胰腺癌是具有高致死率的恶性肿瘤,5年生存率不到5%[1]。胰腺癌是全球主要的致死性癌症之一[2],其侵袭性表型、治疗耐受和缺乏早期诊断方法导致其预后不良。手术切除是目前胰腺癌早期治疗的首选方法,但超过80%的患者被诊断为晚期且无法手术。对于存在远处转移疾病的患者,尽管已努力探寻新的治疗策略,但近年来总体生存率并未显著提高[3]。中草药具有安全、有效及低成本等优势,受到研究者越来越多的关注,如何运用中医药疗法提高胰腺癌患者的生存率是现阶段关注的重点。以清胰化积方为主的中西医综合疗法在治疗晚期胰腺癌的生存方面取得了很好的效果。沈晔华等[4]研究发现清胰化积方可明显提高晚期胰腺癌患者1年、3年和5年的生存率,并延长患者中位生存期。清胰化积方以清热解毒、理气化湿为原则, 在中晚期胰腺癌的治疗中可稳定瘤灶、延长生存期,其机制主要表现在下调免疫抑制因子、改善免疫抑制状态、抑制肿瘤相关成纤维细胞、改善肿瘤微环境、下调转移相关基因、降低S期细胞比例进而抑制肿瘤细胞增殖、调节Notch信号通路抑制蛋白表达等[5]。
目前多向药理学、计算生物学等多学科都已步入大数据时代,网络药理学作为一种研究药物、疾病、靶点之间相互关系的网络分析方法,从网络构建的角度分析、预测和证实药物成分的物质基础,有利于理解药物作用网络的作用机制[6-8]。本研究运用网络药理学的分析方法,获取清胰化积方主要成分对治疗胰腺癌的作用靶点,建立基因调控网络,分析其治疗胰腺癌的作用机制并通过动物实验加以验证。
1 材料和方法 1.1 实验细胞、动物与主要试剂人胰腺癌SW1990细胞系购于中国科学院上海细胞所。SPF级的BALB/c裸小鼠由上海斯莱克实验动物有限公司提供[动物生产许可证编号:SCXK(沪)2012-0002;动物合格证编号:2015000520204],5~8周龄,体质量18~20 g,饲养于上海中医药大学重点动物实验室。动物自由摄取常规饲料(由上海中医药大学动物研究中心供应)。清胰化积方由药蛇六谷、白花蛇舌草、半枝莲、灵芝、薏苡仁、豆蔻和绞股蓝组成,实验用清胰化积方采用中药免煎颗粒剂(江阴天江药业有限公司生产,批号:05112006),加水配制成含生药0.9、1.8、3.6 g/mL的药液,置4 ℃冰箱备用。胰蛋白酶、FBS、DMEM培养基购自美国Gibco公司,青霉素-链霉素溶液、PBS购自上海索莱宝生物科技有限公司,Qiagen 7410购自德国凯杰公司,Prime ScriptTM RT reagent Kit购自日本TaKaRa公司。
1.2 裸小鼠移植瘤模型的建立及处理 1.2.1 裸小鼠移植瘤模型的建立人胰腺癌SW1990细胞用含10% FBS、1%青霉素-链霉素、1%羟乙基哌嗪乙磺酸(hydroxyethyl piperazineethanesulfonic acid,HEPES)的DMEM培养液培养,使用0.25%的胰蛋白酶进行消化, 按1:3的比例传代。取处于对数生长期的SW1990细胞制成单细胞悬液,调整细胞密度为1×107/mL,在无菌条件下用1 mL注射器将细胞接种于裸小鼠右前肢腋下皮下,每只裸小鼠接种量为0.2 mL。
1.2.2 分组和给药造模次日将裸小鼠随机分成4组,即生理盐水对照组和清胰化积方低、中、高剂量组,每组8只。按《中药药理实验方法学》[9]附录3《人和动物间按体表面积折算的等效剂量比率表》计算,低、中、高剂量组中小鼠清胰化积方用量分别为18、36、72 g/kg,予以浓缩含生药分别为0.9、1.8、3.6 g/mL的药液每日灌胃给药0.2 mL,对照组小鼠予生理盐水每日灌胃0.2 mL。隔日测量移植瘤大小。给药10 d后处死小鼠,取出移植瘤,观察后备用。
