2. 海军军医大学(第二军医大学)长海医院病理科, 上海 200433
2. Department of Pathology, Changhai Hospital, Naval Medical University(Second Military Medical University), Shanghai 200433, China
法尼醇X受体(farnesol X receptor,FXR)又称核因子受体1H4(nuclear factor receptor 1H4,NR1H4),与人类肿瘤的发生有关,但关于其在胰腺癌中的作用研究结果并不一致。Lee等[1]研究发现FXR/NR1H4过表达促进胰腺癌发生和淋巴结转移,与患者预后差相关。Hu等[2]从mRNA和蛋白水平发现FXR/NR1H4在胰腺癌组织中的表达高于癌旁组织,是一个不良的预后因素。但Giaginis等[3]的研究结果显示FXR/NR1H4具有抗肿瘤作用且与预后好有关。
为了提高研究结果的可靠性,本研究采用美国ACD公司新型RNAscope 2.5 mRNA原位杂交(in situ hybridization,ISH)技术和免疫组织化学(immunohistochemistry,IHC)技术分别检测176例人胰腺导管腺癌组织芯片中FXR/NR1H4 mRNA和蛋白的表达状态,优化实验条件并进行互相印证,进一步探讨FXR/NR1H4在胰腺导管腺癌组织中的表达及其与患者临床病理特征和预后的关系。
1 材料和方法 1.1 组织标本选取2013年至2016年海军军医大学(第二军医大学)长海医院胰腺外科手术切除的胰腺导管腺癌组织标本176例,同时收集患者临床病理资料。176例患者中男108例,女68例;年龄为32~75岁,平均年龄(60.6±11.5)岁。其中高分化或中分化标本110例,低分化或未分化标本66例;按照国际抗癌联盟TNM分期标准,Ⅰ、Ⅱ期标本138例,Ⅲ、Ⅳ期标本38例。纳入标准:(1)10%甲醛溶液固定的石蜡包埋(formalin-fixed paraffin-embedded,FFPE)胰腺导管腺癌组织标本;(2)经病理学确诊为胰腺导管腺癌;(3)临床病理资料完整;(4)术前未行放射和化学治疗;(5)无其他原发性肿瘤。其中48例胰腺导管腺癌病例有配对的癌旁胰腺组织(距癌组织边缘1~1.5 cm)和正常胰腺组织(经病理学检查证实无癌细胞)。
1.2 组织芯片制作将FFPE胰腺导管腺癌组织标本常规制成H-E切片,确定具有代表性的肿瘤组织及癌旁组织,然后应用Quick-Ray预铸蜡块模块(孔径1.5 mm,阵列10×9)和手工组织芯片制作枪(韩国UniTMA公司)制备组织芯片。
1.3 试剂与耗材NR1H4单克隆抗体(货号:ab187735;稀释比例1:200)、NR1H4多克隆抗体(货号:ab235094;稀释比例1:1 000)、羊抗鼠IgG-FITC(稀释比例1:500)均购自英国Abcam公司,NR1H4多克隆抗体(货号:25055-1-AP;稀释比例1:100)购自美国Proteintech公司,FXR单克隆抗体(货号:SC-25309;稀释比例1:30)购自美国Santa Cruz公司;RNAScope® NR1H4 mRNA探针(货号:494541)、RNAscope® 2.5 HD检测试剂盒(棕色,货号:322310)、阳性对照探针Hs-PPIB(货号:313901)、阴性对照探针DapB(货号:310043)、RNAscope®多通道二代荧光HRP-C1、TSA® Plus-Cyanine 5、RNAscope®多通道二代荧光HRP阻断剂均购自美国ACD公司。DAPI(货号:ab104139)购自英国Abcam公司。
1.4 IHC方法和评分IHC采用超敏低背景的多聚物二步法,分别采用4种FXR/NR1H4一抗进行检测。切片常规脱蜡至水,热诱导抗原修复,阻断内源性过氧化物酶,分别加入适当稀释的一抗和多聚物二抗进行孵育,DAB显色,苏木精衬染,中性树胶封片,显微镜下观察。由2位病理科医师(1位主治医师、1位副主任医师)分别独立对FXR/NR1H4染色强度及范围进行评分。染色强度:无染色为0分,浅黄色为1分,棕黄色为2分,棕褐色为3分;染色范围:<5%为0分,5%~25%为1分,>25%~50%为2分,>50%~75%为3分,>75%为4分。取两者评分的乘积为最终总评分,其中<4分为低表达,≥4分为高表达。
