胃癌的发病率居全球第5位,也是癌症死亡的第3大原因[1]。由于其术后复发率高、化疗耐药性强,局部晚期胃癌患者的预后较差[2]。肿瘤细胞与正常细胞之间的物质代谢存在显著差异,针对这些差异进行抗肿瘤治疗的潜力巨大[3]。甲硫氨酸是人体必需氨基酸之一,在蛋白质合成和甲基化中起重要作用。研究表明,甲硫氨酸饥饿能抑制胃癌细胞的增殖并促进癌细胞凋亡,且不影响正常胃黏膜细胞的生长[4-5]。
我们前期研究发现,使用甲硫氨酸酶在胃癌细胞培养液中裂解甲硫氨酸可以抑制肿瘤细胞有氧糖酵解并促进胃癌细胞凋亡[6]。有研究表明,肿瘤糖酵解活性与肿瘤免疫逃避呈正相关,其机制是促进了肿瘤细胞上程序性死亡配体1(programmed death ligand-1,PD-L1)的表达[7]。PD-L1作为一种重要的免疫检查点,在胃癌中呈高表达[8-9]。已有研究发现在胃癌、直肠癌等多种肿瘤中抑制PD-L1的表达可以抑制肿瘤细胞的生长并促进肿瘤细胞凋亡[10-14]。本研究通过检测甲硫氨酸饥饿培养液和抑制PD-L1表达的siRNA(siPD-L1)对AGS细胞增殖、凋亡及相关蛋白表达的影响,探讨甲硫氨酸饥饿调控PD-L1促进肿瘤细胞凋亡的分子机制。
1 材料和方法 1.1 主要试剂甲硫氨酸饥饿RPMI 1640培养液、RPMI 1640培养液、FBS(美国Gibco公司),胰蛋白酶、青链霉素(北京索莱宝科技有限公司);Lipo3000(美国ThermoFisher公司),siPD-L1(苏州吉玛基因有限公司);CCK-8(武汉博士德生物工程有限公司),吖啶橙(acridine orange,AO)/溴化乙锭(ethidium bromide,EB)双染试剂盒(北京索莱宝科技有限公司),Annexin Ⅴ-FITC/PI双染细胞凋亡检测试剂盒(江苏凯基生物技术股份有限公司);RIPA裂解液、蛋白酶抑制剂(北京康为世纪生物科技有限公司),蛋白磷酸酶抑制剂、BCA蛋白浓度测定试剂盒(北京普利莱基因技术有限公司);PD-L1、Bcl-2、B淋巴细胞瘤2相关X蛋白(B-cell lymphoma-2-related X protein,Bax)、Caspase 3、GAPDH抗体(美国Proteintech公司),IRDye800荧光标记二抗(武汉博士德生物工程有限公司),ECL发光试剂盒(上海碧云天生物技术有限公司),PVDF膜(美国Millipore公司)。
1.2 利用癌症基因组图谱(The Cancer Genome Atlas,TCGA)数据库分析PD-L1在胃癌组织中表达从TCGA网站(https://tcga-data.nci.nih.gov/tcga/)提取RNA测序(RNA-Seq)FPKM(fragments per kilobase of exon model per million mapped fragments)矩阵和胃癌患者的临床信息。使用基因转换文件(Homo_sapiens.GRCh38.94.chr)获得每个Ensembl ID的基因名。使用与之对应的可用数据对样本分析PD-L1的表达差异及其与临床病理特征的关系。
1.3 细胞系与细胞培养AGS人胃腺癌细胞株购自上海普瑞曼生物科技有限公司。培养条件:含有10% FBS的RPMI 1640培养液,100 U/mL青链霉素,在37 ℃、95%相对湿度、5% CO2孵育箱中培养。48~72 h传代1次,选取对数生长期细胞进行实验。
1.4 细胞处理与siRNA转染接种细胞24 h后,细胞生长到融合度约60%时根据Lipo3000转染试剂说明书进行转染。用普通培养液和甲硫氨酸饥饿培养液分别培养未转染细胞、转染siPD-L1的细胞72 h,根据处理方式分为对照组、甲硫氨酸饥饿处理(MET-)组、siPD-L1组、MET-+siPD-L1组;转染对照siRNA(siScr)的细胞只作为蛋白质印迹实验中的阴性对照组。所有转染细胞经检测转染效率可用后进行下一步实验。使用的siRNA均由苏州吉玛公司合成,siPD-L1序列:正义链5′-CCAGCACACUGAGAAUCAATT-3′和反义链5′-UUGAUUCUCAGUGUGCUGGTT-3′;siScr序列:正义链5′-UUCUCCGAACGUGUCACGUTT-3′,反义链5′-ACGUGACACGU UCGGAGAATT-3′。