1.2.3 基因检测和分析提取样品总RNA并使用DNase消化DNA后,使用Illumina HiSeq X Ten(美国Illumina公司)平台进行测序,产生150 bp的双端数据。将测序所产生的raw reads进行质量过滤后产生clean reads数据,使用DESeq(2012) R package[10]的estimateSizeFactors函数对数据进行标准化后,再使用nbinomTest函数计算差异表达基因的P值和差异倍数(fold change,FC),并绘制火山图及热图。
1.3 网络药理学分析 1.3.1 清胰化积方有效成分收集与筛选使用BATMAN-TCM(Bioinformatics Analysis Tool for Molecular Mechanism of Traditional Chinese Medicine,http://bionet.ncpsb.org/batman-tcm/)数据库,以各类清胰化积方有效成分为关键词,设置“药物-靶点”相似性模型阈值Score cutoff≥30、P≤0.05,检索筛选活性成分及对应的潜在靶点。运用Cytoscape软件构建各类药物成分及其对应靶点网络关系图。
1.3.2 清胰化积方靶基因信号通路分析根据收集到的活性成分相对应靶基因及胰腺癌相关靶基因,运用Venn平台(http:bioinformatics.psb.ugent.be/webtools/Venn/)构建韦恩图(Venn diagram)并进行对比分析及重合匹配。利用STRING(https://string-db.org)数据库平台,获取蛋白相互作用关系并构建蛋白质-蛋白质相互作用(protein-protein interaction,PPI)网络图,用Cytoscape绘制作图。通过DAVID(https://david.ncifcrf.gov/)数据库进行京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)信号通路分析功能,预测清胰化积方靶基因富集的信号通路。
1.3.3 腺苷酸活化蛋白激酶(adenosine monophosphate-activated protein kinase,AMPK)通路基因表达水平与预后的关系分析通过基因表达谱数据动态分析(Gene Expression Profiling Interactive Analysis,GEPIA;http://gepia.cancer-pku.cn/index.html)平台[11],对比分析胰腺癌组织和癌旁组织ERK1/2和p38 MAPK信号通路关键基因的转录水平,分析该通路基因与胰腺癌预后的关系。
1.4 统计学处理采用SPSS 22.0软件进行统计学分析。数据均以x±s表示,采用单因素方差分析进行多组间差异的比较,对于有统计学意义的数据采用Dunnett法进行两两比较。检验水准(α)为0.05。采用R 4.0软件制作主成分分析(principal component analysis,PCA)图、MA图(M versus A plot)和聚类热图。
2 结果 2.1 清胰化积方活性成分有效作用靶点筛选使用BATMAN-TCM数据库输入清胰化积方中的7种药材,共得到有效成分149个,对应的靶基因963个。其中白花蛇舌草共7个有效成分,对应234个靶基因;半枝莲共12个有效成分,对应55个靶基因;薏苡仁共3个有效成分,对应78个靶基因;灵芝共34个有效成分,对应539个靶基因;绞股蓝共83个有效成分,对应117个靶基因;豆蔻共10个有效成分,对应468个靶基因。动物实验基因测序结果表明,清胰化积方高剂量组裸小鼠移植瘤组织中共发现6 039个与生理盐水对照组的差异表达基因(P<0.05,|FC|>1.2)。将清胰化积方高剂量组与生理盐水对照组的组间差异表达基因,以及网络药理学分析得到的清胰化积方潜在作用靶点基因与胰腺癌重叠基因进行Venn分析,得到248个共靶标靶基因。