1.5 RNAscope ISH方法和评分先对切片进行RNAscope质量控制检测,结果显示176例样本均符合质量控制要求。RNAscope ISH操作步骤详见ACD公司推荐操作流程和文献[4],简述如下:切片常规脱蜡至水,100 ℃靶标修复25 min;用ImmedgeTM阻水笔在组织周围画阻水圈;蛋白酶消化;阻断内源性过氧化物酶;滴加NR4H1 RNAscope®探针,杂交过夜;依次40 ℃ 30 min、40 ℃ 15 min、40 ℃ 30 min、40 ℃ 15 min、室温30 min、室温15 min放大杂交信号;DAB显色10 min,衬染,中性树胶常规封片,显微镜下观察。RNAscope ISH结果分为5个级别:0分(阴性,无色或<1点/细胞,40倍视野),1分(1~3点/细胞,20~40倍视野),2分(4~10点/细胞,无或极少数点簇,20~40倍视野),3分(>10点/细胞,<10%的阳性细胞有点状团簇,20倍视野),4分(>10点/细胞,≥10%的阳性细胞有点状团簇,20倍视野)。其中0分和1分为低表达,2~4分为高表达。
1.6 RNAscope ISH和IHC荧光双重标记操作步骤详见ACD公司推荐操作流程和文献[4],简述如下:切片脱蜡水化后靶标修复,蛋白酶消化;阻断内源性过氧化物酶;滴加NR1H4单克隆抗体;滴加NR1H4 RNAscope®探针,杂交过夜;杂交信号放大(40 ℃ 30 min、40 ℃ 15 min、40 ℃ 30 min);荧光信号放大(先后加入RNAscope®多通道二代荧光HRP-C1、TSA® Plus-Cyanine 5、RNAscope®多通道二代荧光HRP阻断剂);加入羊抗鼠IgG-FITC;加入DAPI孵育30 s;用Prolong Gold防淬灭封片,4 ℃避光保存。在荧光显微镜下观察RNAscope ISH和IHC定位情况。
1.7 统计学处理采用SPSS 20.0软件和R 3.5.1软件进行统计学分析。计数资料以例数和百分数表示,组间比较采用χ2检验。采用Spearman秩相关分析探讨2种检测方法的相关性。采用Kaplan-Meier生存分析和Cox比例风险回归模型研究FXR/NR1H4 mRNA和蛋白表达与预后的关系。检验水准(α)为0.05。
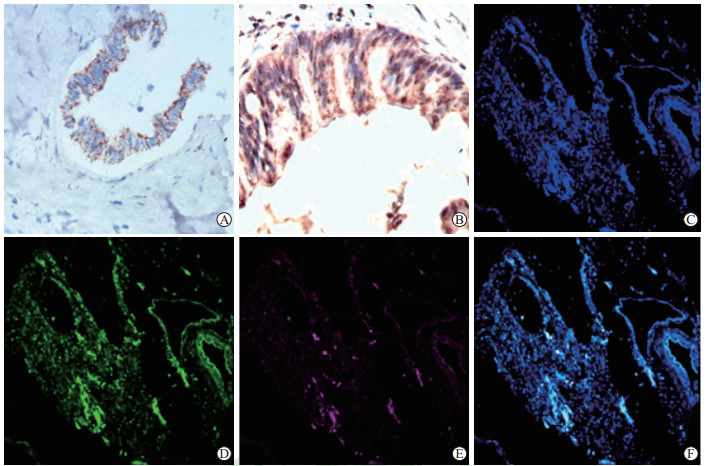
2 结果 2.1 RNAScope ISH和IHC染色质量与共定位分别采用RNAScope ISH和IHC对胰腺导管腺癌组织中FXR/NR1H4的mRNA和蛋白进行染色,发现NR1H4 mRNA呈细胞质内局灶性和散在性颗粒状染色,FXR/NR1H4蛋白呈细胞核和(或)细胞质型染色,前者的背景染色较后者低,分辨率优于后者(图 1A、1B)。应用176例胰腺导管腺癌组织芯片进行RNAscope ISH和IHC荧光双重标记,结果显示mRNA的定位较蛋白更准确(图 1C~1F),两者共阳性率为67.05%(118/176)。
|
图 1 RNAscope ISH和IHC检测胰腺导管腺癌石蜡切片中FXR/NR1H4 mRNA和蛋白的表达 Fig 1 FXR/NR1H4 mRNA and protein expression in paraffin sections of pancreatic ductal adenocarcinoma detected by RNAscope ISH and IHC A: NR1H4 mRNA expression detected by RNAscope ISH; B: FXR/NR1H4 protein expression detected by IHC; C-F: Co-localization of FXR/NR1H4 mRNA and protein (C: DAPI; D: FXR protein, FITC-yellowish green; E: NR1H4 mRNA, Cyanine 5-fuchsia; F: FXR/NR1H4 mRNA and protein co-expression). ISH: In situ hybridization; IHC: Immunohistochemistry; FXR: Farnesol X receptor; NR1H4: Nuclear factor receptor 1H4; DAPI: 4', 6-diamidino-2-phenylindole; FITC: Fluorescein isothiocyanate. Original magnification: ×200 (A), ×400 (B-F) |
