2. Department of Spine Surgery(Ⅱ), Changzheng Hospital, Naval Medical University(Second Military Medical University), Shanghai 200003, China
胸椎后纵韧带骨化症(vertebra-ossification of posterior longitudinal ligament,OPLL)是一种因胸椎后纵韧带异位骨化压迫脊髓和(或)神经根导致肢体感觉和运动障碍及内脏自主神经功能紊乱的疾病。胸椎OPLL发生率低于颈椎OPLL,在日本人群中发病率为1.6%~1.9%[1-2]。对于胸椎OPLL的手术治疗,由于其解剖位置的关系,通过前方入路的直接减压仅局限于颈胸段病变。对于中段胸椎和胸腰段的OPLL可以选择侧方、侧后方和后方入路摘除骨化物直接减压。但脊髓直接减压手术操作难度大,同时术后神经功能恶化和脑脊液漏等并发症的发生率高[3]。因此对于 > 3个节段的骨化多通过去除椎板、同时纠正胸椎后凸间接缓解对脊髓的压迫,手术操作相对安全,但是术后神经功能恢复往往不理想[3]。为了能更安全、有效地治疗严重的多节段胸椎OPLL,本团队尝试使用胸椎后路椎体骨化物复合体前移技术,通过后方入路在不切除骨化物的前提下实现了脊髓完全减压。现对该技术进行介绍,并报道1例接受该技术治疗的多节段胸椎OPLL患者随访13个月的资料,初步评估其治疗效果。
1 资料和方法 1.1 手术操作 1.1.1 术前准备术前影像学检查包括胸椎X线侧位片、胸椎CT三维重建和胸椎MRI。依据胸椎CT三维重建数据测量骨化物的左右径、前后径和范围,指导椎弓根螺钉置入。通过胸椎MRI判断脊髓受压节段及受压程度,明确手术节段。根据术前测得的骨化物前后径判断椎体骨化物复合体的前移距离。对于无“山峰状”骨化凸入椎管的节段,椎体的前推距离与骨化物厚度相当。对于存在“山峰状”骨化的节段,由于节段前骨化物前后径相差较大,若以骨化物最大前后径作为椎体的前移距离将可能造成骨化较前节段椎体过度前移,致使神经张力增高,而对可能伴硬脊膜骨化者过度提拉甚至可能导致硬膜撕裂的发生,因而,我们选取“山峰状”骨化以外骨化物的最大前后径,并以此作为椎体的前移距离。
1.1.2 麻醉及体位患者全身麻醉后取俯卧位,并进行电生理监护。
1.1.3 显露术中X线透视定位手术节段。术区消毒铺单。选择后正中入路显露椎体后方结构,显露过程中用电刀双极电凝及时止血。对于前移节段显露位置需达两侧横突至肋横突关节处,对于前移头端及尾端节段仅需显露至椎弓根螺钉置钉处。显露结束后,双极电凝彻底止血。于椎体前移节段及头端、尾端各节段椎弓根置钉处放置标记,在X线透视下观察钉道位置。
1.1.4 分离两侧肋骨及松解椎间盘于前移节段两侧肋横突关节远端5 mm处骨膜下剥离肋骨,用超声骨刀不完全离断肋骨。保留肋骨腹侧皮质骨,改为2 mm枪钳咬断前方骨皮质,致远端肋骨浮动(图 1A)。三关节咬骨钳咬除前移椎体节段首、尾间隙位置两侧椎间关节,暴露胸段脊髓及神经根,自硬脊膜及神经根之间使用神经根挡板保护神经根,显露前方椎间盘。松解椎间盘,降低前移阻力,主要操作包括:尖刀切开侧后方纤维环,髓核钳取出少量髓核组织,铰刀松解椎间盘,不撑开椎间隙,完成对椎间盘的松解。
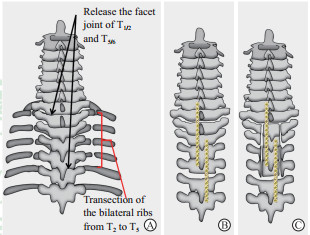
|
图 1 游离椎体骨化物复合体示意图 Fig 1 Illustration of the procedure of isolation of VOC A: Facet joints and ligamentum flavum were resected at T1/2 and T5/6. Bilateral ribs from T2 to T5 were transected at 5 mm distal to the sternocostal joints of each level. B: Two long connecting plates were installed on the spinous processes from C7 to T7 to help fixing the posterior wall of