2. 上海捷瑞生物工程有限公司, 上海 201615
2. Shanghai Generay Biotech Co., Ltd, Shanghai 201615, China
新型冠状病毒肺炎(coronavirus disease 2019,COVID-19)疫情在全球范围内迅速蔓延,截至2020年4月6日,已在全球200多个国家和地区流行,造成超过125万人感染,7万人死亡[1]。在COVID-19疫情出现初期,我国研究人员迅速鉴定出引发此次疫情的病原体为一种新型冠状病毒,并发现它与严重急性呼吸综合征冠状病毒(severe acute respiratory syndrome coronavirus,SARS-CoV)的基因组序列具有约80%的同源性,随后成功分离出该病毒[2-3]。国际病毒分类委员会将该病毒命名为严重急性呼吸综合征冠状病毒2(severe acute respiratory syndrome coronavirus 2,SARS-CoV-2)。虽然SARS-CoV-2的致病性较SARS-CoV弱,但其具有更强的传染性,经多代传播后病毒基因组仍较稳定,变异率较低[4-8]。
SARS-CoV-2属于冠状病毒科β冠状病毒属,是一种球形、有包膜的单正链RNA病毒。其基因组长约30 kb,5’端有甲基化帽子结构、3’端有多聚A尾结构;编码区中含有冠状病毒常见的开放阅读框(open reading frame,ORF)及其他辅助基因,主要编码4种结构蛋白:刺突蛋白(spike protein,S蛋白)、包膜蛋白(envelope protein,E蛋白)、膜蛋白(membrane protein,M蛋白)、核衣壳蛋白(nucleocapsid protein,N蛋白),其中N蛋白由413个氨基酸残基组成,可与病毒基因组RNA形成核衣壳,参与病毒基因组复制和颗粒的组装,同时具有较强的免疫原性,可诱导机体产生高水平的免疫反应[3, 9-11]。无论是N蛋白还是针对N蛋白的IgM抗体,均是SARS-CoV-2感染的重要检测靶标[12]。
本研究将含有SARS-CoV-2 N基因的原核表达质粒在大肠杆菌BL21(DE3)中诱导表达出可溶性的SARS-CoV-2 N重组蛋白,利用Ni柱亲和层析法进行蛋白纯化,并用纯化后的蛋白免疫小鼠制备出抗血清,为进一步研究SARS-CoV-2的致病机制、N蛋白的生物学功能及建立免疫学快速诊断方法等奠定实验基础。
1 材料和方法 1.1 材料SARS-CoV-2 N基因原核表达质粒pET28a-N(6×His标签位于N蛋白的氨基末端)由广东省实验动物检测所新检测技术中心丛锋博士馈赠,真核表达质粒pcDNA3.1-N是将经哺乳动物细胞偏爱密码子优化的SARS-CoV-2 N基因插入真核表达载体pcDNA3.1中构建而成。大肠杆菌BL21(DE3)、人胚肾细胞系293T为海军军医大学(第二军医大学)海军医学系生物医学防护实验室保存,兔抗SARS-CoV-2 N单克隆抗体(货号:40143-R019)购自北京义翘神州科技股份有限公司,兔抗SARS-CoV N多克隆抗体为海军军医大学(第二军医大学)海军医学系生物医学防护实验室前期制备,锰佐剂由北京大学生命科学学院蒋争凡教授馈赠,Ni-NTA琼脂糖纯化树脂购自美国QIAGEN公司,重力流动层析的一次性聚丙烯空柱购自深圳逗点生物技术有限公司,异丙基-β-D-硫代半乳糖苷(isopropyl-β-D-thiogalactopyranoside,IPTG)干粉购自上海翊圣生物科技有限公司,脂质体2000、HRP标记的山羊抗兔IgG、FITC标记的羊抗兔或小鼠IgG购自美国Invitrogen公司,DAPI、RIPA裂解液、蛋白酶抑制剂、BCA蛋白定量试剂盒购自江苏碧云天生物技术研究所,蛋白预染相对分子质量标准购自加拿大Fermentas公司。6~8周龄雌性BALB/c小鼠购自上海灵畅生物科技有限公司,动物生产许可证号:SCXK(沪)2018-0003。
1.2 SARS-CoV-2 N重组蛋白的诱导表达用原核表达质粒pET28a-N转化BL21(DE3)感受态细胞,挑取单菌落,接种于卡那霉素抗性的LB培养基中,37 ℃培养过夜。次日按1︰100比例将上述菌液再接种到LB培养基中,37 ℃培养3 h;分别加入终浓度为0.5、1 mmol/L的IPTG进行诱导表达;诱导后3、5 h收集菌液,离心后用适量PBS重悬细菌沉淀,然后加入等体积的2×SDS上样缓冲液,100 ℃煮10 min,然后行12.5% SDS-PAGE,同时以未诱导表达菌液作为对照。对SDS-PAGE凝胶中的蛋白进行考马斯亮蓝染色。
