2. 海军军医大学(第二军医大学)长海医院肾内科, 上海 200433;
3. 武汉市汉口医院消化科, 武汉 430014;
4. 解放军 905 医院呼吸内科, 上海 200052;
5. 海军军医大学(第二军医大学)长海医院消化内科, 上海 200433;
6. 海军军医大学(第二军医大学)长海医院呼吸与危重型医学科, 上海 200433
2. Department of Nephrology, Changhai Hospital, Naval Medical University(Second Military Medical University), Shanghai 200433, China;
3. Department of Gastroenterology, Hankou Hospital, Wuhan 430014, Hubei, China;
4. Department of Respiratory Medicine, No. 905 Hospital of PLA, Shanghai 200052, China;
5. Department of Gastroenterology, Changhai Hospital, Naval Medical University(Second Military Medical University), Shanghai 200433, China;
6. Department of Respiratory and Critical Care Medicine, Changhai Hospital, Naval Medical University(Second Military Medical University), Shanghai 200433, China
新型冠状病毒肺炎(coronavirus disease 2019,COVID-19)是由一种β属的新型冠状病毒感染的肺炎,其发病机制尚不清楚,病情进展快,容易引起严重急性呼吸窘迫综合征[1-3]。国际病毒分类委员会将这种新型冠状病毒命名为严重急性呼吸综合征冠状病毒2(severe acute respiratory syndrome coronavirus 2,SARS-CoV-2)。目前COVID-19临床治疗经验少,尤其是糖皮质激素的使用时机尚未找到明确的规律。现报道1例重型COVID-19应用糖皮质激素联合免疫球蛋白救治成功的病例并复习相关文献,分享重型COVID-19的临床治疗经验。
1 临床资料 1.1 诊断过程患者男,42岁,武汉市医务工作者。2020年1月16日起无明显诱因出现咳嗽,伴少许白黏痰,不易咳出,无胸痛、呼吸困难,无心慌、胸闷,无腹痛、腹泻,无恶心、呕吐等不适,未予处理。1月22日出现发热,最高38.5 ℃,伴全身酸痛、乏力,纳差,自行对症处理后于当日入住武汉市汉口医院治疗。平素体健,无烟酒嗜好,否认高血压病、糖尿病等慢性病史。起病前曾多次接触过COVID-19患者。
入院体格检查:体温36.8 ℃,脉搏85/min,呼吸频率20/min,血压120/80 mmHg(1 mmHg=0.133 kPa)。神志清,精神软,体表淋巴结未触及肿大,气管居中,双肺呼吸音粗,未闻及明显干湿性啰音,心率85/min,律齐,各瓣膜听诊区未闻及病理性杂音,腹平软,肝脾肋下未及,全腹无压痛及反跳痛,肠鸣音正常,双下肢无水肿。入院后实验室检查:白细胞计数4.3×109/L,淋巴细胞计数1.2×109/L,血红蛋白162 g/L,血小板计数213×109/L,丙氨酸转氨酶48 U/L,血清钠143 mmol/L,降钙素原0.11 ng/mL,超敏CRP 3.39 mg/L(正常参考值:0.00~6.00 mg/L),超敏肌钙蛋白T<3.00 ng/L,红细胞沉降率8.81 mm/1 h(正常参考值:0~15 mm/1 h),吸空气时脉搏血氧饱和度(pulse oxygen saturation,SpO2)98%。流感病毒、副流感病毒及肺炎支原体、衣原体等检测结果均为阴性。咽拭子检测(1月26日)显示SARS-CoV-2核酸阳性。入院后影像学检查:胸部CT示双肺散在少量磨玻璃样渗出影(图 1A)。诊断:COVID-19。