2.2 清胰化积方靶基因KEGG通路分析将248个靶基因输入DAVID数据库进行KEGG通路分析,结果显示248个靶基因主要富集于信号转导、氨基酸代谢和碳水化合物代谢等通路。信号转导通路主要包括MAPK、AMPK、叉头框转录因子O亚型(forkhead box O,FoxO)、cAMP、环磷酸鸟苷(cyclic guanosine monophosphate,cGMP)-蛋白激酶G (protein kinase G,PKG)、钙、缺氧诱导因子1(hypoxia-inducible factor 1,HIF-1)、erb-b2受体酪氨酸激酶(erb-b2 receptor tyrosine kinase,ErbB)、TNF、磷脂酰肌醇(phosphatidylinositol,PI)和血管内皮生长因子(vascular endothelial growth factor,VEGF)通路(图 1)。
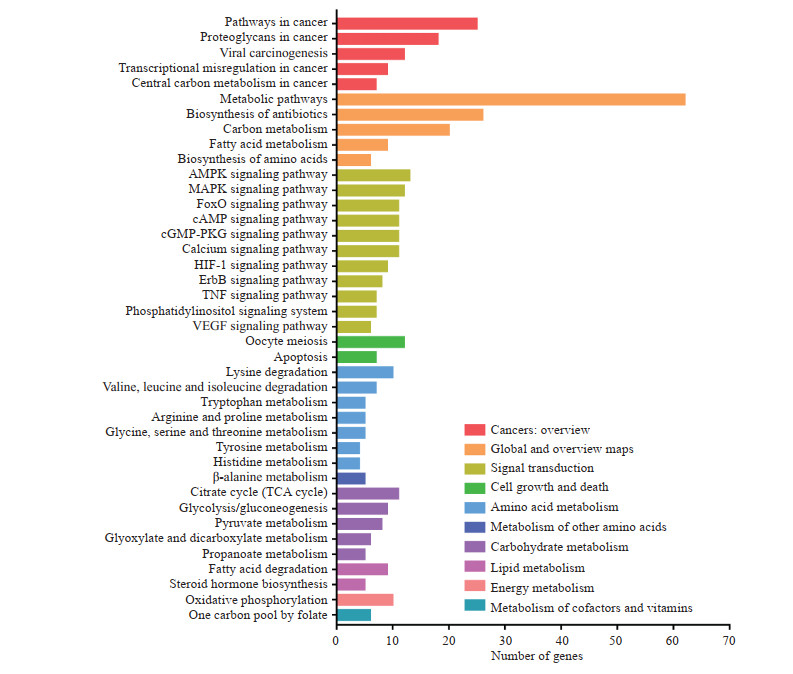
|
图 1 清胰化积方靶基因KEGG通路分析 Fig 1 KEGG pathway analysis of target genes of Qingyi Huaji recipe KEGG: Kyoto Encyclopedia of Genes and Genomes; AMPK: Adenosine monophosphate-activated protein kinase; MAPK: Mitogen-activated protein kinase; FoxO: Forkhead box O; cAMP: Cyclic adenosine monophosphate; cGMP: Cyclic guanosine monophosphate; PKG: Protein kinase G; HIF-1: Hypoxia-inducible factor 1; ErbB: Erb-b2 receptor tyrosine kinase; TNF: Tumor necrosis factor; VEGF: Vascular endothelial growth factor; TCA: Tricarboxylic acid |
2.3 清胰化积方低剂量组和高剂量组差异表达基因分析 2.3.1 各组裸小鼠肿瘤生长曲线
裸小鼠皮下种植造模1 d后,随机分为对照组及清胰化积方低、中、高剂量组,每组8只。随着时间延长,肿瘤逐渐增大,与对照组相比,清胰化积方低、中、高剂量组裸小鼠肿瘤生长明显受到抑制且呈剂量依赖性,治疗10 d时各剂量组肿瘤相对增长率均低于对照组(P均<0.01,图 2)。
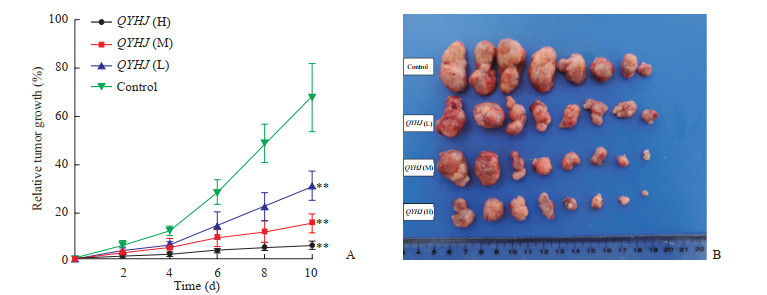
|
图 2 清胰化积方对小鼠移植瘤生长的影响 Fig 2 Effects of QYHJ on growth of xenograft tumor in mice A: Tumor growth rate. Day 0: The day of tumor injection. Day 10: The last day of intervention. B: Tumor specimens dissected after 10 days of intervention on Day 11. QYHJ: Qingyi Huaji recipe; H: High-dose; M: Medium-dose; L: Low-dose. **P < 0.01 vs control group. n=8, x±s |