the T2-5 spinal canal. C: Two longitudinal osteotomy incisions were located to the medial of bilateral pedicles and connected to the previously resected T1/2 and T5/6 facet, isolating the posterior wall of the T2-5 spinal canal. VOC: Vertebra-OPLL complex; OPLL: Ossification of posterior longitudinal ligament |
1.1.5 游离椎板
使用三关节咬骨钳和枪钳咬除前移节段与头端和尾端节段椎板和棘突之间的韧带组织。两侧椎板与棘突交界处的基底处,上下错位各放置一块稳定钢板辅助固定椎板(图 1B)。于两侧椎板与关节突移行处(椎弓根内侧缘)骨槽内填充明胶海绵和脑棉止血(图 1C)。
1.1.6 置入椎弓根螺钉取出之前放置的标记,于前移节段以外椎体拧入长尾椎弓根螺钉。在前移节段置入螺钉时,依据术前测量的骨化物前后径,保留螺钉螺纹数,部分置入椎弓根螺钉,使前移节段螺钉与头尾节段螺钉之间产生高度落差。置入折弯的钛棒(直径5.5 mm),拧紧前移节段的椎弓根螺钉螺帽并锁定。前移节段以外节段仅预先拧入螺帽,不拧紧。
1.1.7 椎体骨化物复合体前移使用多把螺帽或起子同步、缓慢拧紧前移头、尾节段螺帽,以钛棒作为力臂将骨化节段椎体骨化物复合体向腹侧推动。松解椎间盘后,在拧紧各节段螺钉时,无异常阻力增大。术中注意电生理监护结果。完成前移后拧紧并锁定螺帽(图 2)。
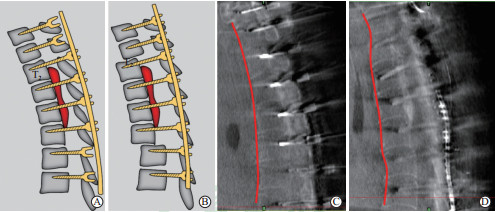
|
图 2 椎体骨化物复合体前移示意图及术中CT透视图 Fig 2 Illustration and intraoperative CT demonstrating thoracic VOC antedisplacement technique A: Screws were placed into the VOC levels and two levels cranial and caudal to the VOC. The screws in the VOC levels were unscrewed to create enough space for the antedisplacement of the VOC. B: Nuts of cranial and caudal levels to the VOC were tightened to force the VOC into an antedisplacement position. C: The intraoperative CT scan with O-arm showing the anterior border of the thoracic spine (red line) before antedisplacement procedure. D: The intraoperative CT scan with O-arm showing the anterior border of the thoracic spine (red line) after antedisplacement procedure. CT: Computed tomography; VOC: Vertebra-OPLL complex; OPLL: Ossification of posterior longitudinal ligament |
1.1.8 关闭切口及术后制动
在两侧开槽内椎弓根螺钉置钉处侧方植骨,冲洗术野后彻底止血,留置负压引流管,逐层关闭手术创口。术后佩戴胸椎支具3个月,保证植骨融合,早期进行肢体功能锻炼。
1.2 病例资料患者女,54岁,主诉双下肢无力、麻木及感觉障碍6个月。近2个月症状进行性加重,出现步态不稳和排便功能障碍。体格检查:胸骨角以下躯体感觉异常(束带感);肛门反射减退;双下肢触觉、痛温觉减退;双下肢肌力3级;双下肢腱反射亢进;双侧Babinski征(+)。