1.3 SARS-CoV-2 N重组蛋白的纯化扩大pET28a-N转化菌的培养体积至200 mL,参照上述方法以0.5 mmol/L IPTG处理5 h以诱导蛋白表达。收集菌液,离心后菌体沉淀以层析裂解液悬浮,然后超声粉碎细菌,高速离心,收集上清,以Ni-NTA琼脂糖纯化树脂为介质,亲和层析纯化SARS-CoV-2 N重组蛋白,按照使用说明书操作。收集的洗脱组分进行SDS-PAGE和考马斯亮蓝染色检测。
1.4 SARS-CoV-2 N重组蛋白的蛋白质印迹分析取适量纯化的SARS-CoV-2 N重组蛋白和牛血清白蛋白(bovine serum albumin,BSA;阴性对照),加入等量的2×样品缓冲液,100 ℃煮10 min,高速离心后取上清,行12.5% SDS-PAGE[13-14]。利用电转移法将凝胶中的蛋白转移到PVDF膜(300 mA,90 min),经5%脱脂奶粉封闭后加入按1︰1 000比例稀释的SARS-CoV-2 N单克隆抗体或SARS-CoV N多克隆抗体,4 ℃孵育过夜。TBST漂洗,加入按1︰1 000比例稀释的HRP标记的山羊抗兔IgG,室温孵育2 h,漂洗后用增强型HRP-DAB底物显色,用化学发光凝胶成像仪检测反应条带。
1.5 小鼠免疫将纯化的SARS-CoV-2 N重组蛋白与锰佐剂按1︰5质量比混合,通过肌内和皮下多点注射接种6~8周龄雌性BALB/c小鼠,每只小鼠注射剂量包括SARS-CoV-2 N重组蛋白40 μg、锰佐剂200 μg,用PBS调整体积至200 μL,共免疫2次,第1次和第2次免疫间隔1周。在小鼠第2次免疫后的第7天,用毛细管从小鼠眼眶取血,离心(8 000×g,10 min)后取血清,用于抗体的间接免疫荧光实验检测。
1.6 细胞培养与转染293T细胞培养于含10% FBS、1 mmol/L L-谷氨酰胺、100 nmol/L非必需氨基酸(non-essential amino acid,NEAA)、100 μg/mL链霉素和100 U/mL青霉素的DMEM培养液中,置于37 ℃、5% CO2培养箱中培养。将293T细胞接种于24孔板中,次日细胞密度约90%时,用SARS-CoV-2 N基因真核表达质粒pcDNA3.1-N转染细胞,转染试剂用脂质体2000,按照试剂说明书操作。
1.7 间接免疫荧光实验将上述转染表达SARS-CoV-2 N重组蛋白的293T细胞接种至96孔板,37 ℃培养过夜[15]。次日用预冷的甲醇固定细胞,3% BSA封闭;然后用SARS-CoV-2 N单克隆抗体、SARS-CoV N多克隆抗体或免疫小鼠血清(分别按1︰200、1︰200、1︰500稀释)作为一抗、FITC标记的羊抗兔或小鼠IgG(1︰500稀释)作为二抗进行间接免疫荧光实验,于荧光显微镜下观察细胞中的免疫荧光强度并拍照。
2 结果 2.1 SARS-CoV-2 N重组蛋白诱导表达的SDS-PAGE分析SDS-PAGE分析结果(图 1)显示,SARS-CoV-2 N重组蛋白的相对分子质量约为55 000,与预期蛋白大小相符,表明目的蛋白诱导表达成功。SARS-CoV-2 N重组蛋白经不同条件诱导后均能在大肠杆菌中表达,而未经诱导的菌液不表达;随着IPTG诱导时间的延长,SARS-CoV-2 N重组蛋白的表达量也稍有增加;不同终浓度IPTG诱导的蛋白表达量无明显差异。
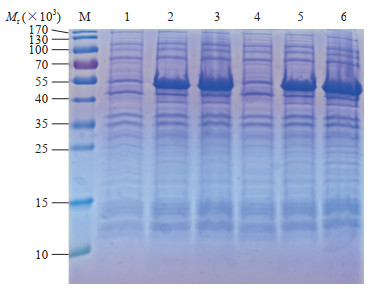
|
图 1 SARS-CoV-2 N重组蛋白在大肠杆菌中诱导表达的SDS-PAGE分析 Fig 1 SDS-PAGE analysis of recombinant SARS-CoV-2 N protein expression induced in Escherichia coli M: The molecular weight standard of protein; 1, 4: The bacteria were not induced; 2: The bacteria were induced by 0.5 mmol/L IPTG for 3 h; 3: The bacteria were induced by 0.5 mmol/L IPTG for 5 h; 5: The bacteria were induced by 1 mmol/L IPTG for 3 h; 6: The bacteria were induced by 1 mmol/L IPTG for 5 h. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; N: Nucleocapsid; SDS-PAGE: Sodium dodecyl sulphate-polyacrylamide gel electrophoresis; IPTG: Isopropyl-β-D-thiogalactopyranoside |
