2. 南方医科大学南方医院增城分院药学部, 广州 511300;
3. 南方医科大学南方医院药剂科, 广州 510515
2. Department of Pharmacy, Zengcheng Branch of Nanfang Hospital, Southern Medical University, Guangzhou 511330, Guangdong, China;
3. Department of Pharmacy, Nanfang Hospital, Southern Medical University, Guangzhou 510515, Guangdong, China
急性淋巴细胞白血病(acute lymphoblastic leukemia,ALL)是一种T、B淋巴细胞在骨髓、血液或组织中异常增殖和蓄积的恶性血液肿瘤。依据血液表型的特点,一般分为T-ALL和B-ALL 2种类型,其中B-ALL占80%~85%[1]。儿童和成人均可发生ALL,其中2~5岁儿童是高发对象[2-3]。目前儿童治愈率已达90%,但成人治愈率还不到40%[4-5],且难治性/复发ALL仍然存在预后不佳的问题。对于难治性/复发ALL,儿童5年生存率为(21.0±1.8)%,成人不到7%[6-7]。因此,开发新治疗策略尽快改善难治性/复发ALL预后仍然是国内外研究的热点。
作为第1代蛋白酶体抑制剂,硼替佐米是一种可逆性的二肽基硼酸盐类似物,已被美国FDA批准用于治疗多发性骨髓瘤(2003年)和套细胞淋巴瘤(2006年)[8]。近年,硼替佐米被报道可作为ALL治疗的新选择[2]。尽管硼替佐米在ALL的临床前研究中显示了良好的效果[9-11],但是在对ALL患者的临床治疗中,单独使用硼替佐米的效果仍欠佳[12]。有临床前研究表明,硼替佐米联用其他一些化学治疗药物如地塞米松、天冬酰胺酶、长春新碱、多柔比星、阿糖胞苷,对急性白血病有良好的协同效果[13],但具体机制仍在研究中。
泛素-蛋白酶体通路(ubiquitin-proteasome pathway,UPP)负责约80%蛋白的降解,涉及细胞内多项重要的生理功能,特别是对细胞增殖和凋亡的调控。研究表明,硼替佐米可调控p53、p21Cip1、p27Kip1、Bax和IκB等细胞周期和凋亡蛋白[14-18]。硼替佐米的抗肿瘤机制是通过抑制26S蛋白酶体活性阻断UPP[19-20]。
诱导细胞凋亡是抗肿瘤药物的一种常规治疗策略[21]。Bcl-2是研究最早的与细胞凋亡相关的一类原癌基因。Bcl-2家族蛋白主要分为抗凋亡蛋白[Bcl-2、Bcl-xL、Bcl-w和髓细胞白血病因子1(myeloid cell leukemia 1,Mcl-1)等]和促凋亡蛋白(Bax、Bak、Bok和Bim等),通过调节抗/促凋亡蛋白的表达水平调控细胞凋亡。此外,越来越多的资料表明硼替佐米可通过激活Bcl-2家族促凋亡蛋白的表达发挥抗肿瘤活性,如BH3-only、Noxa、Puma和Bim[22-23]。但也有研究表明,某些抗凋亡蛋白如Mcl-1的蓄积可降低肿瘤细胞对硼替佐米的敏感性,从而产生耐药。目前,Bcl-2抑制剂obatoclax联合硼替佐米治疗难治性/复发套细胞淋巴瘤的研究正在开展Ⅰ/Ⅱ期临床试验[18]。已有证据表明,硼替佐米联合多种化学治疗药物疗效更佳[24-25]。但是,硼替佐米联合Bcl-2抑制剂在急性B淋巴细胞白血病细胞上的研究甚少,两者是否具有协同细胞毒性作用尚不清楚。因此,本研究拟探究硼替佐米分别联用3种Bcl-2抑制剂(obatoclax、AT-101、ABT-199)是否协同诱导人急性B淋巴细胞白血病细胞株Nalm-6细胞凋亡。
1 材料和方法 1.1 药物地塞米松、牛磺熊去氧胆酸(tauroursodeoxycholate acid,TUDCA)和3种Bcl-2抑制剂(obatoclax、AT-101、ABT-199)均购于美国Selleck Chemicals公司,其他试剂购于美国Sigma-Aldrich公司。
1.2 细胞株来源与培养人急性B淋巴细胞白血病细胞株Nalm-6由南方医科大学南方医院血液科提供。细胞培养条件:37 ℃、5% CO2培养箱。培养液配制:450 mL RPMI 1640培养液(美国Invitrogen公司)+50 mL FBS(美国Invitrogen公司)+5 mL青/链霉素(美国Invitrogen公司)。
1.3 细胞活力实验用MTT比色法检测药物处理后细胞的存活率。Nalm-6细胞接种过夜后加入不同浓度的药物持续培养48 h,加入终浓度为0.5 mg/mL的MTT共孵育4 h。用GENios Pro型酶标仪(美国Tecan公司)在570 nm波长处测定光密度(D)值。细胞存活率计算公式为:细胞存活率(%)=(药物组D值-空白组D值)/(未加药物组D值-空白组D值)×100%。细胞抑制率(%)=100%-细胞存活率(%)。用GraphPad Prism 5.0软件和CompuSyn药物联合指数分析软件分别计算IC50和药物联用指数(combination index,CI)。
1.4 Annexin Ⅴ-FITC染色检测细胞凋亡不同浓度的药物单独或联合作用于Nalm-6细胞48 h后停止培养,收集细胞于离心管中,用预冷的PBS重悬洗2次。按照Annexin Ⅴ-FITC细胞凋亡检测试剂盒(日本同仁化学研究所)操作流程,加入Annexin Ⅴ-FITC后避光室温孵育15 min。用流式细胞仪(BD FACSCantoⅡ)检测细胞凋亡情况。同时用地塞米松作为细胞凋亡阳性对照药物,浓度为180 μmol/L。
