2. 海军军医大学(第二军医大学)东方肝胆外科医院麻醉科, 上海 200438;
3. 中国人民解放军联勤保障部队988 医院普通外科, 郑州 450042
2. Department of Anesthesiology, Eastern Hepatobiliary Surgery Hospital, Naval Medical University(Second Military Medical University), Shanghai 200438, China;
3. Department of General Surgery, No. 988 Hospital of Logistic Support Forces of PLA, Zhengzhou 450042, Henan, China
神经病理性疼痛是由中枢或周围神经损伤诱发免疫炎症反应、激活细胞内信号转导通路导致痛觉过敏而引起的慢性疼痛,严重影响患者生活质量。炎症反应、感染、肿瘤等因素造成的痛觉传导系统易化和抑制失衡在神经病理性疼痛的进展中发挥重要作用[1],但目前尚无有效治疗方法。脊髓背角作为疼痛信号传递的中继站,通过整合不同传入神经元的疼痛信息调控痛觉行为。小胶质细胞广泛存在于脊髓背角,激活后可介导炎性因子、白血病抑制因子、生长因子等释放,进而改变神经元突触的结构和功能[2]。2型大麻素受体(cannabinoid type 2 receptor,CB2R)主要表达于外周免疫细胞,参与免疫调节、炎症反应和细胞迁移等生理过程,研究证实CB2R在脊髓小胶质细胞中存在较多表达,介导内源性大麻类物质的生理功能,且CB2R激动剂能够显著减轻吗啡戒断反应、炎性疼痛等痛觉过敏,但其机制尚不清楚[3]。本研究通过脊神经结扎(spinal nerve ligation,SNL)技术建立神经病理性疼痛小鼠模型,用小干扰RNA(small interfering RNA,siRNA)高效抑制CB2R表达,探讨脊髓内CB2R表达和小胶质细胞活化对神经病理性疼痛的影响及作用机制,为临床缓解神经病理性疼痛提供新的思路。
1 材料和方法 1.1 实验动物与试剂健康雄性C57/BL小鼠(体重20~30 g)180只,购自海军军医大学(第二军医大学)实验动物中心[动物生产许可证号:SCXK(沪)2018-0006],恒温、恒湿条件下饲养。采用SNL方法建立神经病理性疼痛模型,CB2R选择性激动剂AM1241(50 μg/kg,美国Sigma公司)、小胶质细胞抑制剂米诺环素(minocycline,10 μg/kg,美国Sigma公司)均溶解于5 mL生理盐水后于测痛阈或取材前2 h鞘内注射10 μL给药。蛋白质印迹法使用的兔抗CB2R一抗(稀释比例为1:500)、兔抗钙离子结合调节因子1(ionized calcium-binding adapter molecule 1,IBA-1)一抗(稀释比例为1:1 000)均购自英国Abcam公司,兔抗GAPDH一抗(稀释比例为1:5 000)、抗兔二抗(稀释比例为1:5 000)均购自美国CST公司。免疫荧光使用的兔抗IBA-1一抗(稀释比例为1:500)购自日本Wako公司,山羊抗兔IgG荧光二抗(稀释比例为1:1 000)购自美国Invitrogen公司。将小鼠随机分为假手术、SNL、SNL+AM1241、SNL+米诺环素、SNL+siRNA、SNL+siRNA+米诺环素组。
1.2 鞘内置管小鼠麻醉后腰中线备皮消毒,切开长1~2 cm小口,剪开筋膜,在L3棘突斜向L4方向向下剪开肌肉,剪除部分L4棘突,暴露L3~L4的三角间隙。用5 mL注射器针头刺破硬脊膜,夹取PE-10导管向尾端插入,插入时可见甩尾反射,置入约1 cm后见脑脊液流出即封闭管口。依次缝合切口,妥善固定导管于肌肉和皮肤,腹腔注射0.3 mL氨苄青霉素(100 g/L)预防感染,术后小鼠单笼饲养。
1.3 神经病理性疼痛小鼠模型的建立将鞘内置管成功的小鼠麻醉,取俯卧位。沿中线切开皮肤,暴露L5~L6椎体,用咬骨钳咬断左侧L5横突,6-0丝线结扎L5脊神经,然后依次缝合肌肉与皮肤,建立SNL神经病理性疼痛模型。腹腔注射0.3 mL氨苄青霉素(100 g/L)预防感染。假手术组小鼠仅暴露脊神经而不结扎,其余手术操作与SNL组小鼠相同。