术前改良日本骨科协会评分(modified-Japanese Orthopaedic Association score,m-JOA评分)为3分,术前胸椎X线片示T2~T5可见后纵韧带骨化影(图 3A)。术前MRI示椎管前方低信号影,压迫脊髓(图 3B)。CT矢状面示T2~T5连续型OPLL,T2/3与T3/4椎间隙位置骨化物呈“山峰状”,余节段骨化物较为平坦(图 3C);横断面示T2/3与T3/4骨化物前后径分别为9.7 mm和7.5 mm,椎管狭窄率分别为86.6%和68.2%(图 3D、3E)。
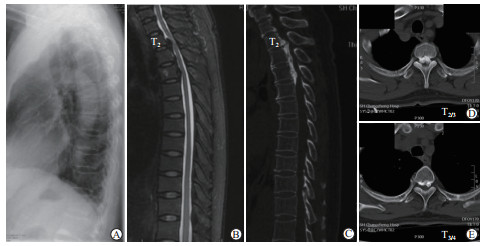
|
图 3 病例术前影像学资料 Fig 3 Preoperative imaging of the patient A: Plain radiograph of thoracic spine; B: Sagittal T2-weighted MRI of the spinal cord presented low-signal masses caused the compression of the spinal cord; C-E: CT view showing T2-T5 multi-level continuous OPLL. MRI: Magnetic resonance imaging; CT: Computed tomography; OPLL: Ossification of posterior longitudinal ligament |
2 结果
患者接受胸椎后路椎体骨化物复合体前移技术,前移节段包括T2~T5,手术耗时480 min,术中出血约1 000 mL,术中输注红细胞悬液400 mL。术后患者肢体麻木感缓解,下肢肌力改善至4级,肋骨离断处有压痛,但不影响呼吸。术后m-JOA评分为9分,m-JOA评分改善率[(术后m-JOA评分-术前m-JOA评分)/(11-术前m-JOA评分)]为75%。末次随访时,患者平地行走及上楼梯不借助支撑,可以慢跑,无行走不稳,m-JOA评分为10分,胸背部压痛消失。末次随访时全脊柱X线片示T2~T5椎体前移(图 4A);MRI检查示脊髓压迫解除,脊髓形态恢复正常,前后脑脊液带恢复(图 4B);CT检查示骨化节段椎管扩大,术后T2/3与T3/4间隙水平椎管狭窄率降至58.8%和45.9%。椎体前移距离为6.4 mm。骨化节段双侧肋骨离断并前后错位,少量胸腔积液(图 4C~4E)。
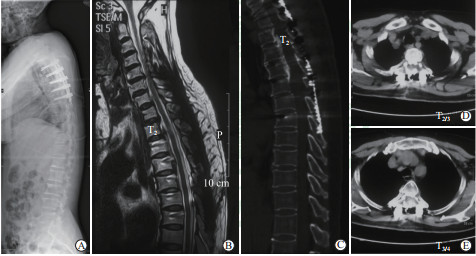
|
图 4 病例末次随访时影像学资料 Fig 4 Imaging of the patient at final follow-up A: Plain radiograph; B: Sagittal T2-weighted MRI presented no compression existed in the spinal canal; C: Sagittal CT scanning showed that the VOC was pushed ventrally and sufficient enlargement of the spinal canal; D, E: Axial CT scanning at T2/3 and T3/4 demonstrated that the displacement of VOC and the fusion of fractured ribs could be noticed at final follow-up. MRI: Magnetic resonance imaging; CT: Computed tomography; VOC: Vertebra-OPLL complex; OPLL: Ossification of posterior longitudinal ligament |
3 讨论