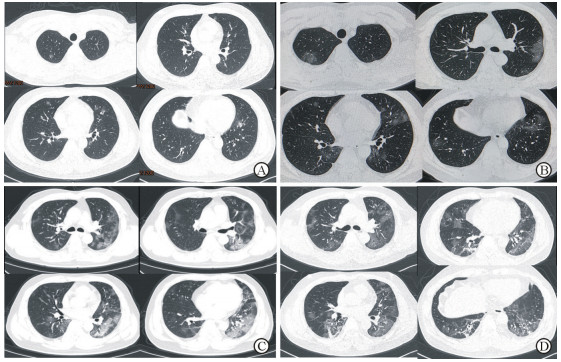
|
图 1 COVID-19患者诊治过程中胸部CT表现 Fig 1 Chest CT results of the COVID-19 patient A: CT results of the chest on January 22 showed a small amount of ground-glass exudation in both lungs; B: CT results of the chest on January 26 showed increased amount of exudation in both lungs; C: CT results of the chest on January 31 showed large amount of exudation in both lungs, more prominent in the left lung; D: CT results of the chest on February 3 showed that bilateral lung exudation was absorbed and local fibrosis was identified. COVID-19: Coronavirus disease 2019; CT: Computed tomography |
1.2 治疗经过
患者入院后予口服奥司他韦(75 mg,每天2次)、静脉输注莫西沙星(0.4 g,每天1次)和头孢哌酮舒巴坦钠(3 g,每12 h 1次)、加强营养等治疗。
患者入院后体温波动于37~38 ℃,其他症状无明显改变。1月26日患者出现胸闷气急,SpO2 94%(吸氧量3 L/min),复查胸部双肺CT提示渗出较前增加(图 1B)。1月28日下午患者体温最高38.4 ℃,气急加重,呼吸频率28/min,SpO2 93%(吸氧量5 L/min),复查血淋巴细胞计数0.7×109/L、超敏CRP 19.4 mg/L、降钙素原0.087 ng/mL、红细胞沉降率44.2 mm/1 h。予以输注甲泼尼龙(40 mg,每天1次)和人免疫球蛋白(10 g,每天1次)。1月29日患者体温正常,气急未好转。1月30日,患者体温40.7 ℃,气急进一步加重,呼吸频率35/min,SpO2 83%(吸氧量10 L/min),血白细胞计数11.2×109/L、淋巴细胞计数0.5×109/L、降钙素原0.202 ng/mL。调整甲泼尼龙剂量(40 mg,每12 h 1次)及人免疫球蛋白剂量(20 g,每天1次),增加胸腺法新(商品名:日达仙,1.6 mg,皮下注射,每天1次)等治疗,患者体温恢复正常,气急稍好转。1月31日患者SpO2 88%(吸氧量10 L/min),复查胸部CT提示双肺大片渗出影,左肺明显(图 1C)。2月1日患者SpO2 92%(吸氧量10 L/min),呼吸频率28/min。2月2日患者SpO2 95%(吸氧量5 L/min),呼吸频率25/min,甲泼尼龙减量至40 mg、每天1次。2月3日患者SpO2 94%(吸氧量3 L/min),血淋巴细胞计数0.3×109/L、超敏CRP正常,复查胸部CT提示肺部炎症较前好转(图 1D)。2月4日患者SpO2 98%(吸氧量3 L/min),甲泼尼龙减量至20 mg、每天1次;2月5日患者SpO2 94%,呼吸困难明显好转,人免疫球蛋白减量至10 g、每天1次。自1月31日起患者体温连续3 d以上正常(图 2),胸闷气急好转。2月4日及2月9日复查咽拭子均显示SARS-CoV-2核酸阴性。
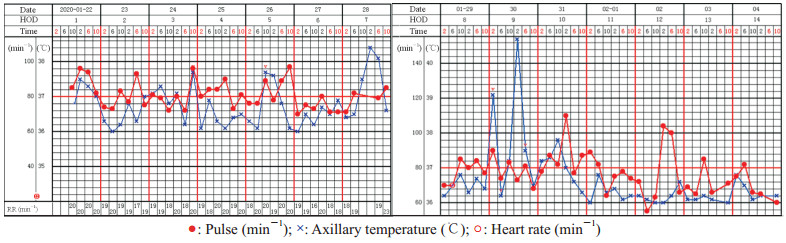
|
图 2 COVID-19患者住院期间的体温变化 Fig 2 Body temperature of the COVID-19 patient during hospitalization COVID-19: Coronavirus disease 2019; HOD: Hospitalization day; RR: Respiratory rate |
2 讨论