1.5 CCK-8检测细胞活力将细胞接种在96孔板中,根据1.4项下方法进行处理,设置5个复孔和调零孔。将10 μL CCK-8溶液添加到96孔板的每个孔中,并在37 ℃孵育2 h。使用酶标仪(VarioskanTM LUX,美国ThermoFisher公司)在450 nm处测光密度。根据公式计算细胞活力:细胞活力(%)=[(实验组光密度值-凋零孔光密度值)/(对照组光密度值-凋零孔光密度值)]×100%。
1.6 AO/EB双染检测细胞凋亡数目根据1.4项下方法处理细胞,用PBS洗2次。在PBS中制备AO/EB染料混合物(100 μg/mL AO和100 μg/mL EB)。在黑暗中室温下孵育5 min,AO渗透所有细胞使细胞核呈绿色,EB仅被丧失细胞膜完整性的细胞吸收并呈红色。用倒置荧光显微镜(IX71,日本Olympus公司)拍照分析。
1.7 流式细胞术检测细胞凋亡率根据1.4项下方法处理细胞,根据说明书用Annexin Ⅴ-FITC/PI细胞凋亡检测试剂盒进行染色。使用流式细胞仪(美国Becton Dickinson公司)分析4个象限的细胞数,计算各组的细胞凋亡率。
1.8 蛋白质印迹法分析细胞PD-L1、Bcl-2、Bax、Caspase 3蛋白表达根据1.4项下方法处理细胞,收集细胞后用RIPA裂解液提取总蛋白,检测蛋白质浓度后进行SDS-PAGE,然后将蛋白转至PVDF膜上。使用含5%脱脂奶粉的TBST封闭液在室温下封闭1 h。加入一抗4 ℃孵育过夜,吸净一抗并清洗后,加入二抗室温孵育1 h,吸净二抗并清洗。用超敏ECL发光试剂盒和化学发光成像系统(美国Bio-Rad公司)进行曝光分析。
1.9 starBase数据库分析PD-L1与抗凋亡蛋白表达相关性用starBase数据库中的RNA-RNA CoExpression工具(http://starbase.sysu.edu.cn/panGeneCoExp.php)分析PD-L1与Bcl-2抗凋亡蛋白家族(BCL2A1、MCL1、BCL2、BCL2L1)之间在mRNA水平表达的相关性。
1.10 统计学处理用GraphPad Prism 7.0软件进行统计学分析并作图。实验均重复3次,数据以x±s表示,组间比较采用独立样本t检验。TCGA数据库中的数据使用非配对t检验统计分析。检验水准(α)为0.05。
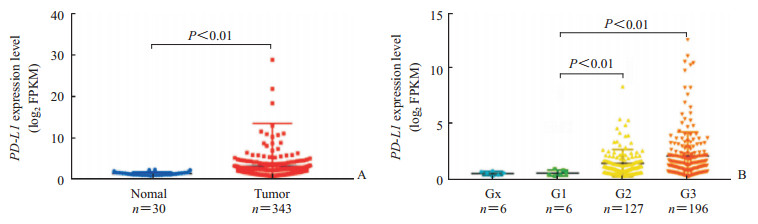
2 结果 2.1 PD-L1在胃癌组织中高表达并与胃癌G分级有关从TCGA数据库中一共提取了30个正常组织样本和343个胃癌组织样本FPKM数据,结果(图 1A)显示PD-L1在胃癌组织中表达水平较正常组织高(P<0.01)。343例胃癌组织样本中去除无临床数据的样本8例,利用剩余335例样本(其中Gx分级6例、G1分级6例、G2分级127例、G3分级196例)分析PD-L1表达与临床病理特征的关系。结果表明,PD-L1的表达与性别、年龄、TNM分期在统计学上均无相关性,但PD-L1的表达随G分级的升高而增加(P<0.01,图 1B)。
|
图 1 TCGA数据库分析PD-L1在胃癌组织中表达(A)及其与胃癌G分级的关系(B) Fig 1 Expression of PD-L1 in gastric cancer tissues (A) and its relationship with G grade of gastric cancer (B) were analyzed using TCGA database TCGA: The Cancer Genome Atlas; PD-L1: Programmed death ligand-1; FPKM: Fragments per kilobase of transcript per million fragments mapped |
