胰腺神经内分泌肿瘤(pancreatic neuroendocrine neoplasm,pNEN)是一组起源于胰腺肽能神经元和神经内分泌细胞的肿瘤[1],其具有发病率低、异质性强、临床表现多变及治疗手段和治疗顺序复杂等特点。准确的术前诊断对pNEN患者治疗策略的选择和预后判断具有重要意义。典型的pNEN诊断并不困难,多数患者发病年龄为40~65岁,呈富血供,表现为动脉期或门静脉期显著强化、延迟期造影剂部分退出[2]。pNEN较少阻塞胰管[3],当pNEN患者出现主胰管扩张等不典型影像学表现时,有时与胰腺其他肿瘤的鉴别有一定困难,加之其发病率较低,经验不足的医师可能会误诊、漏诊。本研究收集了我院2012年6月至2018年10月收治的伴有胰管阻塞的pNEN患者资料,试分析其影像学特征和误诊原因,以期加强对该病的认识和提高术前诊断准确率。
1 资料和方法 1.1 研究对象与一般资料回顾性分析2012年6月至2018年10月在我院接受手术治疗并经病理检查证实为pNEN的200例患者资料。纳入标准:(1)手术病理结果为pNEN,且临床资料完整;(2)术前进行胰腺CT和(或)MRI平扫和增强检查,且可见主胰管扩张(主胰管最大径≥3 mm);(3)检查前未接受过任何治疗。排除标准:图像质量不佳,影响观察。最终纳入25例患者,其中男13例、女12例,年龄为30~74岁,平均年龄为(52±12)岁。1例男性患者有2枚pNEN病灶,因此共纳入病灶26枚。
1.2 检查方法CT设备为日本东芝Aquilion ONE 320排动态容积CT机。扫描条件:管电压120 kV,管电流300 mA。扫描参数:准直器宽度2.5 mm,层厚0.5 mm,重建间隔3.0 mm,螺距5.3,矩阵512×512,视野348 mm×348 mm。增强扫描由肘静脉团注非离子型造影剂碘普罗胺(含碘355 mg/mL)90~95 mL,注射速率5.5 mL/s,之后以同样速率继续注射生理盐水冲洗。分别于注射造影剂后20~25、60~70、110~130 s进行动脉期、门静脉期和延迟期扫描。
磁共振扫描设备为德国西门子Skyra 3.0 T超导型磁共振扫描仪,使用体部线圈。扫描序列:横断位T1加权像重复时间(repetition time,TR)3.97 ms,回波时间(echo time,TE)1.26 ms;T2加权像半傅立叶单次激发快速自旋回波序列TR 1 400 ms,TE 92 ms;脂肪抑制T2快速恢复快速自旋回波序列TR 4 820 ms,TE 83 ms。其他参数:视野400 mm×400 mm,矩阵320×320,采集次数2次,层厚5 mm,层间距1 mm。动态增强扫描采用梯度回波快速三维容积式插入法屏气检查序列,TR 3.46 ms,TE 1.3 ms,层厚3 mm,层间距1 mm,视野320 mm×400 mm,矩阵195×320。扩散加权成像(diffusion-weighted imaging,DWI)中扩散敏感因子(b)值取1 000 s/mm2。增强扫描由静脉团注钆喷酸葡胺(gadolinium-diethylenetriamine pentaacetic acid,Gd-DTPA),剂量0.1 mmol/kg,注射速率3.0 mL/s,注射造影剂结束后用生理盐水以同样速率冲洗。分别于注射造影剂后20~25、60~70、110~130 s进行动脉期、门静脉期和延迟期扫描。