2.2 NR1H4 mRNA在胰腺导管腺癌中的表达
176例胰腺导管腺癌组织中NR1H4 mRNA总体阳性表达率为68.75%(121/176),其中39例NR1H4 mRNA高表达。对48例胰腺导管腺癌病例的癌组织、配对癌旁胰腺组织和正常胰腺组织进行RNAscope ISH检测,发现NR1H4 mRNA在胰腺导管腺癌、间质、癌周组织呈不同程度表达,并且在癌组织中的阳性表达率(66.67%,32/48)高于癌旁胰腺组织(31.25%,15/48)和正常胰腺组织(16.67%,8/48),差异均有统计学意义(χ2=12.05、24.69,P均<0.01)。
2.3 FXR/NR1H4蛋白在胰腺导管腺癌中的表达采用4种不同的抗体对176例胰腺导管腺癌组织中的FXR/NR1H4进行IHC检测,结果显示以ab187735、ab235094、25055-1-AP、SC-25309抗体检测的阳性率分别为68.75%(121/176)、75.57%(133/176)、73.30%(129/176)、71.02%(125/176),总体阳性率为77.27%(136/176),其中89例FXR/NR1H4蛋白高表达。对48例胰腺导管腺癌病例的癌组织、配对癌旁胰腺组织和正常胰腺组织进行IHC检测,发现FXR/NR1H4蛋白在癌组织、癌旁非肿瘤组织中呈不同程度表达,并且在癌组织中的阳性表达率(75.00%,36/48)高于癌旁胰腺组织(35.42%,17/48)和正常胰腺组织(20.83%,10/48),差异均有统计学意义(χ2=15.21、28.22,P均<0.01)。
2.4 FXR/NR1H4 mRNA和蛋白在胰腺导管腺癌中表达的一致性176例胰腺导管腺癌组织中,RNAscope ISH检测121例NR1H4 mRNA阳性、55例阴性,IHC检测136例FXR/NR1H4蛋白阳性、40例阴性。其中RNAscope ISH和IHC检测结果均为阳性118例、均为阴性37例,一致率为88.07%(155/176);RNAscope ISH检测阳性而IHC检测阴性3例,IHC检测阳性而RNAscope ISH检测阴性18例。Spearman秩相关分析结果显示,NR1H4 mRNA表达水平与FXR/NR1H4蛋白表达水平呈正相关(r=0.307,P<0.01)。
2.5 FXR/NR1H4 mRNA和蛋白表达与胰腺导管腺癌患者临床病理特征的关系由表 1可见,FXR/NR1H4 mRNA和蛋白表达均与肿瘤临床分期(χ2=5.391,P=0.020;χ2=4.108,P=0.042)、肿瘤分化程度(χ2=6.560,P=0.010;χ2=4.969,P=0.026)有关,蛋白的表达也与肿瘤大小有关(χ2=4.957,P=0.026);两者与患者性别、年龄、神经浸润、肿瘤部位、淋巴结转移均无关(P均>0.05)。
|
|
表 1 FXR/NR1H4 mRNA和蛋白表达与胰腺导管腺癌患者临床病理特征的关系 Tab 1 Relationship between expression of FXR/NR1H4 mRNA and protein and clinicopathological features of pancreatic ductal adenocarcinoma patients |
2.6 FXR/NR1H4 mRNA和蛋白表达与胰腺导管腺癌患者预后的关系
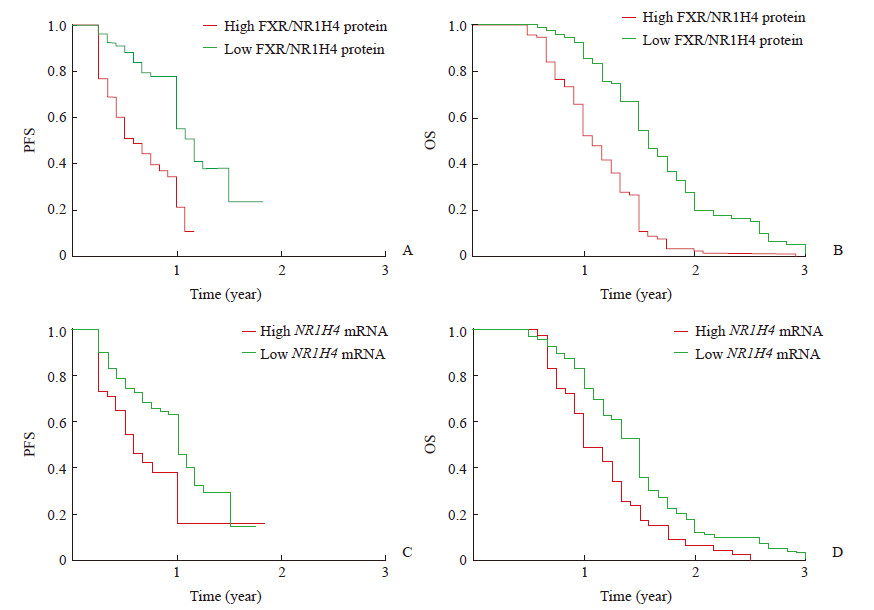
Kaplan-Meier生存分析结果显示,FXR/NR1H4 mRNA和蛋白高表达的患者无进展生存期及总生存期均短于低表达的患者(P均<0.01,图 2)。Cox比例风险回归模型分析结果(表 2)显示,癌组织的分化程度和FXR/NR1H4蛋白的表达水平是胰腺导管腺癌患者无进展生存期的独立危险因素(P均<0.05),远处转移和FXR/NR1H4蛋白的表达水平是总生存期的危险因素(P均<0.05)。
|