2.2 甲硫氨酸饥饿能促进AGS细胞凋亡并抑制PD-L1表达
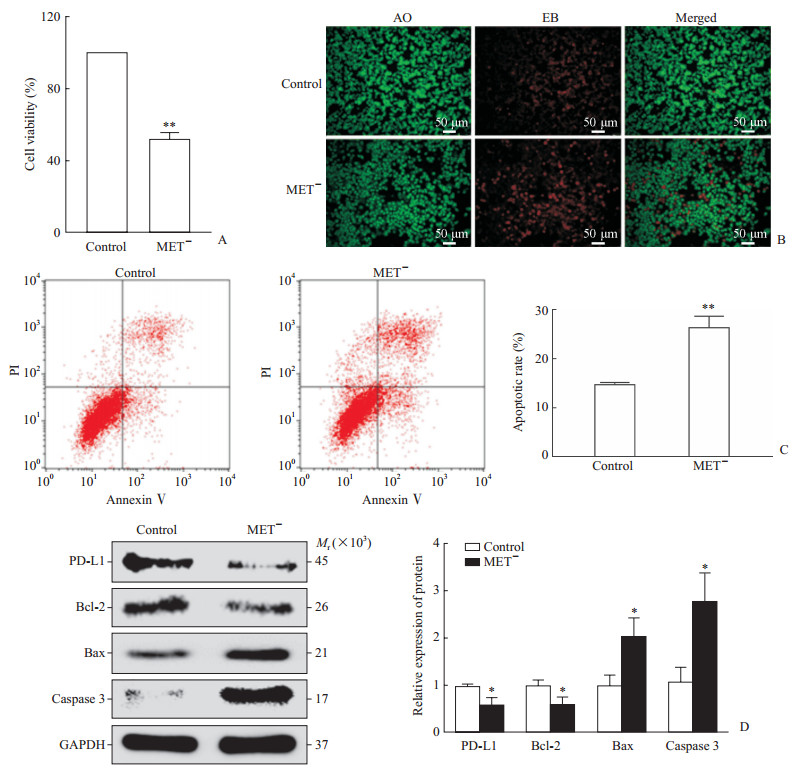
CCK-8结果显示,MET-组的细胞活力低于对照组,差异有统计学意义(P<0.01,图 2A)。AO/EB染色结果显示,MET-组的AGS细胞凋亡数量较对照组明显上升(图 2B)。流式细胞术结果显示,MET-组的AGS细胞凋亡率高于对照组,差异有统计学意义(P<0.01,图 2C)。蛋白质印迹法检测结果显示,MET-组的PD-L1和Bcl-2表达水平较对照组低(P<0.05),而Bax和Caspase 3的表达水平较对照组高(P均<0.05,图 2D)。以上结果提示,甲硫氨酸饥饿培养液能够诱导AGS细胞凋亡并抑制PD-L1蛋白表达。
|
图 2 甲硫氨酸饥饿抑制PD-L1表达促进AGS细胞凋亡 Fig 2 Methionine starvation inhibited PD-L1 expression and promoted apoptosis in AGS cells A: Cell counting kit 8 test showed that methionine starvation (MET-) reduced cell viability; B: AO/EB staining showed that methionine starvation (MET-) increased the cell apoptosis; C: Flow cytometry results showed that methionine starvation (MET-)promoted cell apoptosis; D: Western blotting results showed methionine starvation (MET-) inhibited the expression of PD-L1 and Bcl-2, and promoted the expression of Bax and Caspase 3. AO: Acridine orange; EB: Ethidium bromide; PI: Propidium iodide; PD-L1: Programmed death ligand-1; Bcl-2: B-cell lymphoma-2; Bax: B-cell lymphoma-2-related X protein; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. *P < 0.05, **P < 0.01 vs control group. n=3, x±s |
2.3 抑制PD-L1能促进AGS细胞凋亡
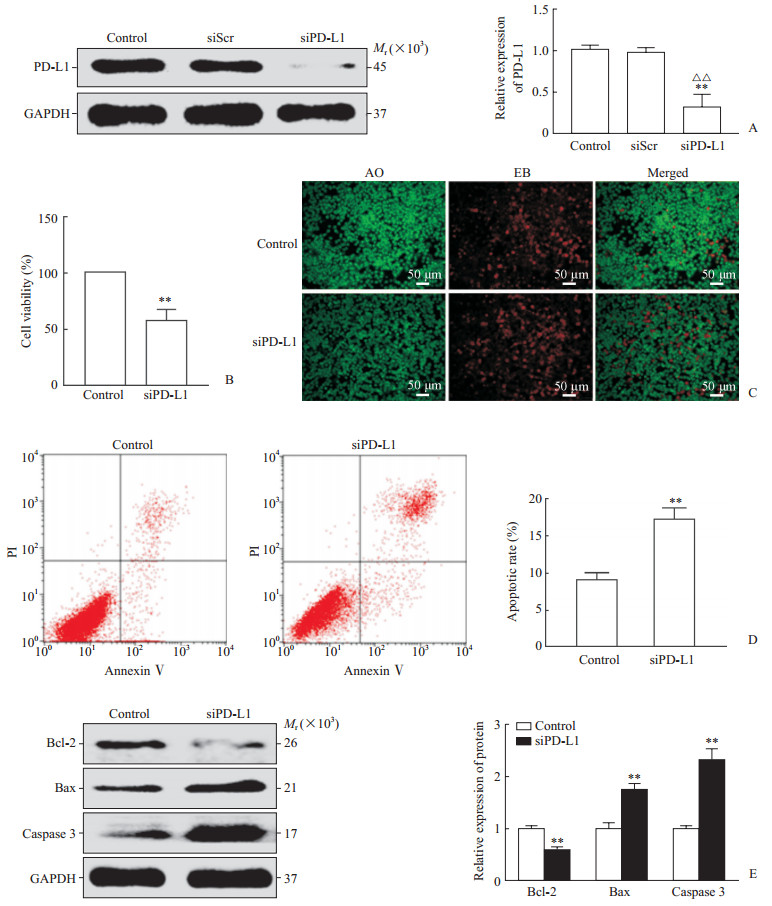
siPD-L1转染AGS细胞后PD-L1的表达下调(P<0.01,图 3A)。CCK-8结果显示,siPD-L1组的细胞活力低于对照组,差异有统计学意义(P<0.01,图 3B)。AO/EB染色结果显示,siPD-L1组的AGS细胞凋亡数量较对照组增多(图 3C)。流式细胞术结果显示,siPD-L1组的细胞凋亡率为高于对照组,差异有统计学意义(P<0.01,图 3D)。蛋白质印迹法检测结果显示,siPD-L1组的Bcl-2表达水平低于对照组,而Bax和Caspase 3的表达水平高于对照组,差异均有统计学意义(P<0.01,图 3E)。以上结果提示,抑制PD-L1表达能够促进AGS细胞凋亡。
|