1.3 图像分析由2名高年资影像诊断医师分别阅片,意见不同时通过讨论达成一致意见。(1)病灶位置:分为胰头、胰颈、胰体、胰尾。(2)病灶大小:取横断位病灶最大层面的长径。(3)病灶性质:分为实性、囊性和囊实性,内部实性成分占比>90%定义为实性,50%~90%为囊实性,<50%为囊性[4]。(4)边界:分为清晰、不清。边界光滑、清晰可见定义为肿瘤边界清晰,边缘有毛刺或肿瘤周界浸润>90°为边界不清[5]。(5)强化方式:分为富血供和非富血供,以病灶周围正常胰腺实质为对比,根据动脉期和门静脉期病灶内实性成分的强化程度进行评估。(6)主胰管扩张程度:主胰管最大径3~6 mm为轻度扩张,>6~9 mm为中度扩张,>9 mm为显著扩张[6]。(7)胰腺萎缩程度:测量瘤体上游除去主胰管后的胰腺实质最大前后径判断有无上游胰腺萎缩。胰腺实质最大前后径<9 mm判定为上游胰腺明显萎缩,9~<12 mm为上游胰腺中度萎缩,12~<15 mm为胰腺实质轻度萎缩[6]。(8)有无胆管扩张、有无周围组织侵犯及远处转移、有无血管侵犯等。
1.4 统计学处理应用SPSS 22.0软件进行统计学分析。计数资料以例数(枚数)和百分数表示,计量资料以x±s表示。
2 结果 2.1 临床特征25例患者中,6例患者为健康体检时发现肿瘤,1例表现为反复胃溃疡,1例表现为高血糖,2例黄疸,余15例主要表现为腹胀、腹痛、腰背痛等非特异性症状。2例患者伴有CA19-9升高,分别为38.6 U/mL和67.7 U/mL,余23例患者肿瘤标志物均正常。依据2010 WHO分类标准[7],26枚pNEN病灶中G1级6枚、G2级19枚、G3级1枚。
2.2 影像学表现18例患者接受CT检查,17例患者接受MRI检查,其中10例患者同时接受CT和MRI检查。(1)病灶位置、大小、性质及边界情况:26枚病灶大小为0.4~9.1 cm,平均(2.5±1.7)cm,中位数为2.3 cm。14枚(53.8%)病灶位于胰头(图 1),4枚(15.4%)位于胰颈,6枚(23.1%)位于胰体,2枚(7.7%)位于胰尾。24枚(92.3%)病灶表现为实性,2枚(7.7%)表现为囊实性。12枚(46.2%)病灶与周围组织界限清晰,14枚(53.8%)病灶边界不清。(2)强化方式:16枚(61.5%)病灶富血供,其中13枚在增强扫描动脉期和门静脉期强化程度高于周围正常胰腺实质,3枚在动脉期强化程度与周围正常胰腺实质相仿、门静脉期强化程度明显高于周围胰腺实质;10枚(38.5%)病灶为非富血供,在增强扫描各期强化程度均相等或低于周围正常胰腺实质。(3)主胰管扩张、胰腺萎缩程度:12枚(46.2%)主胰管轻度扩张,8枚(30.8%)中度扩张,6枚(23.1%)显著扩张。17枚(65.4%)病灶上游胰腺实质重度萎缩,6枚(23.1%)上游胰腺实质中度萎缩,1枚(3.8%)上游胰腺实质轻度萎缩,2枚(7.7%)未见上游胰腺实质萎缩。5枚(19.2%)病灶上游胰腺可见潴留囊肿。(4)胆管扩张、周围组织侵犯、远处转移、血管侵犯情况:5例患者(5枚病灶,19.2%)可见胆总管扩张,2例患者(2枚病灶,7.7%)伴有肝转移,1例患者(1枚病灶,3.8%)伴淋巴结转移。25例患者均未见血管侵犯。
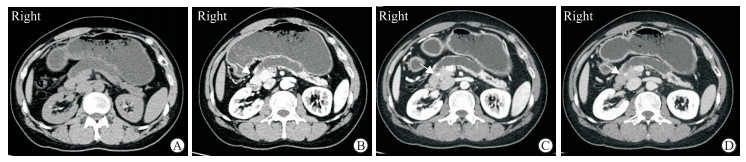
|
图 1 1例胰头pNEN伴胰管梗阻的34岁男性患者CT影像 Fig 1 CT images of a 34-year-old male patient with pNEN of pancreatic head and pancreatic duct obstruction Axial CT plain scan (A) and contrast-enhanced arterial phase (B) images showed severe dilation of the main pancreatic duct (arrow) and marked pancreatic atrophy while the tumor represented as isodense with pancreas. Contrast-enhanced CT portal venous phase (C) and delayed phase (D) images showed well-circumscribed hyperdense lesion (arrows) of pancreatic head. pNEN: Pancreatic neuroendocrine neoplasm; CT: Computed tomography |