图 2 FXR/NR1H4 mRNA和蛋白高表达与低表达的胰腺导管腺癌患者的Kaplan-Meier生存分析 Fig 2 Kaplan-Meier survival analysis of pancreatic ductal adenocarcinoma patients with high or low expression levels of FXR/NR1H4 mRNA and protein A: High expression of FXR/NR1H4 protein is correlated with poor PFS (P < 0.01); B: High expression of FXR/NR1H4 protein is associated with poor OS (P < 0.01); C: High expression of NR1H4 mRNA is associated with poor PFS (P < 0.01); D: High expression of NR1H4 mRNA is associated with poor OS (P < 0.01). FXR: Farnesol X receptor; NR1H4: Nuclear factor receptor 1H4; PFS: Progression-free survival; OS: Overall survival |
|
|
表 2 胰腺导管腺癌患者预后危险因素的Cox比例风险回归分析结果 Tab 2 Cox regression analysis of prognostic risk factors in patients with pancreatic ductal adenocarcinoma |
3 讨论
FXR/NR1H4是一种核受体,在胆汁酸代谢、脂质/胆固醇和葡萄糖稳态相关基因的转录调控中起着关键作用,研究表明FXR/NR1H4在多种正常组织和肿瘤组织中表达[5-12]。本研究收集了176例胰腺导管腺癌样本,采用ACD公司RNAscope 2.5 mRNR ISH和IHC技术分别检测胰腺导管腺癌组织芯片中FXR/NR1H4 mRNA和蛋白的表达,并互相印证。RNAscope具有灵敏度高、特异性强、稳定性高、可视性与定量分析、非常适合FFPE组织切片检测、不限物种应用和多重标记等特点[13-14]。IHC方法的影响因素很多,主要有组织固定、前处理方式(抗原修复)、一抗的选择和检测方法的灵敏性等,本研究中IHC采用超敏低背景的多聚物二步法,并使用了不同公司生产、不同克隆号的4种FXR/NR1H4一抗。RNAscope ISH结果显示NR1H4 mRNA呈细胞质内局灶性和散在性颗粒状染色,IHC结果显示FXR/NR1H4蛋白呈细胞核和(或)细胞质型染色,前者的背景染色较后者低,分辨率优于后者。通过对176例胰腺导管腺癌组织芯片进行RNAscope ISH和IHC荧光双重标记,结果显示mRNA的定位较蛋白更准确,其共阳性率达67.05%(118/176)。RNAscope ISH和IHC检测结果均为阳性118例、均为阴性37例,一致率为88.07%(155/176);Spearman秩相关分析结果显示,NR1H4 mRNA表达水平与FXR/NR1H4蛋白表达水平呈正相关(r=0.307,P<0.01)。上述研究结果表明,RNAscope ISH和IHC检测结果一致性较高,可以互相印证,使研究结果更可靠。
FXR/NR1H4抗原在胰腺导管腺癌细胞中呈不同程度的细胞核、细胞质和核质表达,以细胞核表达为主,其次为核质;而在癌旁胰腺组织和正常胰腺组织中则主要表达在细胞质,且表达量较低[1, 2, 5, 10]。王维斌等[10]认为FXR/NR1H4在正常胰腺组织中普遍低表达,而在胰腺癌细胞中呈不同程度的细胞质和细胞核高表达,胰腺胰岛细胞中亦呈普遍高表达,胰腺神经组织呈阴性。Giaginis等[3]的IHC结果显示,60.0%(33/55)的胰腺癌组织中FXR/NR1H4表达阳性,呈细胞核和(或)细胞质型染色,其中27例(49.1%)FXR/NR1H4高表达,癌旁非肿瘤部位FXR/NR1H4表达阴性。本研究采用48例胰腺导管腺癌组织及配对的癌旁胰腺组织和正常胰腺组织观察FXR/NR1H4 mRNA和蛋白在不同胰腺细胞的表达情况,结果表明FXR/NR1H4 mRNA和蛋白在癌组织中的阳性表达率高于癌旁胰腺组织和正常胰腺组织,与上述研究结果基本一致。