1.4 慢病毒携带siRNA靶向干扰脊髓CB2R表达小鼠模型的建立合成靶向调控小鼠CB2R表达的siRNA序列并将其克隆至慢病毒载体pLVX-shRNA(美国Clontech公司),然后将重组pLVX-shRNA载体与lenti-XTM HTX系统(美国Clontech公司)共同转染到293T细胞,培养48 h后收获慢病毒复合体。测痛阈或取材前24 h通过鞘内注射携带靶向siRNA慢病毒复合体至小鼠脊髓干扰CB2R表达,并采用蛋白质印迹法验证干扰效果[4]。
1.5 机械性痛阈测定分别于SNL术前及术后1、7、14 d测定小鼠机械性痛阈。测定前将小鼠置于茶色、底部为金属网的丙烯酸树脂笼中,适应1 h后,用Von Frey纤维丝(美国North Coast Medical公司)刺激小鼠左后足五趾间柔软部位,缓慢加力,出现缩足反应后即停止接触,此时记为阳性。每2次刺激至少间隔5 min,每个强度刺激3次取平均值,采用up-down法分析机械痛阈值。为避免造成小鼠足底机械性损伤,实验中采用的Von Frey纤维丝最大值为10 g,刺激时间为5 s[5]。
1.6 蛋白质印迹法检测CB2R和IBA-1表达小鼠麻醉后迅速取出L4~L5段脊髓组织,加入200 μL裂解液超声匀浆,然后低温高速离心15 min取上清。用BCA法测定总蛋白浓度,并在99 ℃条件下变性10 min。取30 μg蛋白样本进行10% SDS-PAGE,采用湿转法将蛋白转移至PVDF膜,经5%脱脂奶粉溶液封闭2 h。分别加入兔抗CB2R、兔抗IBA-1、兔抗GAPDH一抗,4 ℃孵育过夜。TBST洗膜3次后,加入抗兔二抗室温孵育2 h,然后进行化学发光显影。采用Photoshop软件测量条带的灰度值,目的蛋白的相对表达量为目的条带灰度值/GAPDH灰度值。
1.7 免疫荧光法检测IBA-1表达小鼠麻醉后依次用0.1 mol/L PBS和4%多聚甲醛溶液经心脏灌流。取L4~L5段脊髓并用4%多聚甲醛溶液后固定,随后移入30%蔗糖溶液脱水。用冷冻切片机(德国Leica公司)按20 μm厚度切片,用5%山羊血清室温封闭2 h,4 ℃条件下加入兔抗IBA-1一抗孵育过夜,PBS洗3次,加入山羊抗兔IgG荧光二抗室温孵育2 h,用荧光显微镜观察并拍照。
1.8 qRT-PCR检测炎性因子mRNA表达小鼠麻醉后置于立体定位仪,于腰部中间皮肤切口,摘除椎板暴露部分脊髓,插入透析针,保持透析管同脊髓在同一平面并固定。打开微透析泵并填充透析液,平衡2 h后收集透析液。采用TRIzol法提取脊髓透析液中总RNA,分光光度计测量RNA浓度和纯度。37 ℃ 15 min、85 ℃ 15 s、4 ℃ 10 min条件下反转录获得cDNA。然后以cDNA为模板、以GAPDH为内参照进行qRT-PCR,引物序列见表 1,反应条件:95 ℃ 15 min,95 ℃ 15 s、60 ℃ 15 s、72 ℃ 40 s循环40次。计算每个样本的ΔCt值并获得目的基因的相对表达量。
|
|
表 1 实时定量聚合酶链反应引物序列 Tab 1 Primer sequences of quantitative real-time polymerase chain reaction |
1.9 电生理检测
随机选择20只正常小鼠麻醉后,取L4~L5段脊髓置于预冷人工脑脊液(artificial cerebrospinal fluid,ACSF)中。ACSF组成:NaCl 117 mmol/L、KCl 3.6 mmol/L、CaCl2 2.5 mmol/L、MgCl2 1.2 mmol/L、NaH2PO4 1.2 mmol/L、NaHCO3 25 mmol/L、葡萄糖11 mmol/L,用95% O2、5% CO2混合气体将ACSF充分饱和。显微镜下清除软脊膜和神经根,用振动切片机按400 μm厚度切片,转移至Gibb槽中33 ℃恒温孵育1 h。然后将脊髓片转移到ACSF持续灌注的记录玻片上,用透明相差显微镜找出半透明胶状质神经元,随后将含有电极内液的电极移至细胞表面负压吸破细胞膜。电极内液组成:Cs2SO4 110 mmol/L、CaCl2 0.5 mmol/L、MgCl2 2 mmol/L、乙二醇二乙醚二胺四乙酸5 mmol/L、4-羟乙基哌嗪乙磺酸5 mmol/L、三乙醇胺5 mmol/L、ATP-Mg 5 mmol/L。通过MultiClamp 700B放大器和pClamp 10.4数据采集软件在0 mV电位下,以电压钳模式记录自发性抑制性突触后电流(spontaneous inhibitory postsynaptic current,sIPSC)。首先记录AM1241干预前后的sIPSC变化,然后在米诺环素持续处理条件下记录AM1241灌流前后的sIPSC变化[6]。