2.2 SARS-CoV-2 N重组蛋白的亲和层析纯化
Ni-NTA亲和层析纯化结果(图 2)显示,纯化后的目的蛋白条带清晰,与预期蛋白的相对分子质量相符;纯化后的蛋白在其他位置均无杂带;随着洗脱时间的延长,目的蛋白的浓度先逐步升高,然后下降,位于第7~11洗脱组分中的目的蛋白浓度最高(图 2A中的7~11泳道)。利用BCA蛋白定量试剂盒测定洗脱组分7中目的蛋白的浓度为1.54 mg/mL,说明该方法可获得纯度和浓度均较高的SARS-CoV-2 N重组蛋白。
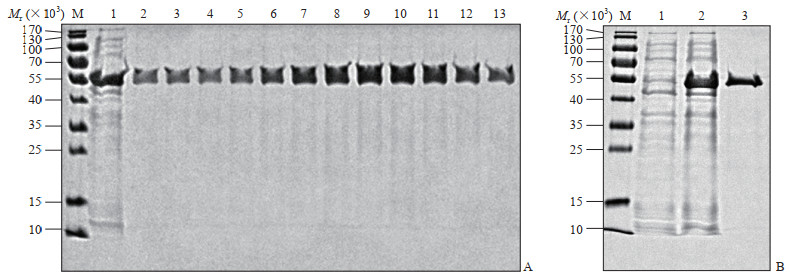
|
图 2 SARS-CoV-2 N重组蛋白的纯化 Fig 2 Purification of recombinant SARS-CoV-2 N protein A: Different fractions after elution (M: The molecular weight standard of protein; 1: The bacteria were induced by 0.5 mmol/L IPTG for 5 h; 2-13: The collected fractions of purified protein after elution); B: Comparison of the proteins before and after purification (M: The molecular weight standard of protein; 1: The bacteria were not induced; 2: The bacteria were induced by 0.5 mmol/L IPTG for 5 h; 3: The representative purified protein). SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; N: Nucleocapsid; IPTG: Isopropyl-β-D-thiogalactopyranoside |
2.3 SARS-CoV-2 N重组蛋白的蛋白质印迹分析鉴定
取上述纯化的蛋白组分7和8样品,经SDS-PAGE分离后转移至PVDF膜,分别以SARS-CoV-2 N单克隆抗体和SARS-CoV N多克隆抗体为一抗、以HRP标记的山羊抗兔IgG为二抗进行蛋白质印迹分析。结果如图 3所示,目标条带清晰,且与预期蛋白大小相符,而在阴性对照组中未见目标条带,说明所表达的SARS-CoV-2 N重组蛋白可与SARS-CoV-2 N单克隆抗体特异性反应,且可与SARS-CoV N多克隆抗体交叉反应。
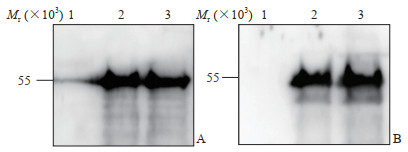
|
图 3 SARS-CoV-2 N重组蛋白的蛋白质印迹分析 Fig 3 Western blotting analysis of recombinant SARS-CoV-2 N protein A: Anti-SARS-CoV-2 N rabbit monoclonal antibodies were used as primary antibodies in Western blotting; B: Anti-SARS-CoV N rabbit polyclonal antibodies were used as primary antibodies in Western blotting. 1: BSA was used as negative control; 2, 3: The representative purified recombinant SARS-CoV-2 N protein was examined. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; N: Nucleocapsid; SARS-CoV: Severe acute respiratory syndrome coronavirus; BSA: Bovine serum albumin |
2.4 小鼠抗血清的间接免疫荧光实验检测
间接免疫荧光实验检测结果显示,与阳性对照组(以SARS-CoV-2 N单克隆抗体为一抗)相似,免疫小鼠血清组和SARS-CoV N多克隆抗体组细胞中均能观察到绿色荧光;而在空白组细胞中未观察到荧光信号(图 4)。结果说明本研究所制备的SARS-CoV-2 N重组蛋白免疫小鼠抗血清能与哺乳动物细胞瞬时表达的SARS-CoV-2 N重组蛋白特异性结合,可用于间接免疫荧光实验检测。
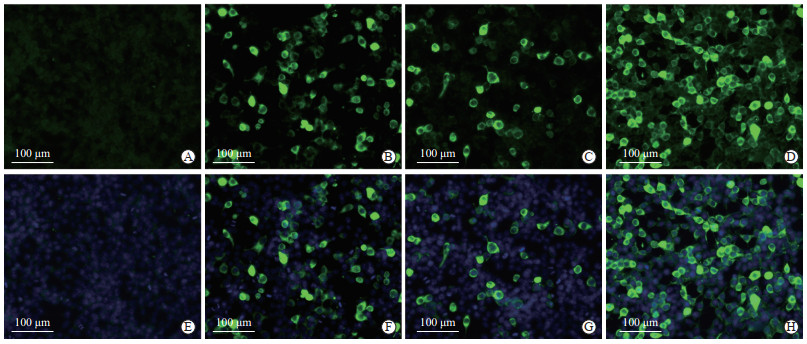
|