1.5 蛋白质印迹分析不同浓度的药物作用于Nalm-6细胞相应时间后,用PBS洗2次并加入含蛋白酶体/磷酸酶抑制剂(美国Merck公司)的RIPA裂解液。等量的蛋白行SDS-PAGE,然后转至PVDF膜(美国Roche公司)。所有一抗4 ℃孵育至少12 h,二抗室温孵育1 h。化学发光液和X-线胶片分别购于美国CST公司和日本富士胶卷公司。抗体来源:Mcl-1(#5453)、Bcl-2(#2870)、Bcl-xL(#2764)、微管相关蛋白1轻链3B(microtubule-associated protein 1 light chain 3B,LC3B;#2775)、p62(#8025)、泛素(#3936)、免疫球蛋白结合蛋白(binding immunoglobulin protein,Bip;#3177S)、磷酸化p38(phosphorylated p38,p-p38;#9212)、磷酸化JNK(phosphorylated JNK,p-JNK;#9251)、C/EBP同源蛋白(C/EBP homologous protein,CHOP;#5554)、内参蛋白β-actin(#4970)均购于美国CST公司,内参蛋白黏着斑蛋白(vinculin,#036M4797V)购于美国Sigma-Aldrich公司,内参蛋白GAPDH(#25778)购于美国Santa Cruz公司。
1.6 qRT-PCR检测硼替佐米和obatoclax的药物浓度分别为2 nmol/L、0.3 μmol/L。加入1 mL TRIzol(美国Invitrogen公司)裂解液提取RNA;按照PrimeScript反转录试剂盒(日本TaKaRa公司)说明书合成cDNA;利用SYBR Select预混液(美国Applied Biosystems公司)标记,放入荧光定量PCR仪(7500型,美国ABI公司)中反应。反应条件:95 ℃变性15 s(1个循环),退火/延伸60 ℃ 60 s(40个循环)。采用2-ΔΔCt法分析目的基因的相对表达水平。引物序列来源于qPrimerDepot数据库,见表 1。
|
|
表 1 引物序列 Tab 1 Primer sequences of genes |
1.7 统计学处理
应用GraphPad Prism 5.0软件进行统计学分析。各实验数据均重复多次,结果以x±s表示,采用方差分析和Tukey’s t检验进行比较。检验水准(α)为0.05。
2 结果 2.1 硼替佐米抑制了Nalm-6细胞的活力不同浓度的硼替佐米与Nalm-6细胞共孵育48 h后,用MTT法检测细胞活力,结果显示随着硼替佐米浓度的增加,Nalm-6细胞的活力逐渐降低,硼替佐米的IC50为(4.5±0.3)nmol/L(图 1)。
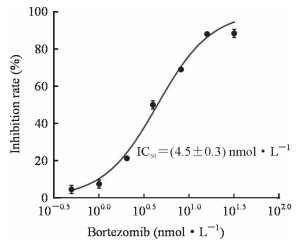
|
图 1 硼替佐米对人急性B淋巴细胞白血病细胞株Nalm-6的毒性作用 Fig 1 Cytotoxic effect of bortezomib on human acute B lymphoblastic leukemia cell line Nalm-6 The IC50 value was calculated by GraphPad Prism 5.0 software. IC50: Half inhibition concentration. n=3, x±s |
2.2 硼替佐米单独处理Nalm-6细胞引起抗凋亡蛋白的蓄积
4 nmol/L硼替佐米处理Nalm-6细胞3 h后,抗凋亡蛋白Mcl-1的蓄积明显增加,Bcl-2轻微上升,Bcl-xL变化不明显(图 2)。

|
图 2 硼替佐米单独处理人急性B淋巴细胞白血病细胞株Nalm-6引起抗凋亡蛋白的蓄积 Fig 2 Accumulation of anti-apoptotic proteins by bortezomib in human acute B lymphoblastic leukemia cell line Nalm-6 Mcl-1: Myeloid cell leukemia 1; Bcl-2: B-cell lymphoma 2; Bcl-xL: B-cell lymphoma-extra large |
2.3 硼替佐米与obatoclax联用对Nalm-6细胞具有协同细胞毒性作用
不同浓度的Bcl-2抑制剂与Nalm-6细胞共孵育48 h,用MTT法检测细胞的存活率。结果显示,Nalm-6细胞的活力随药物剂量的增加而降低;根据CompuSyn软件计算得出,硼替佐米与obatoclax联用的CI<1,提示两药联用有协同作用;而AT-101、ABT-199与硼替佐米联用的CI基本上均>1,提示两药联用没有协同作用(图 3A、3B)。单用硼替佐米或obatoclax均能诱导Nalm-6细胞凋亡,联用后凋亡细胞数明显上升(P均<0.01,图 3C)。
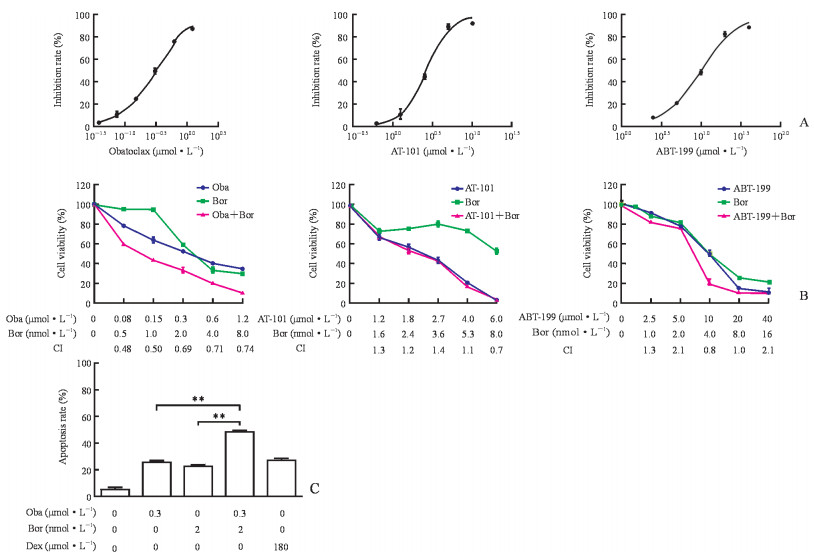
|
图 3 硼替佐米与obatoclax联用对人急性B淋巴细胞白血病细胞株Nalm-6具有协同细胞毒性作用 Fig 3 Obatoclax potentiated the cytotoxicity of human acute B lymphoblastic leukemia cell line Nalm-6 in response to bortezomib A: Cytotoxic effect of obatoclax (IC50=[0.3±0.1]μmol/L), AT-101 (IC50=[3.5±1.5]μmol/L), or ABT-199 (IC50=[9.6±1.6]μmol/L) on Nalm-6 cells. The IC50 value was calculated by GraphPad Prism 5.0 software. B: Cell viability of bortezomib plus obatoclax, AT-101, or ABT-199 was measured by MTT assay (CompuSyn software was used to calculate CI of the two-drug combination, where CI < 1, CI=1, CI > 1, indicate synergism, additive effect and antagonism, respectively). C: Cellular apoptosis was assessed by phosphatidylserine externalization, and cells were stained with Annexin Ⅴ-FITC. Oba: Obatoclax; Bor: Bortezomib; CI: Combination index; Dex: Dexamethasone (positive control); IC50: Half inhibition concentration; FITC: Fluorescin isothiocyanate. **P < 0.01. n=3, x±s |
2.4 Obatoclax抑制Nalm-6细胞的自噬活性
3种Bcl-2抑制剂(obatoclax、AT-101、ABT-199)均可上调Nalm-6细胞中自噬标志蛋白LC3B-Ⅱ的表达,而p62蛋白的蓄积仅在obatoclax作用的细胞中观察到(图 4A)。结果表明obatoclax可下调Nalm-6细胞的自噬活性,ABT-199、AT-101则能够提高自噬活性。同时,obatoclax单用可引起泛素化蛋白的蓄积,与硼替佐米联用后泛素化蛋白蓄积显著增加(图 4B)。

|
图 4 Bcl-2抑制剂对人急性B淋巴细胞白血病细胞株Nalm-6自噬相关蛋白的影响 Fig 4 Effect of Bcl-2 inhibitors on autophagy-related proteins in human acute B lymphoblastic leukemia cell line Nalm-6 A: Conversion of LC3B-Ⅰ to LC3B-Ⅱ and p62 protein expression levels were determined by Western blotting; B: Polyunbiquitinated protein was measured by Western blotting. LC3B: Microtubule-associated protein 1 light chain 3B; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase |
2.5 硼替佐米联用obatoclax诱导内质网应激反应并启动末端凋亡通路
Bip是内质网应激反应的标志蛋白,obatoclax单独作用Nalm-6细胞24 h后,Bip表达轻微上升,硼替佐米单独作用对Bip并无影响,但是硼替佐米与obatoclax联用后Bip表达明显升高,而硼替佐米与AT-101或ABT-199联用并未观察到此现象。p-JNK可启动细胞凋亡,obatoclax单用可引起JNK磷酸化水平增高,联用硼替佐米后JNK磷酸化水平增高更为明显;而AT-101、ABT-199单用或与硼替佐米联用后JNK磷酸化水平变化不明显。相反,这几种药物单用或联用均未能改变p38磷酸化水平。见图 5A。
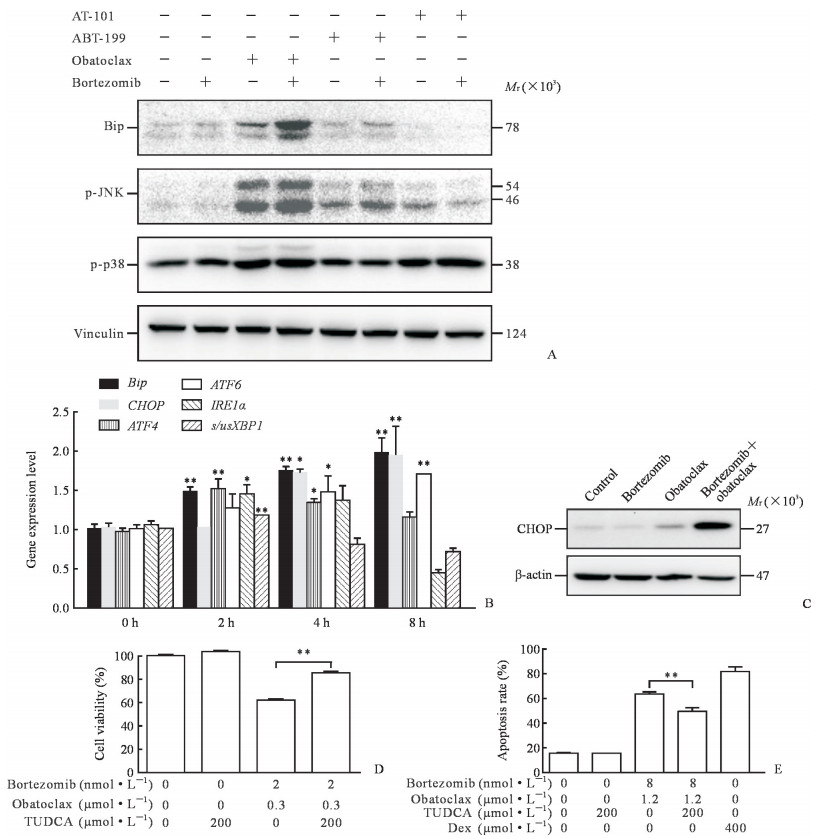
|
图 5 硼替佐米联用obatoclax诱导内质网应激反应并启动末端凋亡通路 Fig 5 Combination of bortezomib with obatoclax induced endoplasmic reticulum stress and terminal pro-apoptotic endoplasmic reticulum stress response A: Protein levels of Bip, p-JNK, p-p38 were detected by Western blotting; B: mRNA levels of Bip, CHOP, ATF4, ATF6, IRE1α and s/usXBP1 were measured by qRT-PCR (*P < 0.05, **P < 0.01 vs 0 h. n=3, x±s); C: Protein level of CHOP was determined by Western blotting; D: Cell viability was determined by MTT assay (**P < 0.01. n=3, x±s); E: Apoptosis rate was detected by flow cytometry (**P < 0.01. n=3, x±s). Bip: Binding immunoglobulin protein; p-JNK: Phosphorylated c-Jun N-terminal kinase; p-p38: Phosphorylated p38; CHOP: C/EBP homologous protein; ATF: Activating transcription factor; IRE1α: Inositol-requiring enzyme 1α; s/usXBP1: Splicing/un-splicing X-box binding protein 1; TUDCA: Tauroursodeoxycholate acid; Dex: Dexamethasone (positive control) |