1.10 统计学处理应用SPSS 21.0软件进行统计学分析。计量资料以x±s表示,多组间比较采用单因素方差分析,事后两两比较采用Bonfferoni t检验,不同组别和时间点痛阈的比较采用多因素重复方差分析。电生理结果用pClamp 10.4软件进行分析,sIPSC频率和振幅在AM1241干预前后比较采用配对t检验。检验水准(α)为0.05,Bonfferoni t检验校正检验水准(α')为0.012 5、0.016 7或0.008 3。
2 结果 2.1 SNL小鼠脊髓组织中CB2R表达变化SNL组小鼠脊髓组织中CB2R表达水平低于假手术组(t=6.733,P<0.012 5);鞘内注射携带靶向CB2R的siRNA慢病毒复合体后,假手术+siRNA组小鼠脊髓组织中CB2R表达低于假手术组(t=6.317,P<0.012 5),SNL+siRNA组CB2R表达也低于SNL组(t=3.017,P<0.012 5),提示鞘内注射携带靶向siRNA慢病毒复合体降低了脊髓组织中CB2R的表达,靶向干扰脊髓CB2R表达小鼠模型建立成功。见图 1。
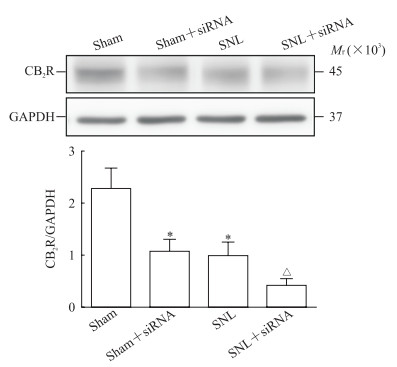
|
图 1 蛋白质印迹法检测小鼠脊髓组织中CB2R表达变化 Fig 1 Expression of CB2R protein in mouse spinal cord tissues detected by Western blotting CB2R: Cannabinoid type 2 receptor; siRNA: Small interfering RNA; SNL: Spinal nerve ligation; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. *P < 0.012 5 vs sham group; △P < 0.012 5 vs SNL group. n=10, x±s |
2.2 各组小鼠机械性痛阈改变
SNL术后1、7、14 d,SNL组小鼠的痛阈均低于假手术组(t=6.254、7.854、7.294,P均<0.016 7),证明神经病理性疼痛的痛觉过敏模型建立成功。鞘内注射CB2R选择性激动剂AM1241后1、7、14 d,SNL+AM1241组小鼠的痛阈与SNL组相比均升高(t=6.574、7.025、6.842,P均<0.008 3),且在术后第7天时变化最明显,因此后续实验均采用SNL术后7 d小鼠进行药物干预。与SNL组相比,SNL+AM1241、SNL+米诺环素组小鼠的痛阈均升高(t=7.081、7.324,P均<0.008 3),而SNL+siRNA组小鼠的痛阈较SNL组降低(t=8.984,P<0.008 3),但鞘内注射米诺环素逆转了SNL+siRNA组小鼠的痛阈降低(t=3.208,P<0.008 3)。提示脊髓CB2R的表达水平影响神经病理性疼痛小鼠的痛觉过敏,而抑制小胶质细胞能减轻其痛觉过敏症状。见图 2。
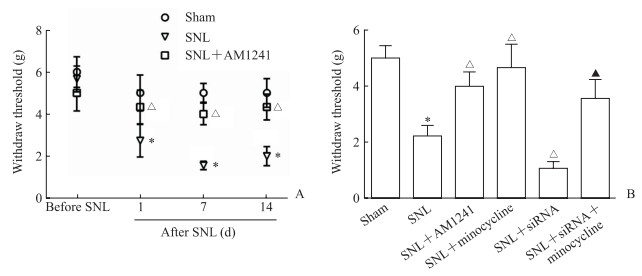
|
图 2 各组小鼠机械性痛阈 Fig 2 Mechanical pain thresholds of mice in each group A: Changes of mechanical pain thresholds at different time points; B: Mechanical pain thresholds 7 d after SNL in six groups. AM1241 is a selective CB2R agonist. Minocycline is a microglia inhibitor. CB2R: Cannabinoid type 2 receptor; siRNA: Small interfering RNA; SNL: Spinal nerve ligation. *P < 0.016 7 (A), *P < 0.008 3 (B) vs sham group; △P < 0.016 7 (A), △P < 0.008 3 (B) vs SNL group; ▲P < 0.008 3 (B) vs SNL+siRNA group. n=10, x±s |
2.3 各组小鼠脊髓背角小胶质细胞活化情况