SARS-CoV-2在人群中普遍易感,病毒感染导致肺炎后,首先引起肺细胞水肿、细胞间隙通透性增加,进而引起组织水肿和蛋白渗出、呼吸膜增厚,影响换气功能;患者早期可出现Ⅰ型呼吸衰竭,随着疾病的进展,肺泡渗出增多,呼吸衰竭进一步加重,严重者可导致死亡[1-3]。COVID-19的病理特点和损伤机制与2003年暴发的严重急性呼吸综合征[4]类似。目前尚无循证学证据支持现有的抗病毒药物对SARS-CoV-2确切有效[5]。加强对症支持、适当给予糖皮质激素、出现呼吸衰竭时予氧疗且必要时机械通气支持是目前COVID-19治疗的主要方法[6]。
关于糖皮质激素在重型病毒性肺炎治疗中的使用时机及剂量问题,目前尚存在争议[7]。根据既往救治流感病毒感染肺炎的经验,糖皮质激素的使用时机非常重要,早期及轻型患者不提倡使用糖皮质激素,在严重急性呼吸综合征、中东呼吸综合征救治中也不建议早期使用糖皮质激素治疗[8-11]。盲目使用糖皮质激素可抑制患者免疫功能,导致病毒负载增加,阻碍机体对病毒的正常清除作用[12-13]。
重型COVID-19患者临床表现为发热、胸闷气喘、氧饱和度下降、胸部影像学快速进展等,尸体解剖报告也发现危重型COVID-19患者体内CD4+/CD8+ T淋巴细胞过度激活,人白细胞DR抗原(human leukocyte antigen DR,HLA-DR)高表达和高度促炎性的Th17细胞增加[14],提示机体免疫反应过强,可能会带来严重的附加损害,此时可考虑使用糖皮质激素,抑制过强的免疫反应[15]。对于糖皮质激素的使用剂量,国家卫生健康委员会和国家中医药管理局发布的《新型冠状病毒感染的肺炎诊疗方案》(试行第六版)推荐使用甲泼尼龙1~2 mg/(kg • d)、疗程3~5 d[6]。
本例COVID-19患者入院时症状未加重,病情稳定,但淋巴细胞数量快速下降,影像学显示患者肺部病灶持续进展加重, 病程第7天体温再次升高并且气急加重,吸氧情况下SpO2下降至93%,患者已进展到重型,这可能与患者免疫功能继续下降、病毒复制加速有关,根据最新指南诊断推荐可使用激素治疗[6]。2020年1月30日,患者病情急剧恶化,体温达40.7 ℃,吸氧量10 L/min时SpO2只有83%,提示体内免疫反应过激,此时应增加糖皮质激素剂量。但患者淋巴细胞计数极低,过量使用糖皮质激素会进一步抑制免疫功能,影响预后。此时,可考虑同时联合使用大剂量的免疫球蛋白,平衡过高抗体水平,同时保持免疫功能无明显下降,减少继发感染风险[16-17]。另外,本例患者淋巴细胞计数呈进行性下降,可能与病情进展及糖皮质激素使用后免疫抑制有关[12]。淋巴细胞功能与数量是抗病毒的关键,也是与患者预后密切相关的重要因素[18],为此,我们加用胸腺法新治疗,以增加T淋巴细胞在各种抗原或致有丝分裂原激活后产生的各种淋巴因子,增强淋巴细胞功能[19],改善临床疗效。
通过该病例的救治,我们初步认为在COVID-19治疗中,早期及轻型患者应尽量避免使用糖皮质激素,当患者出现呼吸衰竭失代偿时可以适当使用糖皮质激素降低免疫指标,但剂量不宜过高,必要时可予大剂量免疫球蛋白帮助平衡免疫功能。同时对淋巴细胞数量过低的患者,使用淋巴细胞功能增加剂可能是有益的策略。我们建议,对轻型和普通型COVID-19尽量不使用糖皮质激素,以对症支持和加强营养为主;对重型患者可使用甲泼尼龙0.5~1 mg/(kg • d);对危重型患者可使用甲泼尼龙1~2 mg/(kg • d),同时联合使用人免疫球蛋白10~20 g/d,疗程5~7 d。
| [1] |
ZHU N, ZHANG D, WANG W, LI X, YANG B, SONG J, et al. A novel coronavirus from patients with pneumonia in China, 2019[J/OL]. N Engl J Med, 2020. doi: 10.1056/NEJMoa2001017.
|
| [2] |
PAULES C I, MARSTON H, FAUCI A S. Coronavirus infections-more than just the common cold[J/OL]. JAMA, 2020. doi: 10.1001/jama.2020.0757.
