神经病理性疼痛是指原发性神经系统损伤或功能异常所引起的慢性疼痛[1]。相关临床研究表明,慢性疼痛患者伴随认知障碍及抑郁、焦虑等情绪障碍的发生率较普通患者高[2-3]。慢性疼痛与抑郁之间的这种相互伴随关系提示二者之间可能存在共同的发病机制。
N-甲基-D-天冬氨酸(N-methyl-D-aspartate,NMDA)受体是一种离子型谷氨酸受体,可通过调节神经元细胞内Ca2+浓度的变化对突触的传导、可塑性及神经细胞变形等产生作用[4]。NMDA受体2B亚基(N-methyl-D-aspartate receptor 2B subunit,NR2B)作为功能性亚基,参与对慢性疼痛或抑郁发病机制的调节[5-6]。研究发现在慢性神经病理性疼痛小鼠模型中,脑源性神经营养因子通过调节前扣带回中NR2B的磷酸化水平从而调控小鼠的疼痛状况[7]。另有研究表明小鼠抑郁模型海马中NR2B的表达明显下降,但通过电休克治疗、面部注射肉毒毒素A及腹腔内注射文拉法辛(5-羟色胺再摄取抑制剂)等措施后,海马中NR2B的表达增加,从而缓解小鼠抑郁样症状[8-10]。
前额叶皮质(prefrontal cortex,PFC)被认为是情感性疾病或慢性疼痛调节的关键脑区[11]。在慢性疼痛小鼠模型中,PFC内谷氨酸水平明显降低,并且使用谷氨酸受体激动剂可缓解小鼠的疼痛症状[12]。另有研究表明,抑郁症患者PFC中NR2B的表达比对照组下降约48%[13];抑郁大鼠模型中PFC内NR2B蛋白的表达也明显降低,且联合应用Zn2+及叶酸增加NR2B表达能产生良好的抗抑郁效果[14]。
右美托咪定(dexmedetomidine,DEX)作为高选择性的α2肾上腺素能受体激动剂,具有抗交感、镇静及镇痛等作用[15]。在痛觉过敏的动物模型中,DEX可抑制脊髓中NR2B磷酸化,调节蛋白激酶C(protein kinase C,PKC)水平变化,从而发挥镇痛作用[16]。有研究表明腹腔注射曲马多及DEX比单用曲马多的抗抑郁效果更好,且在使用电休克冲击疗法治疗抑郁症时,联合使用DEX可通过调节海马NR2B-ERK通路改善患者的学习和记忆功能[17-18]。上述研究说明DEX除了镇痛作用外,可能还具有一定的抗抑郁作用,且可能与NR2B蛋白表达水平相关。本研究在此基础上采用小剂量DEX处理保留性神经损伤(spared nerve injury,SNI)大鼠模型,探讨小剂量DEX对神经病理性疼痛相关抑郁样行为的作用及机制。
1 材料和方法 1.1 实验动物及试剂SD大鼠购于湖南省斯莱克景达实验动物股份有限公司[实验动物生产许可证号:SCXK(湘)2019-0004]。盐酸DEX及七氟烷购于恒瑞医药有限公司。BCA蛋白浓度测定试剂盒及SDS-PAGE凝胶制备试剂盒购于康为世纪生物科技有限公司。NR2B单克隆抗体购于英国Abcam公司,tubulin单克隆抗体购于美国Thermo公司。
1.2 动物分组将22只SD大鼠随机分为4组:假手术组(Sham组,n=6),SNI组(n=6),SNI+生理盐水(normal saline,NS)组(n=5)和SNI+DEX组(n=5)。
1.3 动物建模及给药参照经典SNI模型建模方法[19],使用1.5%~2.0%七氟烷麻醉大鼠,剃毛,消毒,铺单。取右侧股骨中点下方1~2 cm处沿大鼠腹股沟方向切开大鼠皮肤,离断股二头肌后,游离腓总神经及胫神经周围组织并结扎,当大鼠右侧大腿出现抽搐反应时剪断神经,残端再次剪去5 mm左右神经组织,保留腓肠神经,充分止血后,逐层缝合。在建模成功后第8~14天,SNI+DEX组给予腹腔内注射小剂量DEX(20 μg/kg,剂量选择参照文献[20]),而SNI+NS组则给予等体积NS,其余2组未做相关处理。
1.4 大鼠疼痛缩足阈值(pain withdrawal threshold,PWT)测定成功建立模型后,采用不同的von Frey纤维(美国North Coast Medical公司)测量大鼠PWT。将大鼠放置于底部为网状的透明箱内15~20 min,待大鼠安静后,采用不同压力值(0.6、1.0、2.0、4.0、6.0、10.0、15.0 g)的von Frey纤维刺激大鼠右外侧足跟部,使von Frey纤维略微弯曲,维持4 s。当大鼠右腿有舔足、缩足或抬足的动作时则被视为阳性反应,反之则为阴性反应。取2.0 g作为首次测量压力值,若大鼠测量表现为阴性,则记录此次测量为“O”,需要选择高一级的压力值进行刺激;若表现为阳性,则记录为“X”,需要选取低一级的压力值进行测量。每只大鼠需要反复进行最多6次的测量,两次测量之间至少间隔1 min。根据Chaplan等[21]方法估计PWT。建模前1 d,所有大鼠测量基础PWT;Sham组及SNI组在建模后第1、3、7、14天测量PWT;SNI+NS组及SNI+DEX组在建模后第1、3、7、14天测量PWT,并在给予NS或DEX(20 μg/kg)后每天(建模后第8~14天)于给药后2 h测量PWT。