2.3.2 清胰化积方低剂量组和高剂量组差异表达基因筛选
设定差异表达基因筛选条件:P<0.05,|log2FC|>0.26。结果显示,与对照组相比,清胰化积方低剂量组的差异表达基因共2 376个,其中上调1 655个,下调721个;清胰化积方高剂量组的差异表达基因共6 039个,其中上调1 442个,下调1 592个。Venn分析结果表明,清胰化积方低剂量组与高剂量组的重叠基因共1 894个。采用R语言绘制PCA图对清胰化积方对照组、低剂量组和高剂量组进行分析,发现低剂量组和高剂量组主成分性质类似。通过R语言对低剂量组和高剂量组得到的差异表达基因进行MA分析,并采用聚类热图对差异表达基因进行可视化分析,展现差异表达基因的表达分布,结果见图 3。
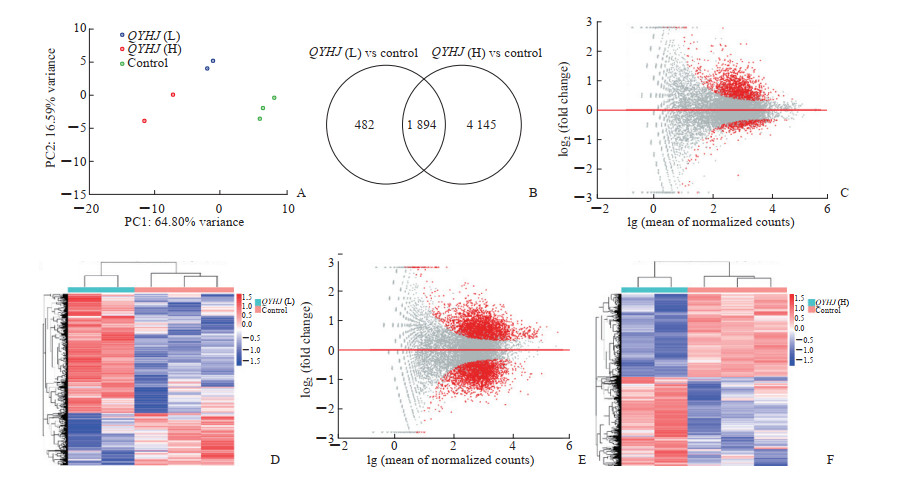
|
图 3 清胰化积方低剂量组和高剂量组差异表达基因的筛选 Fig 3 Screening differentially expressed genes of QYHJ (L) and QYHJ (H) groups A: PCA chart of the control group, QYHJ (L) group and QYHJ (H) group; B: Venn diagram of differentially expressed genes between QYHJ (L) group and QYHJ (H) group; C: MA chart of QYHJ (L) group; D: Heat map of QYHJ (L) group; E: MA chart of QYHJ (H) group; F: Heat map of QYHJ (H) group. QYHJ: Qingyi Huaji recipe; H: High-dose; L: Low-dose; PCA: Principal component analysis; PC: Principal component; MA: M-versus-A plot |
2.3.3 清胰化积方低剂量组和高剂量组差异表达基因富集分析
对清胰化积方低剂量组和高剂量组的差异表达基因进行KEGG分析,其中清胰化积方低剂量组中的差异表达基因主要富集于MAPK、PI3K-Akt、FoxO、Rap1、AMPK等信号通路,清胰化积方高剂量组中的差异表达基因主要富集于MAPK、PI3K-Akt、Rap1、Ras和mTOR等信号通路。两组共同的差异表达基因信号通路见表 1。
|
|
表 1 清胰化积方低剂量组和高剂量组共同的差异表达基因信号通路 Tab 1 Signaling pathways of common differentially expressed genes in QYHJ (L) and QYHJ (H) groups |
2.3.4 MAPK信号通路中差异表达基因的PPI分析
清胰化积方低剂量组富集到MAPK通路的差异表达基因有49个,清胰化积方高剂量组富集到MAPK通路的差异表达基因有106个,输入STRING数据库获得其PPI网络模式图(图 4)。

|