2.3 误诊病例分析
26枚病灶中,14枚(53.8%)术前正确诊断为pNEN,8枚(30.8%)误诊为胰腺癌,2枚(7.7%)误诊为胰腺实性假乳头状瘤,1枚(3.8%)误诊为胰腺浆液性囊腺瘤,1枚(3.8%)误诊为胰腺导管内乳头状黏液瘤。所有误诊病例的CA19-9水平均在正常参考值范围内。分析主要误诊原因为:(1)病灶表现不典型,对疾病的不典型表现认识不足,影像细节观察不到位;(2)诊断时未完整结合患者临床资料;(3)影像学检查手段单一。
2.3.1 伴胰管梗阻pNEN误诊为胰腺癌的影像学特征病例1:女,50岁,病灶位于胰头,大小约3.9 cm,实性,边界清晰,动脉期、门静脉期、延迟期均表现为高于胰腺实质的富血供强化。病例2:女,67岁,病灶位于胰头,大小约2.9 cm,动脉期强化程度与胰腺实质相仿,门静脉期强化程度高于胰腺实质呈富血供表现,延迟期强化稍减退。病例3:男,45岁,病灶位于胰体,大小约2.6 cm,DWI序列可见轻度弥散受限,其余序列病灶显示不清,增强各期强化程度均与周围正常胰腺实质相仿。腹主动脉周围见1枚肿大淋巴结。病例4:男,58岁,病灶位于胰头,大小约2.9 cm,DWI序列可见弥散受限,其余序列病灶显示不清,增强各期强化程度与周围正常胰腺实质相仿,同时可见胆总管、主胰管走行至胰头截断,呈“双管征”。病例5:男,47岁,病灶位于胰体,大小约2.5 cm,增强动脉期见病灶边缘明显强化,与胰腺实质强化程度相仿,病灶内部强化程度弱于胰腺实质,门静脉期及延迟期病灶边缘强化程度高于胰腺实质,呈富血供表现,病灶内部呈渐进性强化。病例6:男,37岁,病灶位于胰头,MRI检查仅见胰体尾胰管明显扩张,至胰头截断,各序列均未见异常信号,病理检查见肿瘤最大径仅0.4 cm。病例7(图 2):女,44岁,病灶位于胰头,大小约1.5 cm,DWI序列可见弥散受限,其余序列病灶显示不清,可见“双管征”并伴有肝转移。病例8:男,53岁,病灶位于胰头,大小约3.1 cm,呈T1低信号、T2不均匀高信号,DWI序列可见弥散受限,动脉期呈不均匀边缘环形强化,门静脉期迅速廓清,内部呈渐进性强化,病灶中央见多发条管状未强化低信号区。
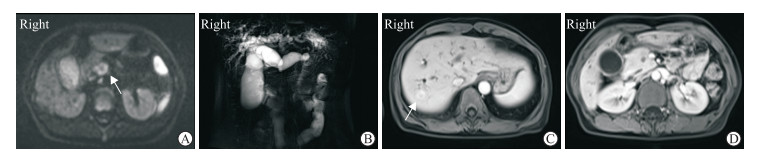
|
图 2 1例pNEN伴胆胰管扩张、肝转移误诊为胰腺癌的44岁女性患者MRI影像 Fig 2 MRI findings of a 44-year-old female patient with pNEN accompanied by cholangiopancreatic duct dilatation and liver metastasis misdiagnosed as pancreatic cancer The lesion represented as hyperintense (arrow) on DWI image (b=1 000 s/mm2) (A) and marked double duct sign can be observed on MRCP image (B). Contrast-enhanced arterial phase showed metastatic lesion (arrow) in the liver (C) while tumor of pancreatic head was isointense with normal