1.5 强迫游泳实验(force swim test,FST)于建模成功后第14天对每只大鼠进行预游泳实验。在安静适宜的环境中,将大鼠放入一个高40 cm、直径25 cm并充满高25 cm自来水[水温维持在(25±1)℃]的透明玻璃圆筒中,每只大鼠预游泳15 min,此次预游泳不做记录,每只大鼠预游泳之后都需清洗透明玻璃圆筒并换水。24 h后,在相同环境中再次以相同的方法使每只大鼠被迫游泳5 min,记录大鼠右腿不动时间(最少不动时间>1 s)。
1.6 蛋白质印迹分析术后第15天处死大鼠,迅速解剖出PFC保存备用。检测时,先进行加入抑制酶试剂、碎裂组织、离心、取上清液、测量蛋白样品浓度及蛋白变性等操作,然后行电泳及转膜。转膜后加入NR2B和tubulin单克隆抗体摇床孵育20 h,再加入二抗孵育1 h。通过化学发光法显示目的条带,使用ImageJ软件进行处理和分析。
1.7 统计学处理采用GraphPad Prism 7.0软件进行统计学分析。计量资料以x±sx表示,使用双因素重复测量方差分析及独立样本t检验进行分析。检验水准(α)为0.05。
2 结果 2.1 慢性神经病理性疼痛模型大鼠伴随抑郁样行为在术后第1、3、7、14天,与Sham组相比,SNI组大鼠PWT均降低(F(1,14)=417,P<0.01;图 1A),说明慢性神经病理性疼痛模型建立成功。在术后第15天,与Sham组大鼠相比,SNI组大鼠FST中右腿静止不动时间延长(t=3.313,P<0.01;图 1B),表明慢性神经病理性疼痛模型大鼠伴有抑郁样行为。

|
图 1 各组大鼠PWT及FST测量结果 Fig 1 Measurement results of PWT and FST of rats in each group A: Comparison of PWT results between Sham group and SNI group; B: Comparison of FST results between Sham group and SNI group; C: Comparison of PWT results between SNI+NS group and SNI+DEX (20 μg/kg) group; D: Comparison of FST results between SNI+NS group and SNI+DEX (20 μg/kg) group. PWT: Pain withdrawal threshold; FST: Force swim test; SNI: Spared nerve injury; NS: Normal saline; DEX: Dexmedetomidine. **P < 0.01 vs Sham group; △△P < 0.01 vs SNI+NS group. n=6 in Sham group and SNI group, n=5 in SNI+NS group and SNI+DEX (20 μg/kg) group; x±sx |
2.2 小剂量DEX可缓解大鼠慢性神经病理性疼痛相关抑郁样行为
与SNI+NS组相比,SNI+DEX组大鼠各时间点PWT无明显变化(P>0.05,图 1C),说明腹腔内注射小剂量DEX(20 μg/kg)并不改变SNI大鼠的痛阈。术后第15天FST结果显示,SNI+DEX组大鼠右腿不动时间比SNI+NS组缩短(t=5.348,P<0.01;图 1D),表明腹腔内注射小剂量DEX能明显改善大鼠慢性神经病理性疼痛伴随的抑郁样行为。
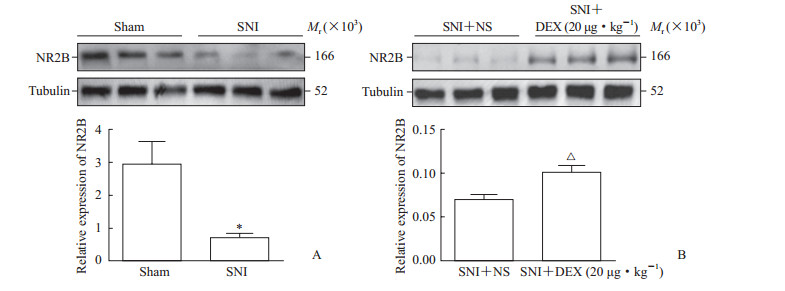
2.3 小剂量DEX可增加慢性神经病理性疼痛大鼠PFC中NR2B蛋白的表达术后第15天取各组大鼠PFC脑区检测NR2B蛋白的表达,结果显示SNI组大鼠PFC中NR2B蛋白的表达比Sham组降低(t=3.236,P<0.05;图 2A),并且SNI+DEX组大鼠PFC中NR2B蛋白的表达比SNI+NS组升高(t=3.627,P<0.05;图 2B)。上述结果表明腹腔内注射小剂量DEX(20 μg/kg)后能明显增加SNI大鼠PFC中NR2B蛋白的表达。
|