图 4 MAPK信号通路相关差异表达基因的PPI分析 Fig 4 PPI analysis of differentially expressed genes related to MAPK signaling pathways A: Qingyi Huaji recipe low-dose group; B: Qingyi Huaji recipe high-dose group. Blue represents downregulated genes, red represents upregulated genes, circles represent the size and degree. MAPK: Mitogen-activated protein kinase; PPI: Protein-protein interaction |
2.3.5 MAPK信号通路中关键基因的筛选和验证
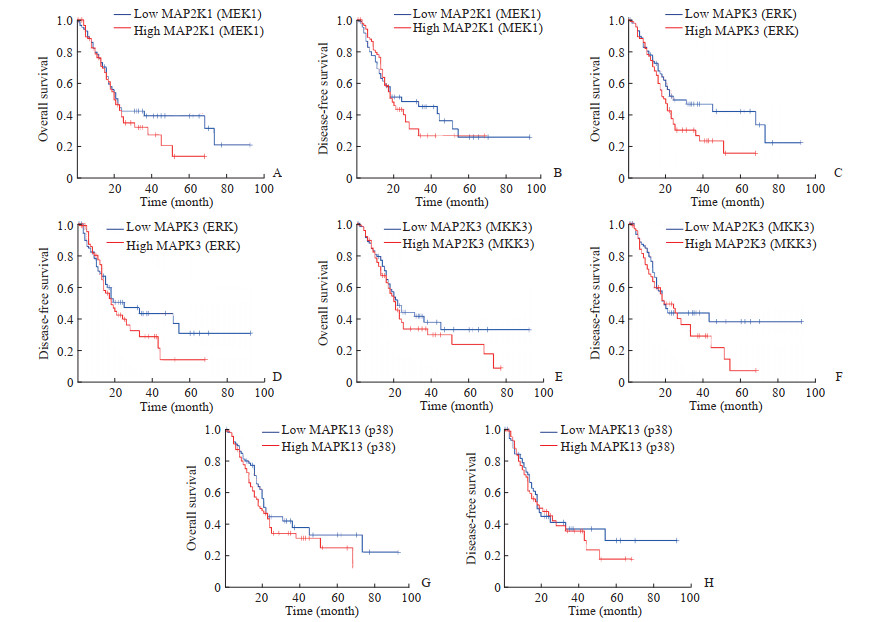
清胰化积方高剂量组中有15个关键基因(Degree>40),分别为AKT1、CDC42、HRAS、KRAS、MAP2K1、MAP2K3、MAP3K1、MAPK11、MAPK12、MAPK13、MAPK3、MAPK8、MYC、NRAS和TP53,其中有7个基因(AKT1、KRAS、MAP3K1、MAPK12、MAPK8、MYC和NRAS)与Venn分析重叠基因一致。利用GEPIA对上述关键基因进行验证,结果表明ERK1/2通路中的关键基因MAP2K1(MEK1)和MAPK3(ERK)在胰腺癌中呈高表达,p38 MAPK信号通路中的关键基因MAP2K3(MKK3)和MAPK13(p38)在胰腺癌中也呈高表达趋势(图 5)。生存分析结果显示,胰腺癌中MAP2K1(MEK1)、MAPK3(ERK)、MAP2K3(MKK3)和MAPK13(p38)高表达时患者存在预后较差的趋势,故它们可作为清胰化积方治疗胰腺癌的潜在预后因子(图 6)。
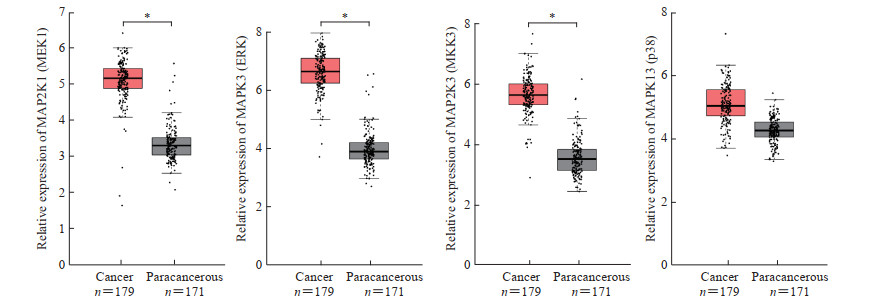
|
图 5 ERK1/2和p38 MAPK信号通路关键基因在胰腺癌和癌旁样本中的表达情况 Fig 5 Expression of key genes of ERK1/2 and p38 MAPK signaling pathways in pancreatic cancer and paracancerous samples ERK: Extracellular-regulated protein kinase; p38 MAPK: p38 mitogen-activated protein kinase; MAP2K1 (MEK1): Mitogen-activated protein kinase kinase 1; MAPK3 (ERK): Mitogen-activated protein kinase 3; MAP2K3 (MKK3): Mitogen-activated protein kinase kinase 3; MAPK13 (p38): Mitogen-activated protein kinase 13. *P < 0.05 |
|
图 6 ERK1/2和p38 MAPK信号通路关键基因与胰腺癌患者预后的关系 Fig 6 Relationship between key genes of ERK1/2 and p38 MAPK signaling pathways and prognosis of pancreatic cancer patients ERK: Extracellular regulated protein kinase; p38 MAPK: p38 mitogen-activated protein kinase; MAP2K1 (MEK1): Mitogen-activated protein kinase kinase 1; MAPK3 (ERK): Mitogen-activated protein kinase 3; MAP2K3 (MKK3): Mitogen-activated protein kinase kinase 3; MAPK13 (p38): Mitogen-activated protein kinase 13 |