与假手术组相比,SNL组小鼠脊髓背角组织中IBA-1荧光定量和蛋白表达均增高(t=15.000、7.760,P均<0.008 3);SNL+AM1241、SNL+米诺环素组小鼠脊髓背角组织中IBA-1荧光定量和蛋白表达与SNL组相比均降低(t=10.230、11.520、4.910、5.280,P均<0.008 3),而其在SNL+siRNA组均增高(t=11.110、5.230,P均<0.008 3),鞘内注射米诺环素逆转了SNL+siRNA组IBA-1荧光定量和蛋白表达的增加(t=14.730、7.950,P均<0.008 3)。提示小胶质细胞活化参与SNL痛觉过敏的产生,而CB2R激活能抑制小胶质细胞活化。见图 3。

|
图 3 各组小鼠脊髓背角小胶质细胞活化改变 Fig 3 Changes of microglia activation in spinal dorsal horn of mice in each group A: Immunofluorescent staining of IBA-1 in spinal dorsal horn; B: Quantitative analysis of positive cells of IBA-1 in spinal dorsal horn; C: Representative images of IBA-1 protein expression in spinal dorsal horn by Western blotting; D: Quantitative analysis of IBA-1 protein expression in spinal dorsal horn by Western blotting. AM1241 is a selective CB2R agonist. Minocycline is a microglia inhibitor. SNL: Spinal nerve ligation; siRNA: Small interfering RNA; CB2R: Cannabinoid type 2 receptor; IBA-1: Ionized calcium-binding adapter molecule 1; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. *P < 0.008 3 vs sham group; △P < 0.008 3 vs SNL group; ▲P < 0.008 3 vs SNL+siRNA group. n=10, x±s |
2.4 各组小鼠脊髓透析液中炎性因子水平
与假手术组相比,SNL组小鼠脊髓透析液中炎性因子TNF-α、IL-1β、IL-6 mRNA表达均增加(t=12.220、12.490、9.880,P均<0.008 3);SNL+AM1241、SNL+米诺环素组TNF-α、IL-1β、IL-6 mRNA表达均较SNL组降低(t=9.690、6.640、6.690,10.900、7.010、7.490;P均<0.008 3);但SNL+siRNA组TNF-α、IL-1β、IL-6 mRNA表达均较SNL组增加(t=7.170、5.970、6.360,P均<0.008 3),而鞘内注射米诺环素逆转了SNL+siRNA组TNF-α、IL-1β、IL-6 mRNA表达的增加(t=6.850、6.730、10.100,P均<0.008 3)。提示脊髓内炎症反应参与SNL痛觉过敏的进展,CB2R激活或小胶质细胞抑制能够减轻脊髓内炎症反应。见图 4。

|
图 4 qRT-PCR检测各组小鼠脊髓透析液中炎性因子水平 Fig 4 Inflammatory factors in dialysate of mouse spinal cord in each group detected by qRT-PCR AM1241 is a selective CB2R agonist. Minocycline is a microglia inhibitor. qRT-PCR: Quantitative real-time polymerase chain reaction; SNL: Spinal nerve ligation; siRNA: Small interfering RNA; CB2R: Cannabinoid type 2 receptor; TNF-α: Tumor necrosis factor α; IL-1β: Interleukin 1β; IL-6: Interleukin 6; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. *P < 0.008 3 vs sham group; △P < 0.008 3 vs SNL group; ▲P < 0.008 3 vs SNL+siRNA group.n=10, x±s |