pancreas (D). The tumor was also invisible on portal venous phase and delayed phase. This patient was misdiagnosed as pancreatic cancer. pNEN: Pancreatic neuroendocrine neoplasm; MRI: Magnetic resonance imaging; DWI: Diffusion-weighted imaging; MRCP: Magnetic resonance cholangiopancreatography |
2.3.2 伴胰管梗阻pNEN误诊为胰腺实性假乳头状瘤的影像学特征
病例9(图 3):女,65岁,病灶位于胰头,大小约9.9 cm,呈T1低信号、T2不均匀高信号,增强见病灶动脉期强化不均匀,门静脉期和延迟期呈渐进性强化,强化程度始终低于远侧胰腺组织。病例10:女,37岁,病灶位于胰头,大小约1.8 cm,T1加权像以低信号为主、内见片状高信号,T2加权像呈等稍低信号,DWI序列呈边缘环形高信号,增强后可见病灶边缘渐进性强化,强化程度高于周围正常胰腺实质,病灶内部强化不明显。
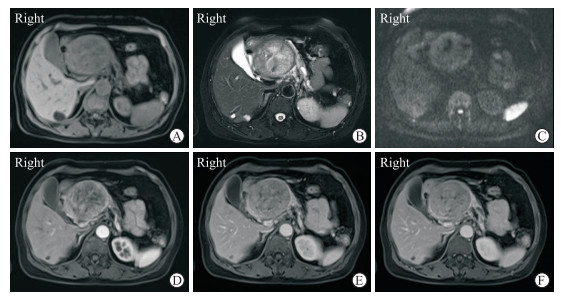
|
图 3 1例pNEN误诊为胰腺实性假乳头状瘤的65岁女性患者MRI影像 Fig 3 MRI findings of a 65-year-old female patient with pNEN misdiagnosed as pancreatic solid pseudopapillary tumor Contrast-enhanced MRI showed a well-circumstanced mass of pancreatic head with main pancreatic duct dilation and pancreatic atrophy. The mass represented as low signal on T1-weighted image (A), inhomogeneous high signal on T2-weighted image (B), inhomogeneous high signal on DWI sequence (C), and gradually uneven enhancement in arterial phase (D), portal venous phase (E) and delayed phase (F). The enhancement of the tumor was always lower than that of normal pancreatic parenchyma at the same level. This patient was misdiagnosed as pancreatic solid pseudopapillary tumor. pNEN: Pancreatic neuroendocrine neoplasm; MRI: Magnetic resonance imaging; DWI: Diffusion-weighted imaging |
2.3.3 伴胰管梗阻pNEN误诊为胰腺浆液性囊腺瘤的影像学特征
病例11:女,59岁,病灶位于胰头,大小约2.4 cm,呈T1低信号、T2不均匀高信号,内部见囊状T2加权像高亮水样信号,边界清晰,增强动脉期病灶强化较明显,门静脉期和延迟期强化略减退,但强化程度仍高于周围正常胰腺实质,病灶内部囊性部分不强化。
2.3.4 伴胰管梗阻pNEN误诊为胰腺导管内乳头状黏液瘤的影像学特征病例12:男,63岁,病灶位于胰头钩突,大小约2.1 cm,DWI序列呈高信号,增强后呈渐进性弱强化,主胰管扩张,胰头分支胰管扩张。