图 3 siPD-L1抑制AGS细胞PD-L1表达并诱导凋亡 Fig 3 siPD-L1 inhibited PD-L1 expression and induced apoptosis in AGS cells A: Western blotting analysis showed that siPD-L1 effectively inhibited PD-L1 expression in AGS cells; B: Cell counting kit 8 test results showed that siPD-L1 reduced cell viability; C: AO/EB staining showed that siPD-L1 increased apoptosis; D: Flow cytometry showed that siPD-L1 increased the apoptotic rate; E: Western blotting analysis showed siPD-L1 inhibited the expression of Bcl-2 and promoted the expression of Bax and Caspase 3. PD-L1: Programmed death ligand-1; siScr: Small interfere scrambled; siPD-L1: Small interfere RNA targeting programmed death ingand-1; AO: Acridine orange; EB: Ethidium bromide; PI: Propidium iodide; Bcl-2: B-cell lymphoma-2; Bax: B-cell lymphoma-2-related X protein; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. **P < 0.01 vs control group; △△P < 0.01 vs siScr group. n=3, x±s |
2.4 甲硫氨酸饥饿与siPD-L1联合后促AGS细胞凋亡作用增强
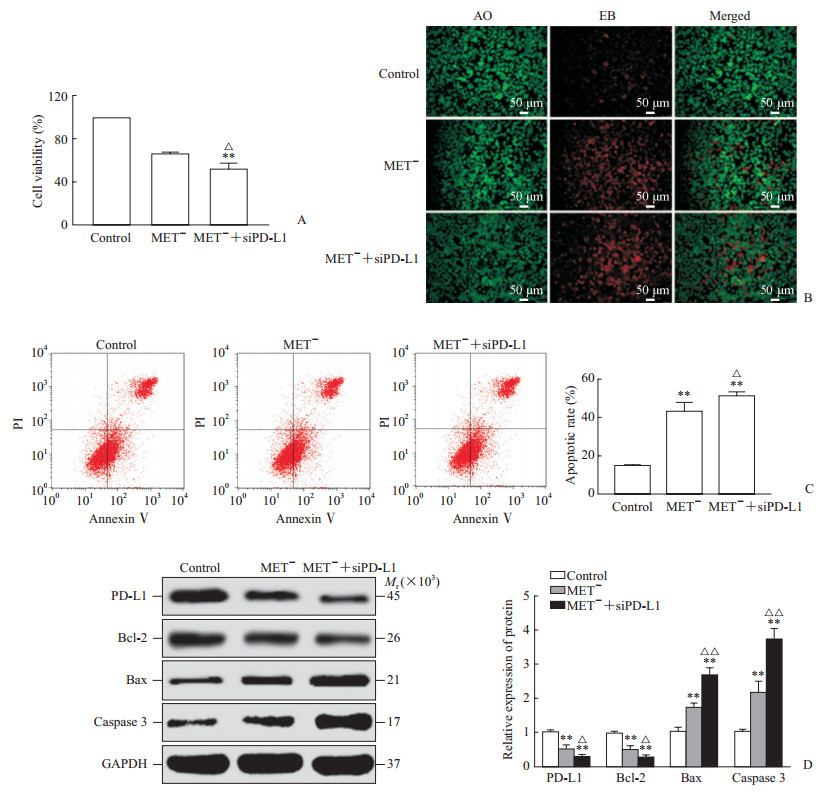
CCK-8结果显示,MET-+siPD-L1组的AGS细胞活力低于对照组及MET-组,差异均有统计学意义(P<0.05或P<0.01,图 4A)。AO/EB染色结果显示,MET-+siPD-L1组的细胞凋亡数量较对照组及MET-组显著增加(图 4B)。流式细胞术检测结果显示,MET-+siPD-L1组细胞凋亡率高于对照组及MET-组,差异均有统计学意义(P<0.05或P<0.01,图 4C)。蛋白质印迹法检测结果显示,MET-+siPD-L1组PD-L1和Bcl-2的表达水平低于对照组及MET-组,而MET-+siPD-L1组Bax和Caspase 3的表达水平均高于对照组及MET-组,差异均有统计学意义(P<0.05或P<0.01,图 4D)。
|
图 4 甲硫氨酸饥饿与siPD-L1联合后促AGS细胞凋亡作用增强 Fig 4 Combination of siPD-L1 and methionine starvation enhaced the effect of promoting apoptosis A: Cell counting kit 8 was used to detect cell viability; B: AO/EB staining was used to detect apoptosis. C: Flow cytometry was used to detect apoptosis; D: Western blotting was used to detect the expression of PD-L1, Bcl-2, Bax and Caspase 3. MET-: Methionine starvation; siPD-L1: Small interfere RNA targeting programmed death ingand-1; AO: Acridine orange; EB: Ethidium bromide; PI: Propidium iodide; PD-L1: Programmed death ligand-1; Bcl-2: B-cell lymphoma-2; Bax: B-cell lymphoma-2-related X protein; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. **P < 0.01 vs control group; △P < 0.05, △△P < 0.01 vs siPD-L1 group. n=3, x±s |