2.5 CB2R激动剂AM1241体外干预对小鼠脊髓背角sIPSC的影响
CB2R激动剂AM1241体外干预能够增强小鼠脊髓背角sIPSC的频率和振幅,与干预前相比差异均有统计学意义(t=7.396、2.741,P=0.000 7、0.040 8,图 5A);而在小胶质细胞抑制剂米诺环素持续处理条件下,AM1241前后小鼠脊髓背角sIPSC的频率和振幅差异均无统计学意义(t=1.313、1.172,P=0.246、0.294,图 5B)。提示CB2R通过抑制小胶质细胞活性增强脊髓抑制性电活动,从而减轻痛觉过敏。
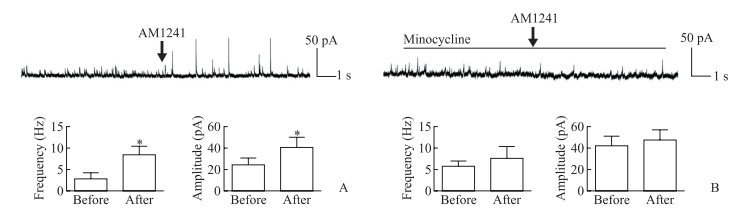
|
图 5 CB2R激动剂AM1241体外干预对小鼠脊髓背角sIPSC的影响 Fig 5 Effects of CB2R agonist AM1241 on sIPSC in spinal dorsal horn of mice in vitro A: sIPSC changes before and after intervention of AM1241; B: sIPSC changes before and after intervention of AM1241 under continuous treatment of minocyline. AM1241 is a selective CB2R agonist. Minocycline is a microglia inhibitor. CB2R: Cannabinoid type 2 receptor; sIPSC: Spontaneous inhibitory postsynaptic current. *P < 0.05 vs before intervention of AM1241.n=10, x±s |
3 讨论
神经病理性疼痛由于复杂的病因和病理生理学改变,常规镇痛药物治疗效果往往不佳。本研究结果显示,SNL致神经病理性疼痛小鼠发生痛觉过敏,是由于脊髓CB2R表达减少增强了小胶质细胞活化,进而介导神经炎性症反应并减弱抑制性电活动,而鞘内注射CB2R激动剂AM1241或小胶质细胞抑制剂米诺环素可缓解其痛觉过敏症状。通过siRNA靶向干扰CB2R表达后,小鼠脊髓背角小胶质细胞活化增强,炎性因子释放增加,痛觉过敏症状加重,鞘内注射米诺环素能够逆转siRNA靶向干扰CB2R表达介导的这些变化。表明CB2R通过抑制脊髓小胶质细胞活化减轻神经炎症反应、增强抑制性电活动,从而改善神经病理性疼痛的痛觉过敏。
CB2R主要在免疫系统、周围神经系统及受损组织中表达,发挥调节免疫反应、抑制炎症、调节能量代谢等作用[7]。研究发现,CB2R在中枢神经系统亦有表达且发挥着重要作用,分布在小胶质细胞、单核巨噬细胞和星形胶质细胞中的CB2R激活可抑制炎性因子释放,从而减弱白细胞的趋化和黏附作用,进而减轻炎症反应[8]。越来越多的研究证明,CB2R可通过调节部分与疼痛密切相关的神经活性物质及受体的功能,间接影响中枢神经元的兴奋性,参与神经病理性疼痛进展[9-10]。CB2R属于G蛋白偶联受体超家族,其激活能抑制腺苷酸环化酶的活性,导致细胞内第二信使cAMP生成减少,从而阻断依赖cAMP的信号通路,延缓痛觉过敏的发展;同时CB2R能够增强细胞内MAPK的磷酸化水平,引起内质网钙离子通道去极化,导致库容性钙离子内流,使细胞质内钙离子浓度升高,影响细胞内信号转导,减轻神经元突触敏化及突触重塑的发生[11]。本研究也证明CB2R激活能够抑制脊髓内炎症反应,并增强神经元的抑制性电活动。