3 讨论CT和MRI在pNEN诊断中具有重要临床意义,尽管它们无法取代病理学检查,但具有无创、介质客观、可重复性高等优势,提供的定性诊断是预判肿瘤侵袭性、制定后续治疗策略的重要依据[8-9]。典型的pNEN多表现为边界清晰、富血供,较少伴有胰胆管扩张或血管侵犯。但pNEN异质性强,形态学、生物学行为多样,文献报道30%~40%的pNEN呈乏血供、边界不清、主胰管扩张等不典型表现[10]。当pNEN影像学表现不典型时,易与胰腺其他肿瘤尤其是胰腺导管腺癌混淆,但其治疗手段、预后并不相同。以胰腺癌为例,pNEN手术切除率比胰腺癌更高,化学治疗效果优于胰腺癌,5年生存率也明显高于胰腺癌[11-12]。此外,考虑到手术带来的创伤和并发症,也有学者认为体积较小的、病理分级较低的、散发性无功能性的pNEN可采取相对保守的治疗策略,如暂时保守观察或行肿瘤剜除术以尽可能保留胰腺功能[2, 13-14];而胰腺癌不论体积大小,凡符合指征均应行标准的根治性切除术。由此可见,术前准确的影像诊断对患者后续治疗策略的选择至关重要。
国内外学者针对乏血供pNEN的鉴别诊断开展了多项研究[11, 15-17],但迄今对伴有胰管梗阻的pNEN病例分析较少。胰管梗阻多因肿瘤过大、与胰管相邻所致,其可导致上游胰管扩张和瘤体上游胰腺实质萎缩,极个别肿瘤也可生长在胰管内[3],致使pNEN患者的影像学表现不典型。本研究回顾性分析了我院收治的200例pNEN患者的影像学资料,发现有25例(12.5%)伴胰管梗阻,与文献报道的胰管梗阻发生率(18.2%)[17]相仿。25例(26枚病灶)伴有胰管梗阻的pNEN患者中,12例(12枚病灶)被误诊,说明目前临床对伴胰管梗阻pNEN的认识仍不充分。
3.1 伴胰管梗阻pNEN的鉴别诊断 3.1.1 胰腺癌胰腺癌为胰腺最常见的实性肿瘤,绝大部分起源于胰管上皮细胞,胰头多见,肿瘤阻塞胰管从而导致远端主胰管扩张是胰腺癌的常见表现,并常伴有扩张胰管周围胰腺组织萎缩。胰腺癌为乏血供肿瘤,增强扫描时强化程度弱于周围正常胰腺组织,边界模糊。胰腺癌恶性程度高,呈围管浸润和噬神经生长,易出现周围侵犯和远处转移。pNEN呈非富血供且伴主胰管扩张时与胰腺癌鉴别较为困难。相比胰腺癌,pNEN边界更清晰,主胰管扩张发生率低,周围组织、血管侵犯和远处转移的发生也较少。文献报道即便是非富血供的pNEN,在动脉期和门静脉期的强化程度也高于胰腺癌[11]。
本组误诊为胰腺癌的8例患者中,2例表现为相对均匀的整体富血供强化,2例表现为边缘明显强化、内部渐进性强化(其中1例病灶边缘在门静脉期及延迟期呈高于胰腺实质的富血供强化),4例表现为与正常胰腺实质强化相仿,8例误诊病例的强化方式与胰腺癌动脉期和门静脉期均呈弱强化的典型强化方式不完全相符。8例误诊胰腺癌病例中,4例病灶在增强各期均无法与正常胰腺组织区分,其余4例病灶的界限较为清晰。此外,本组误诊为胰腺癌的8例伴胰管梗阻pNEN病例虽有不同程度的胰管扩张、胰腺萎缩,但均不伴阻塞性胰腺炎,而如果胰腺癌起源于主胰管或较大分支胰管,易导致阻塞性胰腺炎。我们认为当出现胰管扩张时,“肇事病灶”的强化程度、边界是否清晰及是否伴有阻塞性胰腺炎可为两者的鉴别提供参考。
3.1.2 胰腺实性假乳头状瘤胰腺实性假乳头状瘤好发于年轻女性,以胰头、胰尾较多见,多为囊实性,包膜完整,边界清晰。增强后肿瘤囊性部分不强化,实性部分呈动脉期轻度强化、门静脉期和延迟期渐进性强化,常见实性成分漂浮于囊性成分中而呈“浮云征”。瘤体内可有出血、钙化。肿瘤较大或与主胰管邻近时可导致主胰管扩张。pNEN也可表现为囊实性、乏血供。但文献报道与胰腺实性假乳头状瘤相比,pNEN病灶通常体积更小、动脉期强化更明显[18]。胰腺实性假乳头状瘤出现“浮云征”和钙化的概率高于pNEN,远处转移少于pNEN[19]。本组误诊为胰腺实性假乳头状瘤的2例伴胰管梗阻pNEN患者均为女性,MRI均表现为渐进性强化,未见明确的粗大钙化,亦未见“浮云征”。其中1例为老年女性,并非胰腺实性假乳头状瘤的高发年龄。