2.5 PD-L1与Bcl-2抗凋亡蛋白家族BCL2A1、MCL1、BCL2、BCL2L1的表达水平呈正相关
通过starBase数据库的RNA-RNA CoExpression分析工具分析了375个胃癌组织RNA测序样本,结果(图 5)显示PD-L1的mRNA表达[log2(FPKM+0.01)]与Bcl-2抗凋亡蛋白家族的BCL2A1(r=0.565,P<0.01)、MCL1(r=0.388,P<0.01)、BCL2(r=0.257,P<0.01)、BCL2L1(r=0.153,P<0.01)的mRNA表达[log2(FPKM+0.01)]均呈正相关。这说明,PD-L1与抗凋亡基因密切相关,是一种关键抗凋亡基因。

|
图 5 利用starBase数据库分析PD-L1与Bcl 2抗凋亡蛋白家族mRNA表达的相关性 Fig 5 The starBase database was used to analyze the relationship of PD-L1 with the Bcl 2 anti-apoptotic protein family PD-L1: Programmed death ligand-1; Bcl: B-cell lymphoma; MCL1: Myeloid cell leakemia 1; FPKM: Fragments per kilobase of transcript per million fragments mapped |
3 讨论
甲硫氨酸是人体8种必需氨基酸之一,而肿瘤细胞对甲硫氨酸的需求更加旺盛。研究发现,包括胃癌细胞在内的多种肿瘤细胞在无甲硫氨酸的培养液中不能生长,正常细胞在此条件下却可以正常增殖[15]。可能原因是肿瘤细胞较正常细胞蛋白质合成和转甲基化反应增加, 对甲硫氨酸的需求更多,而肿瘤细胞内甲硫氨酸合成酶的活性较低, 无法像正常细胞一样合成足够的内源性甲硫氨酸[16]。我们的前期研究证实, 消耗胃癌细胞培养液中的甲硫氨酸能抑制肿瘤细胞的生长[17-18],这表明抑制胃癌细胞甲硫氨酸摄入将是有希望的肿瘤治疗靶标。本研究结果显示,在AGS细胞中限制甲硫氨酸摄入使其活力降低,细胞凋亡数、凋亡率和凋亡蛋白表达水平上升,诱导肿瘤细胞发生凋亡。
我们先前的研究发现,使用甲硫氨酸酶在胃癌细胞培养液中裂解甲硫氨酸可以抑制肿瘤细胞的有氧糖酵解[6]。糖酵解作为肿瘤细胞主要产能方式,被抑制后将会产生一系列的分子生物学变化。肿瘤细胞有氧糖酵解的活性与免疫逃避密切相关,而Jiang等[7]研究发现肿瘤细胞中活跃的有氧糖酵解能上调PD-L1的表达,使肿瘤细胞逃避机体的免疫监视。其机制可能是抑制糖酵解作用降低肿瘤细胞中的ATP水平,激活了能量敏感的腺苷酸活化蛋白激酶(adenosine monophosphate-activated protein kinase,AMPK)[19]。AMPK是一种重要的生物能量代谢调节的关键分子,被激活后可以调节自噬、细胞凋亡、细胞周期、细胞代谢和蛋白质合成,从而抑制肿瘤进展[20]。Cha等[21]的研究表明AMPK可以直接磷酸化PD-L1的S195-p基团,从而诱导PD-L1异常糖基化,导致其在内质网中积累,抑制其表达。在本研究中,蛋白质印迹法检测结果显示缺乏甲硫氨酸的AGS细胞PD-L1蛋白表达下调,我们预测甲硫氨酸饥饿有可能经此途径抑制PD-L1表达。