硼替佐米和obatoclax联合处理Nalm-6细胞后,qRT-PCR检测发现Bip、CHOP、活化转录因子(activating transportion factor,ATF4)、ATF6、肌醇需求酶1α(inositol-requiring enzyme 1α,IRE1α)和剪接型/非剪接型X-盒结合蛋白1(splicing/un-splicing X-box binding protein 1,s/usXBP1)等内质网应激反应关键基因的mRNA表达水平均升高(图 5B),说明内质网应激被激活。未受刺激的细胞中基本不表达CHOP,其蛋白表达水平增高是内质网应激诱导凋亡的标志之一,单药并不影响CHOP表达,但是硼替佐米和obatoclax联用后,CHOP蛋白的表达水平明显上升(图 5C)。
MTT实验结果显示,TUDCA对Nalm-6细胞没有毒性,但可明显提高硼替佐米与obatoclax联用组细胞的活性(P<0.01,图 5D);流式细胞术结果与此一致,TUDCA显著降低联用组诱导的细胞凋亡(P<0.01,图 5E)。
3 讨论临床上ALL治疗的经典药物主要开发于20世纪80年代,至今使用已超过30年,近十年研发的新药主要有蛋白酶体抑制剂、雷帕霉素靶蛋白抑制剂、Bcl-2抑制剂、丝裂原活化蛋白激酶激酶抑制剂等[2, 26]。
本实验结果显示硼替佐米、obatoclax、AT-101和ABT-199均可明显抑制人急性B淋巴细胞白血病细胞株Nalm-6的细胞活力。值得思考的是,仅硼替佐米与obatoclax联用才具有协同细胞毒性作用。
硼替佐米抑制了蛋白酶体活性,蛋白降解受阻,这必然会引起一些不必要蛋白的蓄积。已有多项研究证明,硼替佐米可增加Mcl-1蛋白的表达水平[27-29]。本研究发现,硼替佐米单独作用Nalm-6细胞3 h后,Mcl-1蛋白明显上调,Bcl-2蛋白有轻微上升,Bcl-xL蛋白水平基本不变。Fennell等[22]的研究也表明,蛋白酶体抑制剂处理细胞数小时后,Mcl-1表达明显增加。当硼替佐米分别联用3种Bcl-2抑制剂(obatoclax、AT-101和ABT-199)时,出乎意料的结果是,只有obatoclax联用硼替佐米才具有协同细胞毒性作用,而其他2种Bcl-2抑制剂的联用均未观察到相同现象。Obatoclax、AT-101和ABT-199三者是目前研究较为广泛的Bcl-2抑制剂,最经典的作用机制是通过调控Bcl-2等抗/促凋亡蛋白之间的平衡,干扰线粒体膜的完整性,激活内源性途径介导细胞凋亡。Obatoclax、AT-101均可以结合并抑制所有的抗凋亡蛋白,但是AT-101联用硼替佐米并没有发现协同细胞毒性作用。而ABT-199(又名维奈托克)是目前首个蛋白-蛋白相互作用抑制剂,已被批准用于治疗17p缺失突变型慢性淋巴细胞白血病,其与硼替佐米联用同样没有显示出协同细胞毒性作用。因此,抗促凋亡蛋白介导线粒体凋亡并不能完全解释以上研究结果,提示还存在其他协同机制。
我们前期的研究发现,obatoclax作用于人食管癌细胞3 h后,可聚集在溶酶体中并干扰其功能,同时下调组织蛋白酶活性,阻断自噬流[30]。随后,其他实验室也发现obatoclax可影响溶酶体的功能[31-32]。自噬具有双重作用,既可促进细胞生存也可促进细胞死亡。有研究表明,obatoclax诱导的自噬可能与细胞死亡密切相关,当通过基因敲除手段或药理性方法抑制自噬后,发现细胞死亡受到抑制[33-38]。接下来,我们进一步分析发现,obatoclax可增加自噬标志蛋白LC3B-Ⅱ的表达,同时上调p62蛋白的表达,提示自噬流被阻断,自噬活性降低。而在AT-101、ABT-199中并未观察到此现象。自噬和UPP是真核细胞内的两大蛋白降解通路[39]。越来越多的资料证明,自噬和蛋白酶体之间是互补的,当蛋白酶体通路降解受阻时,自噬可被激活,两者相互调节细胞内蛋白降解[40-41]。本实验结果显示,两药联用后泛素蓄积比单用更明显。以上结果表明,obatoclax和硼替佐米共同处理Nalm-6细胞后,自噬和UPP通路两条通路均被阻断,造成大量的泛素化蛋白蓄积。
蛋白的大量蓄积会给内质网带来一定的压力,此时细胞会激活内质网应激反应来自我平衡。只有正确折叠的蛋白才能进入高尔基体修饰,而错误折叠的蛋白需经UPP降解[42]。蛋白酶体抑制剂通过增加错误蛋白的蓄积触发内质网应激从而诱导细胞凋亡。根据前期的实验结果,我们大胆猜测,硼替佐米联用obatoclax通过双重阻断UPP和自噬诱发了内质网应激。
细胞可通过4条不同的途径缓解内质网应激并恢复内质网功能[43]。第一,压力开始前数小时,细胞主要抑制翻译过程,减少新蛋白在内质网上蓄积直至未折叠蛋白反应(unfolded protein response,UPR)的相关蛋白被加工[44]。第二,UPR上调内质网分子伴侣蛋白的表达,如Bip。第三,通过内质网相关性降解途径来降解错误或未折叠蛋白[45-46]。最后,如以上机制仍无法缓解压力,细胞将启动内质网相关程序性死亡[47]。从实验结果中我们可以看到,单用硼替佐米和obatoclax基本不改变Bip蛋白表达,但是两药联用后Bip表达明显上调。当蛋白持续蓄积造成内质网压力过大时,细胞将触发内质网末端凋亡通路。而CHOP的激活转录和JNK的磷酸化均标志着内质网应激反应末端凋亡通路被激活[47]。与预期结果一致的是,我们观察到两药联用后可明显上调p-JNK和CHOP蛋白的表达水平。这些结果均表明,硼替佐米联用obatoclax触发了内质网应激从而诱导了细胞凋亡。为了进一步证明内质网应激在其中扮演的角色,我们在联合用药细胞组中加入适当浓度的TUDCA。TUDCA是一种常见内质网应激反应抑制剂,在体内外均有活性并且已被美国FDA批准上市。它的作用机制主要是促进蛋白质的正确折叠,稳定蛋白质折叠中间体并阻止蛋白质的聚合,从而抑制内质网应激的激活。我们发现TUDCA提高了药物联用组细胞活性,减少了细胞凋亡,结果与预期一致,这可能是TUDCA发挥了其促进蛋白正确折叠等相关功能,相对降低了细胞内蛋白的蓄积量,从而减缓了内质网应激诱导的凋亡。