胸椎OPLL在20世纪70年代被首次报道,其发病率低于颈椎OPLL,流行病学研究显示OPLL的患病率仅为颈椎OPLL的1/4[1, 4]。但是,当胸椎OPLL压迫脊髓出现神经受损的表现后,症状多逐渐恶化[5]。胸椎OPLL保守治疗往往效果不佳,手术是唯一有效的治疗方式[6]。治疗胸椎OPLL的手术方法包括前、后入路直接减压[7-8],后入路间接减压[9-10],360°环形减压[11],以及我国学者提出的“涵洞塌陷法”和“胸椎管后壁切除联合去后凸法”[10, 12]。这些方法在治疗OPLL中均取得了一定的手术效果,但对于胸椎OPLL术后症状改善率差,同时具有极高的致残率。根据系统回顾和多中心研究,这些术式的术后神经功能恢复率均低于60%,并发症发生率极高,其中以硬膜撕裂、神经功能恶化、脑脊液漏发生率最高[13-14]。与此同时,这些治疗方式多适用于3个节段以内的骨化。随着节段的增加,手术并发症的发生率和术后神经功能恶化的发生率骤升[13]。因此,迫切需要一种安全有效的手术方法以改变严重多节段胸椎OPLL的治疗现状。
为治疗严重多节段颈椎OPLL,本团队创新性地提出了颈前路可控性前移减压植骨融合内固定术(anterior controllable antedisplacement and fusion,ACAF)。将颈椎椎体和骨化的后纵韧带作为一个整体,即椎体骨化物复合体(vertebra-OPLL complex),分离后向腹侧移动,重建椎管的空间,在不切除骨化的前提下实现脊髓的直接减压。依据ACAF的手术理念,本团队提出将胸椎椎体、骨化物及部分肋骨作为椎体骨化物复合体,并通过前移椎体骨化物复合体的手术方式达到对胸段脊髓的原位减压。但在手术设计时,由于胸腹腔中存在的诸多脏器和脉管系统,若采用腹侧手术入路,发生手术入路相关并发症的风险极高,因此采用后方入路借助后方钉棒系统产生的杠杆作用,将椎体骨化物复合体从后向前推移,从而降低手术暴露的难度并避免前方入路相关并发症。
传统后路椎板切除减压固定术通过去除椎管后方椎板结构,脊髓向后飘移,间接减压脊髓,由于胸椎生理性后凸的存在,减压效果多不理想[15]。为解决胸椎后凸对手术效果的制约,通过后方加压纠正胸椎后凸角度,增加脊髓后移距离。但由于椎体形态及韧带组织的存在,单纯加压螺钉对后凸的纠正效果不佳,尤其对于“山峰状”的骨化减压效果不满意。孙垂国等[10]通过椎管后壁切除联合去后凸治疗多节段胸椎OPLL,通过椎体后方的楔形截骨,增加纠正后凸角度,取得良好的手术效果。但增加截骨操作后,手术并发症的发生率升高。直接减压及360°环形减压在进行脊髓减压过程中都涉及椎管内操作,通过扩大椎管的空间以去除和塌陷骨化物。同时,当骨化物和硬膜粘连严重,甚至硬膜骨化时,难以分离硬膜与骨化物,去除骨化物和塌陷骨化物的操作极易造成硬膜的损伤,甚至导致神经功能恶化。在手术节段的选择方面,由于手术难度大、并发症发生率高,在 > 3个节段的严重胸椎OPLL治疗中不被选用。因此,直接减压和360°环形减压多适用于短节段OPLL的减压,或是对长节段OPLL术中减压不全节段的骨化作进一步的减压。胸椎前移术最大的优势是在避免去除骨化物及任何椎管前方截骨操作的前提下能实现脊髓直接减压。在手术中,首先游离骨化节段椎管后方椎板结构,并通过钢板稳定。同时,去除前移节段上位及下位间隙的关节突,并通过此间隙松解椎间盘,后者需在神经根挡板的保护下进行操作。操作过程中,不进行截骨及无动力系统的使用,发生硬膜及神经损伤的风险极低。利用螺钉和钛棒的杠杆作用,在电生理监测下将骨化物向腹侧推离,实现脊髓的直接减压,保证了脊髓减压的可控性和安全性。此外,胸椎前移技术不受手术节段的限制,尤其适用于长节段的严重胸椎OPLL的治疗。对于局部骨化物呈“山峰状”者,前移椎体存在减压不足的风险,可通过纠正胸椎后凸获得进一步的减压空间。
离断双侧肋骨是游离椎体骨化物的技术难点之一,其易并发损伤肋间神经及血管的风险。然而在实际操作中,这种损伤的发生概率相对较低。首先,在截骨位置,肋间神经和血管主要行走在肋间间隙的中间,并没有贴合肋骨骨面行走。将肋骨行骨膜下分离,可减少截骨过程中的出血。保留前方骨皮质,改为枪钳咬断,可降低壁层胸膜和重要血管结构损伤的风险。
本例患者术前影像学示骨化最严重节段位于T2/3椎间隙,骨化物呈“山峰状”,骨化前后径为9.7 mm;其他骨化物前后径最大为7.5 mm,最小为5.4 mm。选择以最大骨化物前后径为标准进行椎体前推将导致椎体过度前移。当存在硬膜与骨化物粘连严重、硬膜骨化时,发生硬膜撕裂、脑脊液漏的概率大大升高,而修补腹侧的硬膜操作难度极大,继发脊髓损伤风险高。因此术前规划前推椎体距离为7 mm。术后影像学检查显示,脊髓减压完全,矢状面可见脊髓前后脑脊液带充盈。胸腔可见少量积液(在末次随访时已完全吸收),肋骨游离处无呼吸痛及假关节的形成。无硬膜撕裂、脑脊液漏、神经功能恶化等并发症。