|
| [3] |
MAHASE E. China coronavirus: what do we know so far?[J/OL]. BMJ, 2020, 368: m308. doi: 10.1136/bmj.m308.
|
| [4] |
纪小龙, 尹彤, 申明识. 从SARS患者肺部病变的病理特点探讨SARS的损伤机理[J]. 中华微生物学和免疫学杂志, 2003, 23: 4-7. |
| [5] |
LU H. Drug treatment options for the 2019-new coronavirus (2019-nCoV)[J/OL]. Biosci Trends, 2020.doi: 10.5582/bst.2020.01020.6.
|
| [6] |
国家卫生健康委员会, 国家中医药管理局.新型冠状病毒感染的肺炎诊疗方案(试行第六版)[S/OL].(2020-02-18)[2020-02-18]. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf.
|
| [7] |
YANG J W, FAN L C, MIAO X Y, MAO B, LI M H, LU H W, et al. Corticosteroids for the treatment of human infection with influenza virus: a systematic review and meta-analysis[J]. Clin Microbiol Infect, 2015, 21: 956-963. DOI:10.1016/j.cmi.2015.06.022 |
| [8] |
HUI D S. Systemic corticosteroid therapy may delay viral clearance in patients with Middle East respiratory syndrome coronavirus infection[J]. Am J Respir Crit Care Med, 2018, 197: 700-701. DOI:10.1164/rccm.201712-2371ED |
| [9] |
ZHAO R, WANG H, WANG X, FENG F. Steroid therapy and the risk of osteonecrosis in SARS patients: a dose-response meta-analysis[J]. Osteoporos Int, 2017, 28: 1027-1034. DOI:10.1007/s00198-016-3824-z |
| [10] |
STOCKMAN L J, BELLAMY R, GARNER P. SARS: systematic review of treatment effects[J/OL]. PLoS Med, 2006, 3: e343. doi: 10.1371/journal.pmed.0030343.
|
| [11] |
LEE N, ALLEN CHAN K C, HUI D S, NG E K, WU A, CHIU R W, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients[J]. J Clin Virol, 2004, 31: 304-309. DOI:10.1016/j.jcv.2004.07.006 |
| [12] |
MARTIN-LOECHES I, LISBOA T, RHODES A, MORENO R P, SILVA E, SPRUNG C, et al. Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection[J]. Intensive Care Med, 2011, 37: 272-283. DOI:10.1007/s00134-010-2078-z |
| [13] |
BRUN-BUISSON C, RICHARD J C, MERCAT A, THIÉBAUT A C, BROCHARD L, REVA-SRLF A/H1N1v 2009 Registry Group. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome[J]. Am J Respir Crit Care Med, 2011, 183: 1200-1206. DOI:10.1164/rccm.201101-0135OC |
| [14] |
XU Z, SHI L, WANG Y, ZHANG J, HUANG L, ZHANG C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome[J/OL]. Lancet Respir Med, 2020. doi: 10.1016/s2213-2600(20)30076-x.
|
| [15] |
PEIRIS J S, CHU C M, CHENG V C, CHAN K S, HUNG I F, POON L L, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study[J]. Lancet, 2003, 361: 1767-1772. DOI:10.1016/S0140-6736(03)13412-5 |
| [16] |
DANDACHI D, RODRIGUEZ-BARRADAS M C. Viral pneumonia: etiologies and treatment[J]. J Investig Med, 2018, 66: 957-965. DOI:10.1136/jim-2018-000712 |
| [17] |
RUUSKANEN O, LAHTI E, JENNINGS L C, MURDOCH D R. Viral pneumonia[J]. Lancet, 2011, 377: 1264-1275. DOI:10.1016/S0140-6736(10)61459-6 |
| [18] |
CHEN N, ZHOU M, DONG X, QU J, GONG F, HAN Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study[J]. Lancet, 2020, 395: 15-21. DOI:10.1016/S0140-6736(19)33176-9 |
| [19] |
KING R, TUTHILL C. Immune modulation with thymosin alpha 1 treatment[J]. Vitam Horm, 2016, 102: 151-178. DOI:10.1016/bs.vh.2016.04.003 |
 2020, Vol. 41
2020, Vol. 41