神经病理性疼痛与中枢神经系统疼痛信号传递的易化和抑制通路失衡有关。脊髓Ⅰ板层可接受并传递外周伤害性刺激,当周围神经受损伤后γ-氨基丁酸表达被抑制,导致其介导的抑制性突触传递作用减弱。脊髓Ⅱ板层为胶状质层,内含较多疼痛传递介质,TNF-α、IL-1β等细胞因子能够在神经轴突内逆行或顺行运输,增强兴奋性突触的传递并阻碍抑制性突触的传递[12]。小胶质细胞在中枢神经系统分布广泛,能够介导疼痛信号传递过程中的一系列变化,其在疼痛过程中的作用已被广泛认可。中枢小胶质细胞对外界病理性损伤尤为敏感,如感染、炎症、神经损伤等刺激都能够通过作用于小胶质细胞而改变神经元突触的结构和功能,导致TNF-α、IL-1β、IL-6、白血病抑制因子、生长因子等释放对中枢产生多种效应。其中,内源性IL-1β能显著减少γ-氨基丁酸能神经元的sIPSC[13]。CB2R作为脊髓内小胶质细胞表面重要的信号分子,其活化后通过细胞内第二信使激活PI3K/Akt/脑源性神经营养因子通路减少小胶质细胞内一氧化氮、TNF-α、IL-1β等生成,降低细胞代谢率和减少细胞损伤,从而减轻小胶质细胞活化[14];同时,CB2R亦能通过抑制活性氧/NF-κB通路减缓细胞内的氧化反应,从而降低葡萄糖消耗,减弱小胶质细胞的生理活动[15]。CB2R激活后在脊髓背角能够抑制小胶质细胞活化,减少小胶质细胞标志物IBA-1的过度表达,缓解神经元凋亡,一定程度上抑制突触重塑和长时程增强的发生,延缓痛觉过敏的进展[16]。本研究发现鞘内注射CB2R激动剂AM1241后脊髓背角小胶质细胞活化减弱,同时小胶质细胞介导的炎性因子释放减少;而通过siRNA靶向干扰CB2R表达后小胶质细胞活化增强、炎性因子释放增加,且小胶质细胞抑制剂米诺环素能逆转此现象,进一步证明了CB2R激活对小胶质细胞的抑制作用影响着神经病理性疼痛的进展。
综上所述,CB2R通过抑制脊髓小胶质细胞活化减轻神经炎症反应,并增强脊髓背角抑制性电活动,共同减轻神经病理性疼痛的痛觉过敏,为临床缓解或治疗神经病理性疼痛提供了新的治疗靶点和研究思路。
| [1] |
MEYER L, TALEB O, PATTE-MENSAH C, MENSAH-NYAGAN A G. Neurosteroids and neuropathic pain management:basic evidence and therapeutic perspectives[J/OL]. Front Neuroendocrinol, 2019, 55:100795. doi:10.1016/j.yfrne.2019.100795.
|
| [2] |
SHEN Y, DING Z, MA S, ZOU Y, YANG X, DING Z, et al. Targeting aurora kinase B alleviates spinal microgliosis and neuropathic pain in a rat model of peripheral nerve injury[J]. J Neurochem, 2020, 152: 72-91. DOI:10.1111/jnc.14883 |
| [3] |
WU J, HOCEVAR M, BIE B, FOSS J F, NAGUIB M. Cannabinoid type 2 receptor system modulates paclitaxel-induced microglial dysregulation and central sensitization in rats[J]. J Pain, 2019, 20: 501-514. DOI:10.1016/j.jpain.2018.10.007 |
| [4] |
BIE C Q, LIU X Y, CAO M R, HUANG Q Y, TANG H J, WANG M, et al. Lentivirus-mediated RNAi knockdown of insulin-like growth factor-1 receptor inhibits the growth and invasion of hepatocellular carcinoma via down-regulating midkine expression[J]. Oncotarget, 2016, 7: 79305-79318. DOI:10.18632/oncotarget.13027 |
| [5] |
ZHAO Y, XIN Y, CHU H. MC4R is involved in neuropathic pain by regulating JNK signaling pathway after chronic constriction injury[J/OL]. Front Neurosci, 2019, 13: 919. doi: 10.3389/fnins.2019.00919.