然而,本研究仍存在局限,仅报道了1例患者接受胸椎后路椎体骨化物复合体前移手术的效果及其1年的随访资料。对于肋骨游离这一操作可能带来的胸膜损伤、呼吸痛、假关节形成等并发症的发生率,以及与传统手术治疗的对比仍然需要大样本的回顾性和前瞻性研究进一步分析。另外,前移后内固定的远期稳定性及与生物力学相关的问题,亦需要长期随访研究和动物、尸体解剖实验佐证。下一步我们将侧重于增加大规模的病例和对照,以揭示胸椎后路椎体骨化物复合体前移技术的适用性和安全性。
| [1] |
MORI K, IMAI S, KASAHARA T, NISHIZAWA K, MIMURA T, MATSUSUE Y. Prevalence, distribution, and morphology of thoracic ossification of the posterior longitudinal ligament in Japanese:results of CT-based cross-sectional study[J]. Spine (Phila Pa 1976), 2014, 39: 394-399. DOI:10.1097/BRS.0000000000000153 |
| [2] |
FUJIMORI T, WATABE T, IWAMOTO Y, HAMADA S, IWASAKI M, ODA T. Prevalence, concomitance, and distribution of ossification of the spinal ligaments:results of whole spine CT scans in 1500 Japanese patients[J]. Spine (Phila Pa 1976), 2016, 41: 1668-1676. DOI:10.1097/BRS.0000000000001643 |
| [3] |
ITO Z, MATSUYAMA Y, ANDO M, KAWABATA S, KANCHIKU T, KIDA K, et al. Postoperative paralysis from thoracic ossification of posterior longitudinal ligament surgery risk factor of neurologic injury:nationwide multiinstitution survey[J]. Spine (Phila Pa 1976), 2016, 41: E1159-E1163. DOI:10.1097/BRS.0000000000001585 |
| [4] |
OHTSUKA K, YANAGIHARA M. The epidemiology of the hyperostosis of the spine[J]. Seikeigeka MOOK, 1987, 50: 13-25. |
| [5] |
XU Z W, HU Y C, SUN C G, SHANG X P, LUN D X, LI F, et al. Treatment for thoracic ossification of posterior longitudinal ligament with posterior circumferential decompression[J]. Orthop Surg, 2017, 9: 206-214. DOI:10.1111/os.12331 |
| [6] |
SEICHI A, TAKESHITA K, NAKAMURA K. Choice of surgical procedures for thoracic ossification of the posterior longitudinal ligament[M]. Tokyo: Springer, 2006: 225-230.