关于FXR/NR1H4在肿瘤中的作用目前报道结果并不一致。在胰腺癌中,有研究发现FXR/NR1H4在癌组织中高表达并导致癌症的发生和发展[1-2],也有学者认为FXR/NR1H4具有抑制肿瘤生长的作用[3]。王维斌等[10]研究结果显示FXR/NR1H4在胰腺癌组织中呈不同程度的高表达,与胰腺癌组织病理G分期密切相关(P<0.01),并且FXR/NR1H4高表达患者生存时间较低表达患者长(P<0.05)。本研究结果表明胰腺导管腺癌组织中FXR/NR1H4 mRNA和蛋白表达与肿瘤临床分期、分化程度有关(P<0.05),蛋白表达也与肿瘤大小有关(P<0.05),而与性别、年龄、神经浸润、肿瘤部位、淋巴结转移均无关(P>0.05)。Kaplan-Meier生存分析结果显示,FXR/NR1H4 mRNA和蛋白高表达的患者无进展生存期及总生存期均短于低表达的患者(P均<0.05);Cox比例风险回归模型显示,FXR/NR1H4蛋白高表达是影响预后的独立危险因素(P<0.05)。上述结果与Lee等[1]和Hu等[2]的研究结果一致,表明FXR/NR1H4高表达的患者预后差、生存期短。
综上所述,RNAscope ISH可与IHC技术互相印证,保证检测结果的可靠、精准。FXR/NR1H4在胰腺导管腺癌中的表达与肿瘤临床分期和分化程度有关,其高表达是影响胰腺导管腺癌患者预后的独立危险因素。
| [1] |
LEE J Y, LEE K T, LEE J K, LEE K H, JANG K T, HEO J S, et al. Farnesoid X receptor, overexpressed in pancreatic cancer with lymph node metastasis promotes cell migration and invasion[J]. Br J Cancer, 2011, 104: 1027-1037. DOI:10.1038/bjc.2011.37 |
| [2] |
HU H, WU L L, HAN T, ZHUO M, LEI W, CUI J J, et al. Correlated high expression of FXR and Sp1 in cancer cells confers a poor prognosis for pancreatic cancer:a study based on TCGA and tissue microarray[J]. Oncotarget, 2017, 8: 33265-33275. DOI:10.18632/oncotarget.16633 |
| [3] |
GIAGINIS C, KOUTSOUNAS I, ALEXANDROU P, ZIZI-SERBETZOGLOU A, PATSOURIS E, KOURAKLIS G, et al. Elevated farnesoid X receptor (FXR) and retinoid X receptors (RXRs) expression is associated with less tumor aggressiveness and favourable prognosis in patients with pancreatic adenocarcinoma[J]. Neoplasma, 2015, 62: 332-341. DOI:10.4149/neo_2015_040 |
| [4] |
WANG F, FLANAGAN J, SU N, WANG L C, BUI S, NIELSON A, et al. RNAscope®:a novel in situ RNA analysis platform for formalin-fixed paraffin-embedded tissues[J]. J Mol Diagn, 2012, 14: 22-29. DOI:10.1016/j.jmoldx.2011.08.002 |
| [5] |
PELLICCIARI R, COSTANTINO G, FIORUCCI S. Farnesoid X receptor:from structure to potential clinical applications[J]. J Med Chem, 2005, 48: 5383-5403. DOI:10.1021/jm0582221 |
| [6] |