图 4 SARS-CoV-2 N重组蛋白免疫小鼠抗血清的间接免疫荧光实验结果 Fig 4 Indirect immunofluorescence analysis of the antiserum from immunized mice with recombinant SARS-CoV-2 N protein A-D: Immunofluorescence of cells stained with corresponding antibodies; E-H: The corresponding fields of A-D showing the cells stained with corresponding antibodies and DAPI. A, E: Mock transfected 293T cells (negative control); B, F: pcDNA3.1-N transfected cells were stained with anti-SARS-CoV-2 N rabbit monoclonal antibodies (positive control); C, G: pcDNA3.1-N transfected cells were stained with anti-SARS-CoV N rabbit polyclonal antibodies; D, H: pcDNA3.1-N transfected cells were stained with mouse antiserum. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; N: Nucleocapsid; DAPI: 4', 6-diamidino-2-phenylindole; SARS-CoV: Severe acute respiratory syndrome coronavirus |
3 讨论
本研究通过原核表达系统获得了电泳纯的SARS-CoV-2 N重组蛋白表达产物,蛋白质印迹分析证实该蛋白可以被市售的SARS-CoV-2 N单克隆抗体和实验室前期制备的SARS-CoV N多克隆抗体识别,说明这2种病毒的N蛋白存在相同或相似的抗原表位。进一步通过免疫小鼠,制备出抗血清,经间接免疫荧光实验检测证实所制备的小鼠抗血清能与哺乳动物细胞瞬时表达的SARS-CoV-2 N蛋白发生特异性结合。
冠状病毒的基因组虽然呈现遗传多样性,但N蛋白作为病毒核衣壳中关键的结构与功能蛋白,其功能结构域相对比较保守,含有2个高度碱性、独立折叠的结构域[分别称为氨基末端结构域(N-terminal domain,NTD)和羧基末端结构域(C-terminal domain,CTD)]及1个羧基末端的N3结构域[16-17]。研究表明,N蛋白是个多功能蛋白,其主要功能是使病毒基因组衣壳化,在病毒复制、组装和释放中均起着重要作用[18]。此外,N蛋白还可以调节宿主细胞的多项生理功能,如通过抑制宿主Ⅰ型干扰素的产生来干扰宿主免疫、上调促炎因子环氧酶2(cyclooxygenase 2,COX2)和CXC趋化因子配体10(CXC chemokine ligand 10,CXCL10)的产生、参与细胞信号转导和引起细胞周期失控等[10, 19-21]。我们通过对SARS-CoV和SARS-CoV-2这2种病毒编码的N蛋白序列进行比对分析,发现SARS-CoV-2 N蛋白出现了2处氨基酸残基缺失(Q10处缺失1个丝氨酸残基,S412处缺失1个GA二肽序列)和3个非近似氨基酸残基的变异[22]。这些突变对SARS-CoV-2 N蛋白相关的生物学功能的影响值得深入研究,但本研究结果提示这些氨基酸变异可能并不影响N蛋白的免疫原性。
与单克隆抗体相比,抗血清具有一定的优点,比如制备成本低、过程相对简单、周期短,并且它可以识别出相应抗原的多个抗原表位,从而增强靶蛋白的检测信号,提高检测的灵敏度。有研究指出,针对中东呼吸综合征(Middle East respiratory syndrome,MERS)患者血清中的抗体滴度测定显示,虽然基于N蛋白NTD抗原ELISA实验检测到的抗体出现时间较晚,但其测定结果与S蛋白ELISA实验测定结果呈显著的相关性[22]。SARS-CoV感染期间,可诱导机体产生大量的以IgG为主的抗体反应,且该反应主要针对N蛋白[23-24]。目前针对SARS-CoV-2感染的免疫应答研究非常有限,仅报道了1例COVID-19患者的N蛋白特异性抗体反应,其IgM抗体在疾病发作后第9天达到峰值,在第2周时转为IgG抗体[3],提示SARS-CoV-2的N蛋白也参与了机体的体液免疫。利用N蛋白所具有的强免疫原性,可进行SARS-CoV-2感染的临床检测。本研究为SARS-CoV-2抗原抗体检测提供了理论依据和技术支持,同时也为后续开展N蛋白的生物学功能及SARS-CoV-2基因组选择性包装相关研究提供了重要材料。