3.1.3 胰腺浆液性囊腺瘤胰腺浆液性囊腺瘤多见于老年女性,常发生在胰腺体尾,其典型CT表现为边界清晰、具有多囊分隔的低密度肿块,增强后微小囊腔无强化,分隔结构因富血供而明显强化,部分病灶中心可见星芒状瘢痕。当病灶内部囊腔微小时CT往往难以清晰显示,可误认为是实性肿块,从而误诊为pNEN,此时应进一步行MRI检查,MRI对其微囊结构显示灵敏。当pNEN内部出现囊变时,有时也会误诊为浆液性囊腺瘤。本组误诊为胰腺浆液性囊腺瘤的1例伴胰管梗阻pNEN患者病灶内部见囊状T2加权像高亮水样信号,但观察影像细节可发现pNEN的囊变形态通常不如浆液性囊腺瘤中的囊性灶规则,也不具有薄壁分隔状结构。
3.1.4 胰腺导管内乳头状黏液瘤胰腺导管内乳头状黏液瘤多见于老年男性,来源于导管上皮,呈乳头状生长,分泌黏液,引起主胰管和(或)分支胰管进行性扩张。主胰管型和分支胰管型胰管扩张位置不同,但一般均不存在因肿瘤或其他原因导致的局限性狭窄。pNEN引起的胰管阻塞表现为肿瘤远端的胰管扩张,一般可在增强扫描后发现肿瘤。本组误诊为胰腺导管内乳头状黏液瘤的1例pNEN患者主胰管及胰头分支胰管都见扩张,而且肿瘤位于胰头钩突,呈非富血供强化,较为隐匿,需要影像医师细心观察。
3.2 伴胰管梗阻pNEN的常见影像学误诊原因 3.2.1 对疾病的不典型表现认识不足,影像细节观察不到位pNEN引起胰管梗阻较为少见,但本组12例误诊病例中有5例(41.7%)在动脉期和(或)门静脉期可见富血供强化,诊断医师可能因为pNEN引起胰管梗阻较少见而在鉴别诊断时并未考虑。误诊病例虽然病变不典型,但通过回顾病例发现这些病例的影像细节值得推敲。如误诊为胰腺癌的病例中,病灶未见动脉期、门静脉期均呈弱强化者;误诊为胰腺浆液性囊腺瘤的病例中,病灶内无薄壁分隔状结构;误诊为胰腺导管内乳头状黏液瘤的病例,影像学报告中未提及胰头钩突病灶。我们认为,影像医师应通过阅读大量文献熟悉疾病的不典型表现,建立缜密的影像学诊断思维,在临床工作中细心观察影像细节,以减少不必要的误诊。
3.2.2 诊断时未完整结合患者临床资料影像学诊断并非孤立,需紧密结合临床资料方能提高诊断的准确率。本研究中,误诊为胰腺癌的8例伴胰管梗阻pNEN患者血清CA19-9水平均在正常参考值范围内,且其中4例患者年龄在50岁以下,诊断时应慎重。误诊为胰腺实性假乳头状瘤的病例有1例为老年女性,也不符合胰腺实性假乳头状瘤好发于年轻女性的典型临床特征。因此,在影像诊断工作中,除影像征象外,还需综合考虑患者的临床资料以进行鉴别诊断,提高诊断准确率。
3.2.3 影像学检查手段单一在误诊患者中,仅有2例同时接受CT和MRI检查。事实上,CT和MRI各具优势,CT空间分辨率高,显示病灶的钙化、出血灵敏度高,对pNEN与胰腺实性假乳头状瘤的鉴别颇有帮助。MRI软组织分辨率高,兼具结构与功能序列,本研究中误诊为胰腺癌的病例中有3例表现为DWI序列弥散受限而其他序列病灶显示不清的现象,充分体现了磁共振功能序列对病灶检出的灵敏性。根据影像学诊断经验,我们认为,病灶在单一检查方式下表现不典型时,应考虑结合其他影像学检查手段,提高诊断信心。
综上所述,pNEN伴胰管梗阻少见,影像学表现上常具一定混淆性,有时与其他胰腺肿瘤的鉴别诊断存在难度。应熟悉该病的不典型表现、细心观察影像细节,同时密切结合临床资料,以减少误诊,提高该病的诊断准确率。
| [1] |
KLÖPPEL G. Neuroendocrine neoplasms:dichotomy, origin and classifications[J]. Visc Med, 2017, 33: 324-330. DOI:10.1159/000481390 |