3 讨论
胰腺癌是消化系统中最常见的恶性肿瘤之一[12],目前胰腺癌治疗方法以化学治疗为主,但大多数药物疗效较差、不良反应大[13]。有研究发现,使用辅助化学治疗可改善胰腺癌患者的生活质量并延长生存时间,相比最佳支持治疗对晚期胰腺癌疗效更佳[14]。中医辅助疗法不仅可以延长胰腺肿瘤患者生存时间,同时对生活质量的提高具有重要作用[15]。中药及复方具有多成分、多靶点、多通路的作用特点,作用机制的研究尤为重要。网络药理学是一个基于“疾病-基因-目标-医学”概念的交互式网络,系统并科学地评价药物对疾病网络的干预和影响,以揭示药物对机体作用的机制。该策略的系统性与中医的整体观点及中药配方中的多成分、多途径和多目标的协同作用机制相符,特别适合中药的作用机制研究。
清胰化积方是在胰腺癌“湿热积累”的机制上提出的,是以“清热燥湿”为主要原则的治疗方法,已被用于胰腺癌患者的综合治疗[16],临床和动物研究均显示清胰化积方治疗肝转移的晚期胰腺癌具有明显疗效[17]。本研究选取BALB/c裸鼠皮下种植SW1990细胞诱导胰腺癌的动物模型,该模型模拟胰腺癌肿瘤发生、发展过程,并且符合胰腺癌肿瘤生长微环境,是研究胰腺肿瘤发病及相关药物作用机制的理想模型。本研究发现治疗组相比对照组肿瘤生长受到抑制,说明清胰化积方对胰腺癌的治疗有重要作用。
我们通过网络药理学研究发现,清胰化积方通过抑制MAPK通路治疗胰腺癌,MAP2K1(MEK1)、MAPK3(ERK)、MAP2K3(MKK3)和MAPK13(p38)可作为清胰化积方治疗胰腺癌的潜在预后因子。已有研究发现MAPK通路在癌症中表现出频繁的异常激活,并使肿瘤更易于侵袭和转移[18-19]。抑制MAPK可以阻断胰腺癌细胞的增殖、迁移和侵袭能力[20]。Ras/Raf/MEK/ERK信号通路是调节细胞增殖、分化和存活过程的重要分子[21]。有研究表明RAS/MEK/ERK通路的过度激活通过增强放射疗法诱导的双链DNA断裂的修复来促进肿瘤的放射抵抗,靶向MEK会损害DNA损伤修复途径,并使肿瘤细胞对放射和化学疗法敏感[22]。MEK抑制剂会干扰同源重组和非同源性末端修复途径,从而使胰腺癌细胞对电离辐射更加敏感[23]。Awasthi等[24]提出可以通过特异性抑制MEK1/2激酶活性来增强基于紫杉醇的化学治疗方案的效果。p38 MAPK是炎症的必需激酶,在高糖条件下的胰腺癌细胞和糖尿病伴随胰腺肿瘤的动物模型中,p38 MAPK磷酸化显著增加,使用p38 MAPK抑制剂可显著抑制胰腺癌细胞的增殖和侵袭,抑制肿瘤增长和转移,提高患有胰腺肿瘤的糖尿病小鼠的存活率[25]。高糖可诱导过氧化氢的产生,并进一步通过激活ERK来调节转移相关因子尿激酶纤溶酶原激活剂的表达,从而促进胰腺癌细胞的侵袭[26]。抑制ERK和p38 MAPK信号通路在抑制高血糖驱动的活性氧诱导的胰腺癌进展中起重要作用[27]。因此,MAPK是胰腺癌治疗的关键信号通路。
综上所述,我们通过网络药理学研究结果表明,清胰化积方治疗胰腺癌的作用机制与MAPK信号通路有关,MAP2K1(MEK1)、MAPK3(ERK)、MAP2K3(MKK3)和MAPK13(p38)等可作为评价清胰化积方治疗胰腺癌疗效的潜在预后因子。清胰化积方治疗胰腺癌的具体作用机制仍需大量的体内外实验进行探究。
| [1] |