| [1] |
World Health Organization. Coronavirus disease (COVID-19) outbreak situation[EB/OL]. (2020-04-06)[2020-04-06]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
|
| [2] |
WU F, ZHAO S, YU B, CHEN Y M, WANG W, SONG Z G, et al. A new coronavirus associated with human respiratory disease in China[J]. Nature, 2020, 579: 265-269. DOI:10.1038/s41586-020-2008-3 |
| [3] |
ZHOU P, YANG X L, WANG X G, HU B, ZHANG L, ZHANG W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin[J]. Nature, 2020, 579: 270-273. DOI:10.1038/s41586-020-2012-7 |
| [4] |
ZHOU F, YU T, DU R, FAN G, LIU Y, LIU Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China:a retrospective cohort study[J]. Lancet, 2020, 395: 1054-1062. DOI:10.1016/S0140-6736(20)30566-3 |
| [5] |
LI Q, GUAN X, WU P, WANG X, ZHOU L, TONG Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia[J]. N Engl J Med, 2020, 382: 1199-1207. DOI:10.1056/NEJMoa2001316 |
| [6] |
HUANG C, WANG Y, LI X, REN L, ZHAO J, HU Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China[J]. Lancet, 2020, 395: 497-506. DOI:10.1016/S0140-6736(20)30183-5 |
| [7] |
LU R, ZHAO X, LI J, NIU P, YANG B, WU H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus:implications for virus origins and receptor binding[J]. Lancet, 2020, 395: 565-574. DOI:10.1016/S0140-6736(20)30251-8 |
| [8] |
CHAN J F, YUAN S, KOK K H, TO K K, CHU H, YANG J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission:a study of a family cluster[J]. Lancet, 2020, 395: 514-523. DOI:10.1016/S0140-6736(20)30154-9 |
| [9] |
SADASIVAN J, SINGH M, SARMA J D. Cytoplasmic tail of coronavirus spike protein has intracellular targeting signals[J]. J Biosci, 2017, 42: 231-244. DOI:10.1007/s12038-017-9676-7 |
| [10] |
ABOAGYE J O, YEW C W, NG O W, MONTEIL V M, MIRAZIMI A, TAN Y J. Overexpression of the nucleocapsid protein of Middle East respiratory syndrome coronavirus up-regulates CXCL10[J/OL]. Biosci Rep, 2018, 38: BSR20181059. doi: 10.1042/BSR20181059.