PD-L1也称为CD274、B7-H1,是一种跨膜蛋白,通常在抗原呈递细胞和肿瘤细胞的表面表达。在多种肿瘤细胞中PD-L1的表达升高,其与T细胞上的PD-1受体特异性结合激活下游信号转导,抑制T细胞增殖、细胞因子的产生和释放及细胞毒性,肿瘤细胞利用这种机制来阻止免疫系统的攻击[8-9, 14]。研究显示,PD-L1的表达与胃癌的恶性程度和预后呈负相关[8-9]。本研究提取分析了TCGA数据库的RNA测序数据进行验证,发现在胃癌组织中PD-L1呈高表达状态,并与G分级有关,表明PD-L1是治疗胃癌有效靶点。目前,PD-L1靶向药物阿维单抗(avelumab)已经进入胃癌Ⅲ期临床试验,PD-L1治疗胃癌具有广阔的前景[22]。
PD-L1除了具有免疫抑制作用外,还参与了肿瘤细胞的其他功能,研究证实PD-L1促进胃癌上皮间质转化并抑制癌细胞凋亡[10]。有研究发现上调PD-L1的表达可以减弱靶向PD-L1的miR-140-3p,促进肿瘤细胞凋亡[23]。本研究结果表明,抑制AGS细胞PD-L1的表达同样能抑制胃癌细胞增殖,诱使细胞凋亡率和凋亡蛋白表达水平上升。在肿瘤细胞中,细胞凋亡过程失调导致其不受控制的增殖[24]。有研究发现,抗凋亡蛋白表达有助于癌细胞抵抗机体免疫系统的监视[25]。这些研究结果提示PD-L1与细胞凋亡的关系密切,其机制为:PD-L1作为抗凋亡信号受体将该信号传递给癌细胞,影响第一凋亡信号受体(Fas cell surface death receptor,Fas)-Fas配体(Fas ligand,FasL)的相互作用,拮抗T细胞、药物诱导癌细胞发生凋亡[26];PD-L1还具有信号转导能力,通过调节肿瘤细胞哺乳动物雷帕霉素靶蛋白信号活性影响有氧糖酵解相关的mRNA表达,调控葡萄糖代谢,促进肿瘤进展[27-29];此外,PD-L1可以与ITGB6蛋白相互作用,进而激活信号转导及转录激活蛋白3,与蛋白激酶B、P53、Bcl-2等基因启动子结合发挥相应的抑制凋亡的生物学功能[14]。通过分析PD-L1与Bcl-2家族抗凋亡基因相关性发现,PD-L1与BCL2A1、MCL1、BCL2、BCL2L2表达呈正相关,表明PD-L1是一种关键的抗凋亡基因。为了验证甲硫氨酸饥饿是否经PD-L1诱导凋亡,我们还使用甲硫氨酸饥饿培养液和siPD-L1联合处理AGS细胞,结果显示其在降低细胞活力、诱导细胞凋亡方面的作用更强。
综上所述,本研究证明甲硫氨酸饥饿通过抑制PD-L1的表达诱导胃癌AGS细胞凋亡,揭示了针对胃癌甲硫氨酸依赖性这种特异性代谢治疗手段的有效性。甲硫氨酸饥饿调控PD-L1的具体机制和PD-L1诱导凋亡的信号转导通路仍然需要进一步研究。
| [1] |
BRAY F, FERLAY J, SOERJOMATARAM I, SIEGEL R L, TORRE L A, JEMAL A. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68: 394-424. DOI:10.3322/caac.21492 |
| [2] |
SUN P, XIANG J B, CHEN Z Y. Meta-analysis of adjuvant chemotherapy after radical surgery for advanced gastric cancer[J]. Brit J Surg, 2009, 96: 26-33. DOI:10.1002/bjs.6408 |
| [3] |
VANDER HEIDEN M G, DEBERARDINIS R J. Understanding the intersections between metabolism and cancer biology[J]. Cell, 2017, 168: 657-669. DOI:10.1016/j.cell.2016.12.039 |
| [4] |