| [2] |
PERRI G, PRAKASH L R, KATZ M H G. Pancreatic neuroendocrine tumors[J]. Curr Opin Gastroenterol, 2019, 35: 468-477. DOI:10.1097/MOG.0000000000000571 |
| [3] |
KAWAMOTO S, SHI C, HRUBAN R H, CHOTI M A, SCHULICK R D, FISHMAN E K, et al. Small serotonin-producing neuroendocrine tumor of the pancreas associated with pancreatic duct obstruction[J]. AJR Am J Roentgenol, 2011, 197: W482-W488. DOI:10.2214/AJR.10.5428 |
| [4] |
JEON S K, LEE J M, JOO I, LEE E S, PARK H J, JANG J Y, et al. Nonhypervascular pancreatic neuroendocrine tumors:differential diagnosis from pancreatic ductal adenocarcinomas at MR imaging-retrospective cross-sectional study[J]. Radiology, 2017, 284: 77-87. DOI:10.1148/radiol.2016160586 |
| [5] |
BELOUSOVA E, KARMAZANOVSKY G, KRIGER A, KALININ D, MANNELLI L, GLOTOV A, et al. Contrast-enhanced MDCT in patients with pancreatic neuroendocrine tumours:correlation with histological findings and diagnostic performance in differentiation between tumour grades[J]. Clin Radiol, 2017, 72: 150-158. DOI:10.1016/j.crad.2016.10.021 |
| [6] |
SHI C, SIEGELMAN S S, KAWAMOTO S, WOLFGANG C L, SCHULICK R D, MAITRA A, et al. Pancreatic duct stenosis secondary to small endocrine neoplasms:a manifestation of serotonin production?[J]. Radiology, 2010, 257: 107-114. DOI:10.1148/radiol.10100046 |
| [7] |
RINDI G, ARNOLD R, BOSMAN F T, CAPELLA C, KLIMSTRA D S, KLÖPPEL G, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system[M]//BOSMAN F T, CARNEIRO F, HRUBAN R H, THEISE N D. WHO classification of tumours in the digestive system. 4th edition. Lyon:IARC, 2010:13-14.