图 2 各组大鼠PFC中NR2B蛋白的表达情况 Fig 2 The expression of NR2B protein in the PFC of rats in each group A: Comparison of NR2B expression in the PFC between Sham group and SNI group; B: Comparison of NR2B expression in the PFC between SNI+NS group and SNI+DEX (20 μg/kg) group. PFC: Prefontal cortex; NR2B: N-methyl-D-aspartate receptor 2B subunit; SNI: Spared nerve injury; NS: Normal saline; DEX: Dexmedetomidine. *P < 0.05 vs Sham group; △P < 0.05 vs SNI+NS group. n=6 in Sham group and SNI group, n=5 in SNI+NS group and SNI+DEX (20 μg/kg) group; x±sx |
3 讨论
SNI大鼠模型已被广泛应用于慢性疼痛与抑郁共病的机制研究中。本研究成功建立SNI大鼠模型后,发现SNI组大鼠的PWT较Sham组降低,但是在术后第8~14天给予SNI大鼠持续腹腔内注射小剂量DEX(20 μg/kg),大鼠PWT无明显改变,提示此剂量DEX无明显的镇痛效果。在FST中,我们发现SNI组大鼠右腿静止不动时间较Sham组大鼠延长,提示慢性疼痛大鼠伴随着抑郁样行为。
在FST中,我们还发现与SNI+NS组大鼠相比,SNI+DEX组大鼠右腿静止不动时间缩短,说明腹腔内注射小剂量DEX可缓解慢性神经病理性疼痛诱导的抑郁样行为。DEX通过α2肾上腺素能受体发挥多种不同的生理效应。有文献提示α2肾上腺素能受体的减少或基因敲除都会造成大鼠抑郁样或焦虑样行为的增加,表明其在情绪保护上发挥着重要的作用[22]。另外在小鼠抑郁模型中,单次静脉注射DEX后小鼠悬尾实验中不动时间明显缩短,抑郁症状改善且呈剂量依赖性,但抗抑郁效应不及全选择性α受体激动剂,并且在使用α2受体拮抗剂后可逆转其抗抑郁效果[23-24]。α2受体广泛存在于大脑各处,也是DEX作用的主要靶点,在未来研究中需进一步探讨其与抑郁症状之间的相互关系。
此外,本研究还发现SNI组大鼠PFC中NR2B蛋白的表达较Sham组有所下降,腹腔内注射小剂量DEX后SNI大鼠PFC中NR2B的表达与注射NS组相比明显增加。该结果提示腹腔内注射小剂量DEX(20 μg/kg)可能通过上调PFC中NR2B的表达而缓解慢性神经病理性疼痛伴随的抑郁样行为。PFC中蕴含大量的NR2B,参与PFC功能成熟过程,各种原因导致的PFC中NR2B功能减弱会直接抑制长时程增强效应的形成,而导致长时程抑制,从而造成情绪、学习及认知等功能障碍[25]。在抑郁症患者或抑郁动物模型中,PFC中NR2B蛋白表达降低是抑郁症的起因还是其特征,目前尚无定论,需要后续研究进一步验证两者之间的关系。
DEX作为被FDA批准用于镇痛的药物之一,已广泛应用于临床。Liu等[20]发现连续7 d或14 d腹腔内注射DEX(20 μg/kg)无明显的镇痛效果,我们参考该结果设计了本研究。20 μg/kg剂量可排除DEX缓解疼痛后所带来的抑郁缓解效应,从而证明腹腔内注射小剂量DEX可缓解慢性神经病理性疼痛所伴随的抑郁样行为。本实验虽然初步发现小剂量DEX(20 μg/kg)腹腔内注射具有抗抑郁作用,但对于其他剂量DEX的抗抑郁作用并未进一步探讨,且腹腔内注射DEX虽然可通过毛细血管吸收而作用于大脑,但相对于脑室内注射具有不确定性。
综上所述,慢性神经病理性疼痛使大鼠产生抑郁样症状,并且使PFC中NR2B的表达降低,而小剂量DEX(20 μg/kg)在不改变其疼痛的情况下,可能通过增加PFC中NR2B的表达缓解其抑郁症状。
| [1] |
JENSEN T S, BARON R, HAANPÄÄ M, KALSO E, LOESER J D, RICE A S C, et al. A new definition of neuropathic pain[J]. Pain, 2011, 152: 2204-2205. DOI:10.1016/j.pain.2011.06.017 |
| [2] |
温志娟, 王德, 屈明芬, 高勇, 徐昕, 谭树颖, 等. 慢性疼痛患者认知障碍的研究进展[J]. 中国疼痛医学杂志, 2015, 21: 374-376. DOI:10.3969/j.issn.1006-9852.2015.05.012 |
| [3] |
KNASTER P, KARLSSON H, ESTLANDER A M, KALSO E. Psychiatric disorders as assessed with SCID in chronic pain patients:the anxiety disorders precede the onset of pain[J]. Gen Hosp Psychiatry, 2012, 34: 46-52. DOI:10.1016/j.genhosppsych.2011.09.004 |