根据多个实验室及我们的研究,蛋白酶体抑制剂联用Bcl-2抑制剂在多种血液恶性肿瘤中均发现有协同作用,包括套细胞淋巴瘤、多发性骨髓瘤、弥漫性大B细胞淋巴瘤等[48-50]。另外,硼替佐米联合多种抗肿瘤药物对ALL更为敏感,如地塞米松、天冬酰胺酶、长春新碱、多柔比星、阿糖胞苷[13],但其内在协同机制仍不清晰。目前,硼替佐米和obatoclax联用治疗ALL的研究尚未见报道,其内在协同机制更不清楚。本研究发现硼替佐米联用obatoclax可双重阻断自噬和UPP,造成大量蛋白蓄积并触发内质网应激,最终引起细胞凋亡。这些结果表明硼替佐米与obatoclax联用有望成为ALL治疗的新策略之一。
| [1] |
SIEGEL R L, MILLER K D, JEMAL A. Cancer statistics, 2017[J]. CA Cancer J Clin, 2017, 67: 7-30. DOI:10.3322/caac.21387 |
| [2] |
MAN L M, MORRIS A L, KENG M. New therapeutic strategies in acute lymphocytic leukemia[J]. Curr Hematol Malig Rep, 2017, 12: 197-206. DOI:10.1007/s11899-017-0380-3 |
| [3] |
PUI C H, ROBISON L L, LOOK A T. Acute lymphoblastic leukaemia[J]. Lancet, 2008, 371: 1030-1043. DOI:10.1016/S0140-6736(08)60457-2 |
| [4] |
BUONTEMPO F, ORSINI E, LONETTI A, CAPPELLINI A, CHIARINI F, EVANGELISTI C, et al. Synergistic cytotoxic effects of bortezomib and CK2 inhibitor CX-4945 in acute lymphoblastic leukemia: turning off the prosurvival ER chaperone BIP/Grp78 and turning on the pro-apoptotic NF-kappaB[J]. Oncotarget, 2016, 7: 1323-1340. |
| [5] |
MÖRICKE A1, REITER A, ZIMMERMANN M, GADNER H, STANULLA M, DÖRDELMANN M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2 169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95[J]. Blood, 2008, 111: 4477-4489. DOI:10.1182/blood-2007-09-112920 |
| [6] |
NGUYEN K, DEVIDAS M, CHENG S C, LA M, RAETZ E A, CARROLL W L, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study[J]. Leukemia, 2008, 22: 2142-2150. DOI:10.1038/leu.2008.251 |
| [7] |
DINNER S, LEE D, LIEDTKE M. Current therapy and novel agents for relapsed or refractory acute lymphoblastic leukemia[J]. Leuk Lymphoma, 2014, 55: 1715-1724. DOI:10.3109/10428194.2013.856428 |
| [8] |
CHAUHAN D, SINGH A, BRAHMANDAM M, PODAR K, HIDESHIMA T, RICHARDSON P, et al. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma[J]. Blood, 2008, 111: 1654-1664. DOI:10.1182/blood-2007-08-105601 |
| [9] |
MESSINGER Y, GAYNON P, RAETZ E, HUTCHINSON R, DUBOIS S, GLADE-BENDER J, et al. Phase Ⅰ study of bortezomib combined with chemotherapy in children with relapsed childhood acute lymphoblastic leukemia (ALL): a report from the therapeutic advances in childhood leukemia (TACL) consortium[J]. Pediatr Blood Cancer, 2010, 55: 254-259. DOI:10.1002/pbc.22456 |