|
| [11] |
VERHEIJE M H, HAGEMEIJER M C, ULASLI M, REGGIORI F, ROTTIER P J, MASTERS P S, et al. The coronavirus nucleocapsid protein is dynamically associated with the replication-transcription complexes[J]. J Virol, 2010, 84: 11575-11579. DOI:10.1128/JVI.00569-10 |
| [12] |
DONG C, NI L, YE F, CHEN M L, FENG Y, DENG Y Q, et al. Characterization of anti-viral immunity in recovered individuals infected by SARS-CoV-2[J/OL]. medRxiv, 2020. doi: 10.1101/2020.03.17.20036640.
|
| [13] |
QIN Z L, ZHAO P, ZHANG X L, YU J G, CAO M M, ZHAO L J, et al. Silencing of SARS-CoV spike gene by small interfering RNA in HEK 293T cells[J]. Biochem Biophys Res Commun, 2004, 324: 1186-1193. DOI:10.1016/j.bbrc.2004.09.180 |
| [14] |
QIN Z L, ZHAO P, CAO M M, QI Z T. siRNAs targeting terminal sequences of the SARS-associated coronavirus membrane gene inhibit M protein expression through degradation of M mRNA[J]. J Virol Methods, 2007, 145: 146-154. DOI:10.1016/j.jviromet.2007.05.017 |
| [15] |
QIN Z L, JU H P, GAO T T, WANG W B, REN H, ZHAO P, et al. Two conserved histidines (His490 and His621) on the E2 glycoprotein of hepatitis C virus are critical for CD81-mediated cell entry[J]. J Gen Virol, 2015, 96: 1389-1399. DOI:10.1099/vir.0.000091 |
| [16] |
HUANG Q, YU L, PETROS A M, GUNASEKERA A, LIU Z, XU N, et al. Structure of the N-terminal RNA-binding domain of the SARS CoV nucleocapsid protein[J]. Biochemistry, 2004, 43: 6059-6063. DOI:10.1021/bi036155b |
| [17] |
LUO H, YE F, CHEN K, SHEN X, JIANG H. SR-rich motif plays a pivotal role in recombinant SARS coronavirus nucleocapsid protein multimerization[J]. Biochemistry, 2005, 44: 15351-15358. DOI:10.1021/bi051122c |
| [18] |
CHANG C K, HOU M H, CHANG C F, HSIAO C D, HUANG T H. The SARS coronavirus nucleocapsid protein-forms and functions[J]. Antiviral Res, 2014, 103: 39-50. DOI:10.1016/j.antiviral.2013.12.009 |
| [19] |
CUI L, WANG H Y, JI Y, YANG J, XU S, HUANG X, et al. The nucleocapsid protein of coronaviruses acts as a viral suppressor of RNA silencing in mammalian cells[J]. J Virol, 2015, 89: 9029-9043. DOI:10.1128/JVI.01331-15 |
| [20] |
MCBRIDE R, VAN ZYL M, FIELDING B C. The coronavirus nucleocapsid is a multifunctional protein[J]. Viruses, 2014, 6: 2991-3018. DOI:10.3390/v6082991 |
| [21] |
YAN X, HAO Q, MU Y, TIMANI K A, YE L, ZHU Y, et al. Nucleocapsid protein of SARS-CoV activates the expression of cyclooxygenase-2 by binding directly to regulatory elements for nuclear factor-kappa B and CCAAT/enhancer binding protein[J]. Int J Biochem Cell Biol, 2006, 38: 1417-1428. DOI:10.1016/j.biocel.2006.02.003 |
| [22] |
WANG W, WANG H, DENG Y, SONG T E, LAN J, WU G, et al. Characterization of anti-MERS-CoV antibodies against various recombinant structural antigens of MERS-CoV in an imported case in China[J/OL]. Emerg Microbes Infect, 2016, 5: e113. doi: 10.1038/emi.2016.114.
|
| [23] |
LEUNG D T, TAM F C, MA C H, CHAN P K, CHEUNG J L, NIU H, et al. Antibody response of patients with severe acute respiratory syndrome (SARS) targets the viral nucleocapsid[J]. J Infect Dis, 2004, 190: 379-386. DOI:10.1086/422040 |
| [24] |
WOO P C, LAU S K, WONG B H, TSOI H W, FUNG A M, CHAN K H, et al. Detection of specific antibodies to severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein for serodiagnosis of SARS coronavirus pneumonia[J]. J Clin Microbiol, 2004, 42: 2306-2309. DOI:10.1128/JCM.42.5.2306-2309.2004 |
 2020, Vol. 41
2020, Vol. 41