|
| [8] |
LEWIS R B, LATTIN G E Jr, PAAL E. Pancreatic endocrine tumors:radiologic-clinicopathologic correlation[J]. Radiographics, 2010, 30: 1445-1464. DOI:10.1148/rg.306105523 |
| [9] |
SUNDIN A, VULLIERME M P, KALTSAS G, PLÖCKINGER U; Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS consensus guidelines for the standards of care in neuroendocrine tumors:radiological examinations[J]. Neuroendocrinology, 2009, 90: 167-183. DOI:10.1159/000184855 |
| [10] |
HE M, LIU Z, LIN Y, WAN J, LI J, XU K, et al. Differentiation of atypical non-functional pancreatic neuroendocrine tumor and pancreatic ductal adenocarcinoma using CT based radiomics[J]. Eur J Radiol, 2019, 117: 102-111. DOI:10.1016/j.ejrad.2019.05.024 |
| [11] |
REN S, CHEN X, WANG Z, ZHAO R, WANG J, CUI W, et al. Differentiation of hypovascular pancreatic neuroendocrine tumors from pancreatic ductal adenocarcinoma using contrast-enhanced computed tomography[J/OL]. PLoS One, 2019, 14: e0211566. doi: 10.1371/journal.pone.0211566.
|
| [12] |
SINGH S, MOODY L, CHAN D L, METZ D C, STROSBERG J, ASMIS T, et al. Follow-up recommendations for completely resected gastroenteropancreatic neuroendocrine tumors[J]. JAMA Oncol, 2018, 4: 1597-1604. DOI:10.1001/jamaoncol.2018.2428 |
| [13] |
SCOTT A T, HOWE J R. Evaluation and management of neuroendocrine tumors of the pancreas[J]. Surg Clin North Am, 2019, 99: 793-814. DOI:10.1016/j.suc.2019.04.014 |
| [14] |
MORI M, BENEDETTI G, PARTELLI S, SINI C, ANDREASI V, BROGGI S, et al. CT radiomic features of pancreatic neuroendocrine neoplasms (panNEN) are robust against delineation uncertainty[J]. Phys Med, 2019, 57: 41-46. DOI:10.1016/j.ejmp.2018.12.005 |
| [15] |
WANG Z, CHEN X, WANG J, CUI W, REN S, WANG Z. Differentiating hypovascular pancreatic neuroendocrine tumors from pancreatic ductal adenocarcinoma based on CT texture analysis[J]. Acta Radiol, 2020, 61: 595-604. DOI:10.1177/0284185119875023 |
| [16] |
KARMAZANOVSKY G, BELOUSOVA E, SCHIMA W, GLOTOV A, KALININ D, KRIGER A. Nonhypervascular pancreatic neuroendocrine tumors:spectrum of MDCT imaging findings and differentiation from pancreatic ductal adenocarcinoma[J]. Eur J Radiol, 2019, 110: 66-73. DOI:10.1016/j.ejrad.2018.04.006 |
| [17] |
LI J, LU J, LIANG P, LI A, HU Y, SHEN Y, et al. Differentiation of atypical pancreatic neuroendocrine tumors from pancreatic ductal adenocarcinomas:using whole-tumor CT texture analysis as quantitative biomarkers[J]. Cancer Med, 2018, 7: 4924-4931. DOI:10.1002/cam4.1746 |
| [18] |
SINGH A, HINES J J, FRIEDMAN B. Multimodality imaging of the pancreatic neuroendocrine tumors[J]. Semin Ultrasound CT MR, 2019, 40: 469-482. DOI:10.1053/j.sult.2019.04.005 |
| [19] |
WANG C, CUI W, WANG J, CHEN X, TONG H, WANG Z. Differentiation between solid pseudopapillary neoplasm of the pancreas and hypovascular pancreatic neuroendocrine tumors by using computed tomography[J]. Acta Radiol, 2019, 60: 1216-1223. DOI:10.1177/0284185118823343 |
 2020, Vol. 41
2020, Vol. 41