| [10] |
MESSINGER Y H, GAYNON P S, SPOSTO R, VAN DER GIESSEN J, ECKROTH E, MALVAR J, et al. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study[J]. Blood, 2012, 120: 285-290. |
| [11] |
HOUGHTON P J, MORTON C L, KOLB E A, LOCK R, CAROL H, REYNOLDS C P, et al. Initial testing (stage 1) of the proteasome inhibitor bortezomib by the pediatric preclinical testing program[J]. Pediatr Blood Cancer, 2008, 50: 37-45. DOI:10.1002/pbc.21214 |
| [12] |
CORTES J, THOMAS D, KOLLER C, GILES F, ESTEY E, FADERL S, et al. Phase Ⅰ study of bortezomib in refractory or relapsed acute leukemias[J]. Clin Cancer Res, 2004, 10: 3371-3376. DOI:10.1158/1078-0432.CCR-03-0508 |
| [13] |
HORTON T M, GANNAVARAPU A, BLANEY S M, D'ARGENIO D Z, PLON S E, BERG S L. Bortezomib interactions with chemotherapy agents in acute leukemia in vitro[J]. Cancer Chemother Pharmacol, 2006, 58: 13-23. DOI:10.1007/s00280-005-0135-z |
| [14] |
MAKI C G, HUIBREGTSE J M, HOWLEY P M. In vivo ubiquitination and proteasome-mediated degradation of p53[J]. Cancer Res, 1996, 56: 2649-2654. |
| [15] |
BLAGOSKLONNY M V, WU G S, OMURA S, EL-DEIRY W S. Proteasome-dependent regulation of p21WAF1/CIP1 expression[J]. Biochem Biophys Res Commun, 1996, 227: 564-569. DOI:10.1006/bbrc.1996.1546 |
| [16] |
PAGANO M, TAM S W, THEODORAS A M, BEER-ROMERO P, DEL SAL G, CHAU V, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27[J]. Science, 1995, 269: 682-685. DOI:10.1126/science.7624798 |
| [17] |
RODRIGUEZ M S, WRIGHT J, THOMPSON J, THOMAS D, BALEUX F, VIRELIZIER J L, et al. Identification of lysine residues required for signal-induced ubiquitination and degradation of IkappaB-alpha in vivo[J]. Oncogene, 1996, 12: 2425-2435. |
| [18] |
CHANG Y C, LEE Y S, TEJIMA T, TANAKA K, OMURA S, HEINTZ N H, et al. Mdm2 and bax, downstream mediators of the p53 response, are degraded by the ubiquitin-proteasome pathway[J]. Cell Growth Differ, 1998, 9: 79-84. |
| [19] |
CIECHANOVER A, FINLEY D, VARSHAVSKY A. Ubiquitin dependence of selective protein degradation demonstrated in the mammalian cell cycle mutant ts85[J]. Cell, 1984, 37: 57-66. DOI:10.1016/0092-8674(84)90300-3 |
| [20] |
CHAUHAN D, CATLEY L, LI G, PODAR K, HIDESHIMA T, VELANKAR M, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from bortezomib[J]. Cancer Cell, 2005, 8: 407-419. |
| [21] |