CHATURVEDI S, HOFFMAN R M, BERTINO J R. Exploiting methionine restriction for cancer treatment[J]. Biochem Pharmacol, 2018, 154: 170-173. DOI:10.1016/j.bcp.2018.05.003 |
| [5] |
XIN L, YANG W F, ZHANG H T, LI Y F, LIU C. The mechanism study of lentiviral vector carrying methioninase enhances the sensitivity of drug-resistant gastric cancer cells to cisplatin[J]. Br J Cancer, 2018, 118: 1189-1199. DOI:10.1038/s41416-018-0043-8 |
| [6] |
周立强, 李世豪, 刘力, 周祺, 袁宜武, 辛林. 重组甲硫氨酸酶调控PI3K/Akt/Glut-1通路抑制有氧糖酵解促进胃癌细胞凋亡[J]. 南方医科大学学报, 2020, 40: 27-33. |
| [7] |
JIANG Z, LIU Z, LI M, CHEN C, WANG X. Increased glycolysis correlates with elevated immune activity in tumor immune microenvironment[J]. EBioMedicine, 2019, 42: 431-442. DOI:10.1016/j.ebiom.2019.03.068 |
| [8] |
HASHIMOTO T, KUROKAWA Y, TAKAHASHI T, MIYAZAKI Y, TANAKA K, MAKINO T, et al. Predictive value of MLH1 and PD-L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer[J]. Gastric Cancer, 2019, 22: 785-792. DOI:10.1007/s10120-018-00918-4 |
| [9] |
MORIHIRO T, KURODA S, KANAYA N, KAKIUCHI Y, KUBOTA T, AOYAMA K, et al. PD-L1 expression combined with microsatellite instability/CD8+ tumor infiltrating lymphocytes as a useful prognostic biomarker in gastric cancer[J/OL]. Sci Rep, 2019, 9: 4633. doi: 10.1038/s41598-019-41177-2.
|
| [10] |
FAN Y, CHE X, HOU K, ZHANG M, WEN T, QU X, et al. MiR-940 promotes the proliferation and migration of gastric cancer cells through up-regulation of programmed death ligand-1 expression[J]. Exp Cell Res, 2018, 373: 180-187. DOI:10.1016/j.yexcr.2018.10.011 |
| [11] |
GHEBEH H, LEHE C, BARHOUSH E, AL-ROMAIH K, TULBAH A, AL-ALWAN M, et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: role of B7-H1 as an anti-apoptotic molecule[J/OL]. Breast Cancer Res, 2010, 12: R48. doi: 10.1186/bcr2605.
