17] |
SUDOUR-BONNANGE H, LEROY X, CHAUVET M P, CLASSE M, ROBIN P M, LEBLOND P. Cutaneous metastases during an aggressive course of Xp11.2 translocation renal cell carcinoma in a teenager[J]. Pediatr Blood Cancer, 2014, 61: 1698-1700. DOI:10.1002/pbc.25015 |
| [18] |
KAKOKI K, MIYATA Y, MOCHIZUKI Y, IWATA T, OBATAKE M, ABE K, et al. Long-term treatment with sequential molecular targeted therapy for Xp11.2 translocation renal cell carcinoma: a case report and review of the literature[J/OL]. Clin Genitourin Cancer, 2017, 15: e503-e506. DOI: 10.1016/j.clgc.2016.12.026.
|
 2021, Vol. 42
2021, Vol. 42