HASSAN M, WATARI H, ABUALMAATY A, OHBA Y, SAKURAGI N. Apoptosis and molecular targeting therapy in cancer[J/OL]. Biomed Res Int, 2014, 2014: 150845. doi: 10.1155/2014/150845.
|
| [22] |
FENNELL D A, CHACKO A, MUTTI L. BCL-2 family regulation by the 20S proteasome inhibitor bortezomib[J]. Oncogene, 2008, 27: 1189-1197. DOI:10.1038/sj.onc.1210744 |
| [23] |
POULAKI V, MITSIADES C S, KOTOULA V, NEGRI J, MCMILLIN D, MILLER J W, et al. The proteasome inhibitor bortezomib induces apoptosis in human retinoblastoma cell lines in vitro[J]. Invest Ophthalmol Vis Sci, 2007, 48: 4706-4719. DOI:10.1167/iovs.06-1147 |
| [24] |
GOY A, HERNANDEZ-ILZALITURRI F J, KAHL B, FORD P, PROTOMASTRO E, BERGER M. A phase Ⅰ/Ⅱ study of the pan Bcl-2 inhibitor obatoclax mesylate plus bortezomib for relapsed or refractory mantle cell lymphoma[J]. Leuk Lymphoma, 2014, 55: 2761-2768. DOI:10.3109/10428194.2014.907891 |
| [25] |
HU X, XU J, SUN A, SHEN Y, HE G, GUO F. Successful T-cell acute lymphoblastic leukemia treatment with proteasome inhibitor bortezomib based on evaluation of nuclear factor-kappa B activity[J]. Leuk Lymphoma, 2011, 52: 2393-2395. DOI:10.3109/10428194.2011.593271 |
| [26] |
ZHAO J, WANG C, SONG Y, LIU Y, FANG B. Treatment of refractory/relapsed adult acute lymphoblastic leukemia with bortezomib-based chemotherapy[J]. Int J Gen Med, 2015, 8: 211-214. |
| [27] |
SIEGEL R, NAISHADHAM D, JEMAL A. Cancer statistics, 2012[J]. CA Cancer J Clin, 2012, 62: 10-29. DOI:10.3322/caac.20138 |
| [28] |
QIN J Z, ZIFFRA J, STENNETT L, BODNER B, BONISH B K, CHATURVEDI V, et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells[J]. Cancer Res, 2005, 65: 6282-6293. DOI:10.1158/0008-5472.CAN-05-0676 |
| [29] |
ALMOND J B, COHEN G M. The proteasome: a novel target for cancer chemotherapy[J]. Leukemia, 2002, 16: 433-443. DOI:10.1038/sj.leu.2402417 |
| [30] |
YU L, WU W K, GU C, ZHONG D, ZHAO X, KONG Y, et al. Obatoclax impairs lysosomal function to block autophagy in cisplatin-sensitive and -resistant esophageal cancer cells[J]. Oncotarget, 2016, 7: 14693-14707. |
| [31] |
CHAMPA D, ORLACCHIO A, PATEL B, RANIERI M, SHEMETOV A A, VERKHUSHA V V, et al. Obatoclax kills anaplastic thyroid cancer cells by inducing lysosome neutralization and necrosis[J]. Oncotarget, 2016, 7: 34453-34471. |
| [32] |
STAMELOS V A, FISHER N, BAMRAH H A, VOISEY C, PRICE J C, FARRELL W E, et al. The BH3 mimetic obatoclax accumulates in lysosomes and causes their alkalinization[J/OL]. PLoS One, 2016, 11: e150696. doi: 10.1371/journal.pone.0150696.