FALASCA M, KIM M, CASARI I. Pancreatic cancer: current research and future directions[J]. Biochim Biophys Acta, 2016, 1865: 123-132. |
| [2] |
SIEGEL R L, MILLER K D, JEMAL A. Cancer statistics, 2019[J]. CA Cancer J Clin, 2019, 69: 7-34. DOI:10.3322/caac.21551 |
| [3] |
ADAMSKA A, DOMENICHINI A, FALASCA M. Pancreatic ductal adenocarcinoma: current and evolving therapies[J/OL]. Int J Mol Sci, 2017, 18: 1338. doi: 10.3390/ijms18071338.
|
| [4] |
沈晔华, 刘鲁明, 孟志强, 陈震, 林钧华, 周振华, 等. 清胰化积方为主综合治疗晚期胰腺癌64例生存分析[J]. 中医杂志, 2009, 50: 39-42. |
| [5] |
王凤娇, 陈联誉, 陈震, 刘鲁明. 概述清胰化积方抑制胰腺癌发生发展的现代研究机制[J]. 上海中医药杂志, 2017, 51: 95-98. |
| [6] |
GE Q, CHEN L, TANG M, ZHANG S, LIU L, GAO L, et al. Analysis of mulberry leaf components in the treatment of diabetes using network pharmacology[J]. Eur J Pharmacol, 2018, 833: 50-62. DOI:10.1016/j.ejphar.2018.05.021 |
| [7] |
SHAWKY E. Prediction of potential cancer-related molecular targets of North African plants constituents using network pharmacology-based analysis[J/OL]. J Ethnopharmacol, 2019, 238: 111826. doi: 10.1016/j.jep.2019.111826.
|
| [8] |
LIANG J W, WANG M Y, OLOUNFEH K M, ZHAO N, WANG S, MENG F H. Network pharmacology-based identification of potential targets of the flower of Trollius chinensis Bunge acting on anti-inflammatory effects[J/OL]. Sci Rep, 2019, 9: 8109. doi: 10.1038/s41598-019-44538-z.
|
| [9] |
李仪奎.中药药理实验方法学[M].2版.上海: 上海科学技术出版社, 2006: 附录3.
|
| [10] |
ANDERS S, HUBER W. Differential expression of RNA-Seq data at the gene level—the DESeq package[Z/OL]. (2013-02-24)[2019-08-30]. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.359.7464&rep=rep1&type=pdf.