|
| [7] |
FUJIMURA Y, NISHI Y, NAKAMURA M, TOYAMA Y, SUZUKI N. Anterior decompression and fusion for ossification of the posterior longitudinal ligament of the upper thoracic spine causing myelopathy:using the manubrium splitting approach[J]. Spinal Cord, 1996, 34: 387-393. DOI:10.1038/sc.1996.70 |
| [8] |
OHTSUKA K, TERAYAMA K, YANAGIHARA M, WADA K, KASUGA K, MACHIDA T, et al. An epidemiological survey on ossification of ligaments in the cervical and thoracic spine in individuals over 50 years of age[J]. Nihon Seikeigeka Gakkai Zasshi, 1986, 60: 1087-1098. |
| [9] |
TSUZUKI N, WADANO Y, KIKUCHI S I. Extensive cervicothoracic laminoplastic decompression of the spinal cord:a new method of posterior decompression for thoracic myelopathy caused by ossification of the posterior longitudinal ligament[M]. Tokyo: Springer, 1997: 185-186.
|
| [10] |
孙垂国, 陈仲强, 郭昭庆, 齐强, 李危石, 曾岩, 等. 胸椎管后壁切除联合去后凸治疗多节段胸椎后纵韧带骨化症[J]. 中华骨科杂志, 2015, 35: 6-10. DOI:10.3760/cma.j.issn.0253-2352.2015.01.002 |
| [11] |
TOMITA K, KAWAHARA N, BABA H, KIKUCHI Y, NISHIMURA H. Circumspinal decompression for thoracic myelopathy due to combined ossification of the posterior longitudinal ligament and ligamentum flavum[J]. Spine (Phila Pa 1976), 1990, 15: 1114-1120. DOI:10.1097/00007632-199011010-00006 |
| [12] |
刘晓光, 刘忠军, 陈仲强, 姜亮, 韦峰, 党耕町. "涵洞塌陷法"360°脊髓环形减压术治疗胸椎管狭窄症[J]. 中华骨科杂志, 2010, 30: 1059-1062. DOI:10.3760/cma.j.issn.0253-2352.2010.11.010 |
| [13] |
IMAGAMA S, ANDO K, TAKEUCHI K, KATO S, MURAKAMI H, AIZAWA T, et al. Perioperative complications after surgery for thoracic ossification of posterior longitudinal ligament:a nationwide multicenter prospective study[J]. Spine (Phila Pa 1976), 2018, 43: E1389-E1397. DOI:10.1097/BRS.0000000000002703 |
| [14] |
ONISHI E, YASUDA T, YAMAMOTO H, IWAKI K, OTA S. Outcomes of surgical treatment for thoracic myelopathy:a single-institutional study of 73 patients[J]. Spine (Phila Pa 1976), 2016, 41: E1356-E1363. DOI:10.1097/BRS.0000000000001622 |
| [15] |
MATSUMOTO M, CHIBA K, TOYAMA Y, TAKESHITA K, SEICHI A, NAKAMURA K, et al. Surgical results and related factors for ossification of posterior longitudinal ligament of the thoracic spine:a multi-institutional retrospective study[J]. Spine (Phila Pa 1976), 2008, 33: 1034-1041. DOI:10.1097/BRS.0b013e31816c913b |
 2020, Vol. 41
2020, Vol. 41