|
| [6] |
MANIEZZI C, TALPO F, SPAIARDI P, TOSELLI M, BIELLA G. Oxytocin increases phasic and tonic GABAergic transmission in CA1 region of mouse hippocampus[J/OL]. Front Cell Neurosci, 2019, 13: 178. doi: 10.3389/fncel.2019.00178.
|
| [7] |
CHEN D J, GAO M, GAO F F, SU Q X, WU J. Brain cannabinoid receptor 2:expression, function and modulation[J]. Acta Pharmacol Sin, 2017, 38: 312-316. DOI:10.1038/aps.2016.149 |
| [8] |
YUILL M B, HALE D E, GUINDON J, MORGAN D J. Anti-nociceptive interactions between opioids and a cannabinoid receptor 2 agonist in inflammatory pain[J/OL]. Mol Pain, 2017, 13: 1744806917728227. doi: 10.1177/1744806917728227.
|
| [9] |
DONVITO G, NASS S R, WILKERSON J L, CURRY Z A, SCHURMAN L D, KINSEY S G, et al. The endogenous cannabinoid system:a budding source of targets for treating inflammatory and neuropathic pain[J]. Neuropsychopharmacology, 2018, 43: 52-79. DOI:10.1038/npp.2017.204 |
| [10] |
GRENALD S A, YOUNG M A, WANG Y, OSSIPOV M H, IBRAHIM M M, LARGENT-MILNES T M, et al. Synergistic attenuation of chronic pain using mu opioid and cannabinoid receptor 2 agonists[J]. Neuropharmacology, 2017, 116: 59-70. DOI:10.1016/j.neuropharm.2016.12.008 |
| [11] |
REYES-RESINA I, NAVARRO G, AGUINAGA D, CANELA E I, SCHOEDER C T, ZAŁUSKI M, et al. Molecular and functional interaction between GPR18 and cannabinoid CB2 G-protein-coupled receptors.Relevance in neurodegenerative diseases[J]. Biochem Pharmacol, 2018, 157: 169-179. DOI:10.1016/j.bcp.2018.06.001 |
| [12] |
SDRULLA A D, GUAN Y, RAJA S N. Spinal cord stimulation:clinical efficacy and potential mechanisms[J]. Pain Pract, 2018, 18: 1048-1067. DOI:10.1111/papr.12692 |
| [13] |
TSUDA M, KOGA K, CHEN T, ZHUO M. Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex[J]. J Neurochem, 2017, 141: 486-498. DOI:10.1111/jnc.14001 |
| [14] |
LIN L, YIHAO T, ZHOU F, YIN N, QIANG T, HAOWEN Z, et al. Inflammatory regulation by driving microglial M2 polarization: neuroprotective effects of cannabinoid receptor-2 activation in intracerebral hemorrhage[J/OL]. Front Immunol, 2017, 8: 112. doi: 10.3389/fimmu.2017.00112.
|
| [15] |
DOS-SANTOS-PEREIRA M, GUIMARÃES F S, DEL-BEL E, RAISMAN-VOZARI R, MICHEL P P. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption[J]. Glia, 2020, 68: 561-573. DOI:10.1002/glia.23738 |
| [16] |
SHIUE S J, PENG H Y, LIN C R, WANG S W, RAU R H, CHENG J K. Continuous intrathecal infusion of cannabinoid receptor agonists attenuates nerve ligation-induced pain in rats[J]. Reg Anesth Pain Med, 2017, 42: 499-506. DOI:10.1097/AAP.0000000000000601 |
 2020, Vol. 41
2020, Vol. 41