ZHU Y, LI F, GUO G L. Tissue-specific function of famesoid X receptor in liver and intestine[J]. Pharmacol Res, 2011, 63: 259-265. DOI:10.1016/j.phrs.2010.12.018 |
| [7] |
CIPRIANI S, MENCARELLI A, PALLADINO G, FIORUCCI S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats[J]. J Lipid Res, 2010, 51: 771-784. DOI:10.1194/jlr.M001602 |
| [8] |
HAGEMAN J, HERREMA H, GROEN A K, KUIPERS F. A role of the bile salt receptor FXR in atheroselerosis[J]. Arterioscler Thromb Vasc Biol, 2010, 30: 1519-1528. DOI:10.1161/ATVBAHA.109.197897 |
| [9] |
CHAWLA A, REPA J J, EVANS R M, MANGELSDORF D J. Nuclear receptors and lipid physiology:opening the X-files[J]. Science, 2001, 294: 1866-1870. DOI:10.1126/science.294.5548.1866 |
| [10] |
王维斌, 董良博, 赵邦博, 卢军, 赵玉沛. 法尼醇X受体表达与胰腺癌患者预后及病理分期相关[J]. 基础医学与临床, 2018, 38: 394-399. DOI:10.3969/j.issn.1001-6325.2018.03.022 |
| [11] |
WANG Y D, CHEN W D, MOORE D D, HUANG W. FXR:a metabolic regulator and cell protector[J]. Cell Res, 2008, 18: 1087-1095. DOI:10.1038/cr.2008.289 |
| [12] |
KIM K H, CHOI S, ZHOU Y, KIM E Y, LEE J M, SAHA P K, et al. Hepatic FXR/SHP axis modulates systemic glucose and fatty acid homeostasis in aged mice[J]. Hepatology, 2017, 66: 498-509. DOI:10.1002/hep.29199 |
| [13] |
DUNCAN D J, SCOTT M, SCORER P, BARKER C. Assessment of PD-L1 mRNA and protein expression in non-small cell lung cancer, head and neck squamous cell carcinoma and urothelial carcinoma tissue specimens using RNAScope and immunohistochemistry[J/OL]. PLoS One, 2019, 14: e0215393. doi: 10.1371/journal.pone.0215393.
|
| [14] |
FEDERMANN B, FRAUENFELD L, PERTSCH H, BORGMANN V, STEINHILBER J, BONZHEIM I, et al. Highly sensitive and specific in situ hybridization assay for quantification of SOX11 mRNA in mantle cell lymphoma reveals association of TP53 mutations with negative and low SOX11 expression[J]. Haematologica, 2020, 105: 754-764. DOI:10.3324/haematol.2019.219543 |
 2020, Vol. 41
2020, Vol. 41