|
| [12] |
WANG F, MA J, LIU J, JIN H, HUANG D. Synthetic small peptides acting on B7H1 enhance apoptosis in pancreatic cancer cells[J]. Mol Med Rep, 2012, 6: 553-557. DOI:10.3892/mmr.2012.970 |
| [13] |
CHAKRABARTI J, HOLOKAI L, SYU L, STEELE N G, CHANG J, WANG J, et al. Hedgehog signaling induces PD-L1 expression and tumor cell proliferation in gastric cancer[J]. Oncotarget, 2018, 9: 37439-37457. DOI:10.18632/oncotarget.26473 |
| [14] |
CAO D, QI Z, PANG Y, LI H, XIE H, WU J, et al. Retinoic acid-related orphan receptor c regulates proliferation, glycolysis, and chemoresistance via the PDL1/ITGB6/STAT3 signaling axis in bladder cancer[J]. Cancer Res, 2019, 79: 2604-2618. DOI:10.1158/0008-5472.CAN-18-3842 |
| [15] |
KANO Y, SAKAMOTO S, KASAHARA T, KUSUMOTO K, HIDA K, SUDA K, et al. Methionine dependency of cell growth in normal and malignant hematopoietic cells[J]. Cancer Res, 1982, 42: 3090-3092. |
| [16] |
GRAZIOSI L, MENCARELLI A, RENGA B, D'AMORE C, BRUNO A, SANTORELLI C, et al. Epigenetic modulation by methionine deficiency attenuates the potential for gastric cancer cell dissemination[J]. J Gastrointest Surg, 2013, 17: 39-49. DOI:10.1007/s11605-012-1996-1 |
| [17] |
XIN L, LIU C, YANG W F, ZHANG H T, ZENG F, CAO J Q. Recombinant methioninase inhibits selfrenewal and proliferation of gastric cancer stem cells[J]. Int J Clin Exp Med, 2017, 10: 6091-6099. |
| [18] |
XIN L, CAO W X, FEI X F, WANG Y, LIU W T, LIU B Y, et al. Applying proteomic methodologies to analyze the effect of methionine restriction on proliferation of human gastric cancer SGC7901 cells[J]. Clin Chim Acta, 2007, 377: 206-212. DOI:10.1016/j.cca.2006.09.027 |
| [19] |
JONES R G, PLAS D R, KUBEK S, BUZZAI M, MU J, XU Y, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint[J]. Mol Cell, 2005, 18: 283-293. DOI:10.1016/j.molcel.2005.03.027 |
| [20] |
ZHENG L, YANG W, WU F, WANG C, YU L, TANG L, et al. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma[J]. Clin Cancer Res, 2013, 19: 5372-5380. DOI:10.1158/1078-0432.CCR-13-0203 |
| [21] |
CHA J H, YANG W H, XIA W, WEI Y, CHAN LC, LIM S O, et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1[J/OL]. Mol Cell, 2018, 71: 606-620, e7. doi: 10.1016/j.molcel.2018.07.030.
|
| [22] |
MOEHLER M, RYU M H, DVORKIN M, LEE K W, COŞKUN H Ş, WONG R, et al. Maintenance avelumab versus continuation of first-line chemotherapy in gastric cancer:JAVELIN Gastric 100 study design[J]. Future Oncol, 2019, 15: 567-577. DOI:10.2217/fon-2018-0668 |
| [23] |
JIANG W, LI T, WANG J, JIAO R, SHI X, HUANG X, et al. miR-140-3p suppresses cell growth and induces apoptosis in colorectal cancer by targeting PD-L1[J]. Onco Targets Ther, 2019, 12: 10275-10285. DOI:10.2147/OTT.S226465 |
| [24] |
AHMED K, TABUCHI Y, KONDO T. Hyperthermia:an effective strategy to induce apoptosis in cancer cells[J]. Apoptosis, 2015, 20: 1411-1419. DOI:10.1007/s10495-015-1168-3 |
| [25] |
THOMAS S, QUINN B A, DAS S K, DASH R, EMDAD L, DASGUPTA S, et al. Targeting the Bcl-2 family for cancer therapy[J]. Expert Opin Ther Targets, 2013, 17: 61-75. DOI:10.1517/14728222.2013.733001 |
| [26] |
AZUMA T, YAO S, ZHU G, FLIES A S, FLIES S J, CHEN L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells[J]. Blood, 2008, 111: 3635-3643. DOI:10.1182/blood-2007-11-123141 |
| [27] |
CHANG C H, QIU J, O'SULLIVAN D, BUCK M D, NOGUCHI T, CURTIS J D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression[J]. Cell, 2015, 162: 1229-1241. DOI:10.1016/j.cell.2015.08.016 |
| [28] |
PALMER C S, OSTROWSKI M, BALDERSON B, CHRISTIAN N, CROWE S M. Glucose metabolism regulates T cell activation, differentiation, and functions[J/OL]. Front Immunol, 2015, 6: 1. doi: 10.3389/fimmu.2015.00001.eCollection2015.
|
| [29] |
CLARK C A, GUPTA H B, SAREDD Y G, PANDESWARA S, LAO S, YUAN B, et al. Tumorintrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma[J]. Cancer Res, 2016, 76: 6964-6974. DOI:10.1158/0008-5472.CAN-16-0258 |
 2020, Vol. 41
2020, Vol. 41