| [4] |
BLISS T V, COLLINGRIDGE G L. A synaptic model of memory:long-term potentiation in the hippocampus[J]. Nature, 1993, 361: 31-39. DOI:10.1038/361031a0 |
| [5] |
WEI F, WANG G D, KERCHNER G A, KIM S J, XU H M, CHEN Z F, et al. Genetic enhancement of inflammatory pain by forebrain NR2B overexpression[J]. Nat Neurosci, 2001, 4: 164-169. DOI:10.1038/83993 |
| [6] |
BURNOUF S, MARTIRE A, DERISBOURG M, LAURENT C, BELARBI K, LEBOUCHER A, et al. NMDA receptor dysfunction contributes to impaired brain-derived neurotrophic factor-induced facilitation of hippocampal synaptic transmission in a Tau transgenic model[J]. Aging Cell, 2013, 12: 11-23. DOI:10.1111/acel.12018 |
| [7] |
ZHANG Y, JI F, WANG G, HE D, YANG L, ZHANG M. BDNF activates mTOR to upregulate NR2B expression in the rostral anterior cingulate cortex required for inflammatory pain-related aversion in rats[J]. Neurochem Res, 2018, 43: 681-691. DOI:10.1007/s11064-018-2470-6 |
| [8] |
DONG J, MIN S, WEI K, LI P, CAO J, LI Y. Effects of electroconvulsive therapy and propofol on spatial memory and glutamatergic system in hippocampus of depressed rats[J]. J ECT, 2010, 26: 126-130. DOI:10.1097/YCT.0b013e3181a9947a |
| [9] |
LI Y, LIU J, LIU X, SU C J, ZHANG Q L, WANG Z H, et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain[J]. Neurosci Bull, 2019, 35: 661-672. DOI:10.1007/s12264-019-00367-8 |
| [10] |
YILMAZ N, DEMIRDAS A, YILMAZ M, SUTCU R, KIRBAS A, CURE M C, et al. Effects of venlafaxine and escitalopram treatments on NMDA receptors in the rat depression model[J]. J Membr Biol, 2011, 242: 145-151. DOI:10.1007/s00232-011-9385-3 |
| [11] |
ONG W Y, STOHLER C S, HERR D R. Role of the prefrontal cortex in pain processing[J]. Mol Neurobiol, 2019, 56: 1137-1166. DOI:10.1007/s12035-018-1130-9 |
| [12] |
KELLY C J, HUANG M, MELTZER H, MARTINA M. Reduced glutamatergic currents and dendritic branching of layer 5 pyramidal cells contribute to medial prefrontal cortex deactivation in a rat model of neuropathic pain[J/OL]. Front Cell Neurosci, 2016, 10: 133. doi: 10.3389/fncel.2016.00133.