|
| [11] |
TANG Z, LI C, KANG B, GAO G, LI C, ZHANG Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses[J]. Nucleic Acids Res, 2017, 45(W1): W98-W102. DOI:10.1093/nar/gkx247 |
| [12] |
SUN Q, ZHANG B, HU Q, QIN Y, XU W, LIU W, et al. The impact of cancer-associated fibroblasts on major hallmarks of pancreatic cancer[J]. Theranostics, 2018, 8: 5072-5087. DOI:10.7150/thno.26546 |
| [13] |
BRUNNER T B, KUNZ-SCHUGHART L A, GROSSE-GEHLING P, BAUMANN M. Cancer stem cells as a predictive factor in radiotherapy[J]. Semin Radiat Oncol, 2012, 22: 151-174. DOI:10.1016/j.semradonc.2011.12.003 |
| [14] |
ZHANG Y L, ZHANG X, MIAO X Z, YUAN Y Y, GAO J, LI X, et al. Coptisine suppresses proliferation and inhibits metastasis in human pancreatic cancer PANC-1 cells[J]. J Asian Nat Prod Res, 2020, 22: 452-463. DOI:10.1080/10286020.2019.1585820 |
| [15] |
LI M, WANG M M, GUO X W, WU C Y, LI D R, ZHANG X, et al. Different survival benefits of chinese medicine for pancreatic cancer: how to choose?[J]. Chin J Integr Med, 2018, 24: 178-184. DOI:10.1007/s11655-017-2971-1 |
| [16] |
WANG P, CHEN Z, MENG Z Q, LUO J M, LIN J H, ZHOU Z H, et al. Ski acts as therapeutic target of Qingyihuaji formula in the treatment of SW1990 pancreatic cancer[J]. Integr Cancer Ther, 2010, 9: 50-58. DOI:10.1177/1534735409359179 |
| [17] |
YIN J H, SHI W D, ZHU X Y, CHEN Z, LIU L M. Qingyihuaji formula inhibits progress of liver metastases from advanced pancreatic cancer xenograft by targeting to decrease expression of Cyr61 and VEGF[J]. Integr Cancer Ther, 2012, 11: 37-47. DOI:10.1177/1534735411400315 |
| [18] |
GU D N, JIANG M J, MEI Z, DAI J J, DAI C Y, FANG C, et al. microRNA-7 impairs autophagy-derived pools of glucose to suppress pancreatic cancer progression[J]. Cancer Lett, 2017, 400: 69-78. DOI:10.1016/j.canlet.2017.04.020 |
| [19] |
LIU Y, TONG L, LUO Y, LI X, CHEN G, WANG Y. Resveratrol inhibits the proliferation and induces the apoptosis in ovarian cancer cells via inhibiting glycolysis and targeting AMPK/mTOR signaling pathway[J]. J Cell Biochem, 2018, 119: 6162-6172. DOI:10.1002/jcb.26822 |
| [20] |
HU M, CHEN X, MA L, MA Y, LI Y, SONG H, et al. AMPK inhibition suppresses the malignant phenotype of pancreatic cancer cells in part by attenuating aerobic glycolysis[J]. J Cancer, 2019, 10: 1870-1878. DOI:10.7150/jca.28299 |
| [21] |
SAMATAR A A, POULIKAKOS P I. Targeting RAS-ERK signalling in cancer: promises and challenges[J]. Nat Rev Drug Discov, 2014, 13: 928-942. DOI:10.1038/nrd4281 |
| [22] |
TOULANY M, ⅡDA M, KEINATH S, IYI F F, MUECK K, FEHRENBACHER B, et al. Dual targeting of PI3K and MEK enhances the radiation response of K-RAS mutated non-small cell lung cancer[J]. Oncotarget, 2016, 7: 43746-43761. DOI:10.18632/oncotarget.9670 |
| [23] |
ESTRADA-BERNAL A, CHATTERJEE M, HAQUE S J, YANG L, MORGAN M A, KOTIAN S, et al. MEK inhibitor GSK1120212-mediated radiosensitization of pancreatic cancer cells involves inhibition of DNA double-strand break repair pathways[J]. Cell Cycle, 2015, 14: 3713-3724. DOI:10.1080/15384101.2015.1104437 |
| [24] |
AWASTHI N, MONAHAN S, STEFANIAK A, SCHWARZ M A, SCHWARZ R E. Inhibition of the MEK/ERK pathway augments nab-paclitaxel-based chemotherapy effects in preclinical models of pancreatic cancer[J]. Oncotarget, 2017, 9: 5274-5286. |
| [25] |
WANG L, BAI Y Y, YANG Y, HU F, WANG Y, YU Z, et al. Diabetes mellitus stimulates pancreatic cancer growth and epithelial-mesenchymal transition-mediated metastasis via a p38 MAPK pathway[J]. Oncotarget, 2016, 7: 38539-38550. DOI:10.18632/oncotarget.9533 |
| [26] |
LI W, MA Z, MA J, LI X, XU Q, DUAN W, et al. Hydrogen peroxide mediates hyperglycemia-induced invasive activity via ERK and p38 MAPK in human pancreatic cancer[J]. Oncotarget, 2015, 6: 31119-31133. DOI:10.18632/oncotarget.5045 |
| [27] |
CAO L, CHEN X, XIAO X, MA Q, LI W. Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and migration of pancreatic cancer cells via suppression of the ERK and p38 MAPK signaling pathways[J]. Int J Oncol, 2016, 49: 735-743. DOI:10.3892/ijo.2016.3559 |
 2020, Vol. 41
2020, Vol. 41