|
| [33] |
BASIT F, CRISTOFANON S, FULDA S. Obatoclax (GX15-070) triggers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes[J]. Cell Death Differ, 2013, 20: 1161-1173. DOI:10.1038/cdd.2013.45 |
| [34] |
BONAPACE L, BORNHAUSER B C, SCHMITZ M, CARIO G, ZIEGLER U, NIGGLI F K, et al. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance[J]. J Clin Invest, 2010, 120: 1310-1323. DOI:10.1172/JCI39987 |
| [35] |
HEIDARI N, HICKS M A, HARADA H. GX15-070 (obatoclax) overcomes glucocorticoid resistance in acute lymphoblastic leukemia through induction of apoptosis and autophagy[J/OL]. Cell Death Dis, 2010, 1: e76. doi: 10.1038/cddis.2010.53.
|
| [36] |
MARTIN A P, MITCHELL C, RAHMANI M, NEPHEW K P, GRANT S, DENT P. Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy[J]. Cancer Biol Ther, 2009, 8: 2084-2089. DOI:10.4161/cbt.8.21.9895 |
| [37] |
MARTIN A P, PARK M A, MITCHELL C, WALKER T, RAHMANI M, THORBURN A, et al. BCL-2 family inhibitors enhance histone deacetylase inhibitor and sorafenib lethality via autophagy and overcome blockade of the extrinsic pathway to facilitate killing[J]. Mol Pharmacol, 2009, 76: 327-341. DOI:10.1124/mol.109.056309 |
| [38] |
RUBINSZTEIN D C. The roles of intracellular proteindegradation pathways in neurodegeneration[J]. Nature, 2006, 443: 780-786. DOI:10.1038/nature05291 |
| [39] |
WU W K, CHO C H, LEE C W, WU Y C, YU L, LI Z J, et al. Macroautophagy and ERK phosphorylation counteract the antiproliferative effect of proteasome inhibitor in gastric cancer cells[J]. Autophagy, 2010, 6: 228-238. DOI:10.4161/auto.6.2.11042 |
| [40] |
PANKIV S, CLAUSEN T H, LAMARK T, BRECH A, BRUUN J A, OUTZEN H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy[J]. J Biol Chem, 2007, 282: 24131-24145. DOI:10.1074/jbc.M702824200 |
| [41] |
RASHID H O, YADAV R K, KIM H R, CHAE H J. ER stress: autophagy induction, inhibition and selection[J]. Autophagy, 2015, 11: 1956-1977. DOI:10.1080/15548627.2015.1091141 |
| [42] |
JÄGER R, BERTRAND M J, GORMAN A M, VANDENABEELE P, SAMALI A. The unfolded protein response at the crossroads of cellular life and death during endoplasmic reticulum stress[J]. Biol Cell, 2012, 104: 259-270. DOI:10.1111/boc.201100055 |
| [43] |
SCHRÖDER M, KAUFMAN R J. ER stress and the unfolded protein response[J]. Mutation Res, 2005, 569: 29-63. |
| [44] |
FUJITA E, KOUROKU Y, ISOAI A, KUMAGAI H, MISUTANI A, MATSUDA C, et al. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD (Ⅰ) and autophagy/lysosome ERAD (Ⅱ)[J]. Hum Mol Genet, 2007, 16: 618-629. DOI:10.1093/hmg/ddm002 |
| [45] |
HOUCK S A, REN H Y, MADDEN V J, BONNER J N, CONLIN M P, JANOVICK J A, et al. Quality control autophagy degrades soluble ERAD-resistant conformers of the misfolded membrane protein GnRHR[J]. Mol Cell, 2014, 54: 166-179. DOI:10.1016/j.molcel.2014.02.025 |
| [46] |
YOSHIDA H. ER stress and diseases[J]. FEBS J, 2007, 274: 630-658. DOI:10.1111/j.1742-4658.2007.05639.x |
| [47] |
URANO F, WANG X, BERTOLOTTI A, ZHANG Y, CHUNG P, HARDING H P, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1[J]. Science, 2000, 287: 664-666. DOI:10.1126/science.287.5453.664 |
| [48] |
DASMAHAPATRA G, LEMBERSKY D, RAHMANI M, KRAMER L, FRIEDBERG J, FISHER R I, et al. Bcl-2 antagonists interact synergistically with bortezomib in DLBCL cells in association with JNK activation and induction of ER stress[J]. Cancer Biol Ther, 2009, 8: 808-819. DOI:10.4161/cbt.8.9.8131 |
| [49] |
WRIGHT J J. Combination therapy of bortezomib with novel targeted agents: an emerging treatment strategy[J]. Clin Cancer Res, 2010, 16: 4094-4104. DOI:10.1158/1078-0432.CCR-09-2882 |
| [50] |
RAO R, NALLURI S, FISKUS W, SAVOIE A, BUCKLEY K M, HA K, et al. Role of CAAT/enhancer binding protein homologous protein in panobinostat-mediated potentiation of bortezomib-induced lethal endoplasmic reticulum stress in mantle cell lymphoma cells[J]. Clin Cancer Res, 2010, 16: 4742-4754. DOI:10.1158/1078-0432.CCR-10-0529 |
 2020, Vol. 41
2020, Vol. 41