|
| [13] |
FEYISSA A M, CHANDRAN A, STOCKMEIER C A, KAROLEWICZ B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2009, 33: 70-75. DOI:10.1016/j.pnpbp.2008.10.005 |
| [14] |
DOU M, GONG A, LIANG H, WANG Q, WU Y, MA A, et al. Improvement of symptoms in a rat model of depression through combined zinc and folic acid administration via up-regulation of the Trk B and NMDA[J]. Neurosci Lett, 2018, 683: 196-201. DOI:10.1016/j.neulet.2018.07.036 |
| [15] |
MIKAMI M, ZHANG Y, KIM B, WORGALL T S, GROEBEN H, EMALA C W. Dexmedetomidine's inhibitory effects on acetylcholine release from cholinergic nerves in guinea pig trachea: a mechanism that accounts for its clinical benefit during airway irritation[J/OL]. BMC Anesthesiol, 2017, 17: 52. doi: 10.1186/s12871-017-0345-z.
|
| [16] |
ZHENG Y, CUI S, LIU Y, ZHANG J, ZHANG W, ZHANG J, et al. Dexmedetomidine prevents remifentanil-induced postoperative hyperalgesia and decreases spinal tyrosine phosphorylation of N-methyl-D-aspartate receptor 2B subunit[J]. Brain Res Bull, 2012, 87(4/5): 427-431. |
| [17] |
吴翠翠, 李艳辉, 张新敏, 麻海春. 小剂量曲马多与右美托咪定对神经病理性疼痛大鼠的抗抑郁效果[J]. 中国疼痛医学杂志, 2015, 21: 662-667. DOI:10.3969/j.issn.1006-9852.2015.09.006 |
| [18] |
GAO X, ZHUANG F Z, QIN S J, ZHOU L, WANG Y, SHEN Q F, et al. Dexmedetomidine protects against learning and memory impairments caused by electroconvulsive shock in depressed rats:involvement of the NMDA receptor subunit 2B (NR2B)-ERK signaling pathway[J]. Psychiatry Res, 2016, 243: 446-452. DOI:10.1016/j.psychres.2016.07.020 |
| [19] |
DECOSTERD I, WOOLF C J. Spared nerve injury:an animal model of persistent peripheral neuropathic pain[J]. Pain, 2000, 87: 149-158. DOI:10.1016/S0304-3959(00)00276-1 |
| [20] |
LIU L, JI F, LIANG J, HE H, FU Y, CAO M. Inhibition by dexmedetomidine of the activation of spinal dorsal horn glias and the intracellular ERK signaling pathway induced by nerve injury[J]. Brain Res, 2012, 1427: 1-9. DOI:10.1016/j.brainres.2011.08.019 |
| [21] |
CHAPLAN S R, BACH F W, POGREL J W, CHUNG J M, YAKSH T L. Quantitative assessment of tactile allodynia in the rat paw[J]. J Neurosci Methods, 1994, 53: 55-63. DOI:10.1016/0165-0270(94)90144-9 |
| [22] |
SCHRAMM N L, MCDONALD M P, LIMBIRD L E. The α2A-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety[J]. J Neurosci, 2001, 21: 4875-4882. DOI:10.1523/JNEUROSCI.21-13-04875.2001 |
| [23] |
STONE E A, LIN Y, SARFRAZ Y, QUARTERMAIN D. Antidepressant-like action of intracerebral 6-fluoronorepinephrine, a selective full α-adrenoceptor agonist[J]. Int J Neuropsychopharmacol, 2011, 14: 319-331. DOI:10.1017/S1461145710000507 |
| [24] |
MILLAN M J. The role of monoamines in the actions of established and "novel" antidepressant agents:a critical review[J]. Eur J Pharmacol, 2004, 500(1/2/3): 371-384. |
| [25] |
FLORES-BARRERA E, THOMASES D R, HENG L J, CASS D K, CABALLERO A, TSENG K Y. Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic protein kinase A and D1 dopamine receptor signaling[J]. Biol Psychiatry, 2014, 75: 508-516. DOI:10.1016/j.biopsych.2013.07.033 |
 2020, Vol. 41
2020, Vol. 41


