我国自2010年开展首例经导管主动脉瓣置换术(transcatheter aortic valve replacement,TAVR)以来,目前已经积累了1 000余例TAVR手术经验,随着新型瓣膜的研发应用,国内多家中心陆续开展了TAVR[1]。目前已上市的国产瓣膜有2种,一种是Venus-A瓣膜,属自膨胀瓣膜,主要经股动脉途径置入,用于以主动脉瓣狭窄为主的患者;另一种为J-Valve瓣膜,亦属于自膨胀瓣膜,经心尖途径置入,既可用于单纯主动脉瓣关闭不全的患者,亦可用于单纯主动脉瓣狭窄的患者。由于主动脉瓣邻近房室结和希氏束,使新发传导异常和(或)房室传导阻滞成为TAVR术后常见的并发症之一。本研究旨在总结2种瓣膜TAVR术围手术期心电图的变化情况,从而对不同电传导障碍的患者进行适当的临床干预。
1 资料和方法 1.1 研究对象选择2017年12月至2018年12月在我院心血管外科就诊并行TAVR的重度主动脉瓣狭窄、重度主动脉瓣关闭不全患者。(1)纳入标准:符合重度主动脉瓣狭窄或重度主动脉瓣关闭不全诊断;(2)纽约心脏协会(New York Heart Association,NYHA)心功能分级Ⅲ~Ⅳ级或合并心源性休克;(3)存在主动脉瓣置换术禁忌证或主动脉瓣手术高危者。排除标准:(1)合并其他系统疾病且预期寿命<1年者;(2)重度左心功能不全,左心室射血分数(left ventricular ejection fraction,LVEF)<20%。本研究符合赫尔辛基宣言原则[2],并通过我院医学伦理委员会审批(CHEC2018-049)。
1.2 诊断标准重度主动脉瓣狭窄诊断标准:超声心动图检查示主动脉瓣瓣口面积(aortic valve area,AVA)<1.0 cm2,或平均跨瓣压差≥40 mmHg(1 mmHg=0.133 kPa),或跨主动脉瓣最大血流速度≥4 m/s。对于低血流速度、低跨瓣压差、LVEF<50%的主动脉瓣狭窄患者,即AVA<1.0 cm2、跨主动脉瓣最大血流速度<4 m/s、平均跨瓣压差<40 mmHg的患者,行多巴酚丁胺负荷试验后AVA<1.0 cm2同时跨主动脉瓣最大血流速度≥4 m/s即为重度主动脉瓣狭窄;对于低血流速度、低跨瓣压差、LVEF>50%的患者,即AVA<1.0 cm2、跨主动脉瓣最大血流速度<4 m/s、平均跨瓣压差<40 mmHg的患者,若患者左心室壁明显肥厚,左心腔较小,每搏输出量<35 mL/m2,且测量时患者血压正常,亦为重度主动脉瓣狭窄。
重度主动脉瓣关闭不全诊断标准:伴有劳力性呼吸困难、心绞痛或其他心力衰竭症状者,LVEF正常或下降,存在重度左心室扩张[左心室舒张末期内径(left ventricular end-diastolic diameter,LVEDD)>50 mm]。
1.3 研究方法记录患者性别、年龄、TAVR使用瓣膜情况。于TAVR术前、术后60 min、术后7~12 d查心电图并记录心率、心律失常的表现。
2 结果 2.1 患者基线情况本研究共纳入20例患者,男11例、女9例,年龄范围为71~88岁,平均年龄为(77.8±4.9)岁,其中4例为二叶式主动脉瓣狭窄、4例为单纯主动脉瓣关闭不全。单纯重度主动脉瓣狭窄12例,应用Venus-A瓣膜行TAVR治疗;重度主动脉瓣关闭不全8例,应用J-Valve瓣膜。20例患者均合并严重心力衰竭,术前NYHA心功能分级Ⅲ级15例、Ⅳ级5例;合并有高血压16例,糖尿病3例,冠心病12例,慢性阻塞性肺疾病2例;术前合并心房颤动6例,左束支传导阻滞1例,右束支传导阻滞2例。合并使用药物治疗情况:使用血管紧张素转化酶抑制剂者3例,血管紧张素Ⅱ受体拮抗剂者8例,他汀类者5例,利尿剂者5例。
2.2 心电图随访情况20例患者均成功施行TAVR,术前、术后典型心电图结果见图 1、图 2。
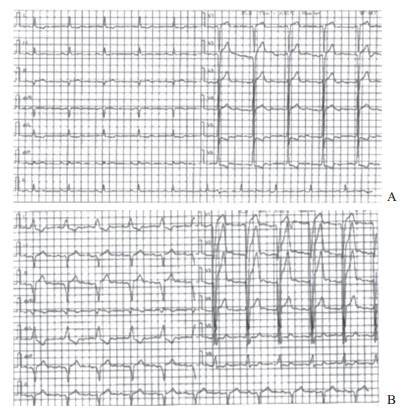
|
图 1 1例应用J-Valve瓣膜行TAVR治疗的88岁男性患者术前、术后24 h心电图表现 A:术前诊断为窦性心律; B:术后24 h诊断为窦性心律、Ⅰ度房室传导阻滞(PR间期为230 ms)、完全性左束支传导阻滞(QRS间期为154 ms). TAVR:经导管主动脉瓣置换术 |

|
图 2 1例应用Venus-A瓣膜行TAVR治疗的78岁男性患者术前、术后24 h、术后半年心电图表现 A:术前诊断为窦性心律; B: TAVR术后24 h诊断为窦性心律、完全性左束支传导阻滞(QRS间期为130 ms); C:术后半年诊断为窦性心律(QRS间期为98 ms). TAVR:经导管主动脉瓣置换术 |
应用Venus-A瓣膜行TAVR的患者,2例在围手术期新发Ⅲ度房室传导阻滞(均于术后7~12 d自行恢复正常传导功能),6例新发左束支传导阻滞,2例新发右束支传导阻滞。应用J-Valve瓣膜的患者在围手术期均未发生Ⅲ度房室传导阻滞,4例新发左束支传导阻滞,2例新发右束支传导阻滞,3例新发多源室性心动过速及室性早搏。
3 讨论目前指南仍推荐TAVR适用于高风险的重度主动脉瓣狭窄患者,但TAVR的适应证有望逐渐扩大,可以包括中危的主动脉瓣狭窄患者[3],也有研究发现TAVR亦可用于二叶式主动脉瓣狭窄[4-7]和单纯主动脉瓣关闭不全患者[8],本研究纳入TAVR治疗患者中也有二叶式主动脉瓣狭窄和单纯主动脉瓣关闭不全患者各4例。
目前被批准应用于临床的瓣膜装置有3种:球扩式Edwards-Sapien瓣膜(Edwards-Sapien Valve,ESV)、自膨胀式Medtronic CoreValve瓣膜系统(Medtronic CoreValve System,MCRS)和无需球扩系统的Boston Lotus瓣膜,MCRS最新一代的产品有Edwards Sapien XT、Edwards Sapien 3和CoreValve Evolut R。目前我国自主研发上市的2种自膨胀瓣膜分别是Venus-A瓣膜和J-Valve瓣膜。在TAVR的操作过程中,传导系统受到急剧缺血与炎症损伤,随之是缓慢的恢复过程[7],这可能与迟发的部分传导阻滞有关[9]。另外自膨胀瓣膜及球扩式瓣膜系统的设计特点、瓣膜系统置入深度都会直接导致传导系统急性机械损伤[10-11];位于传导系统附近的钙化位置也成为主动脉瓣狭窄患者新发左束支传导阻滞及重度房室传导阻滞的关键原因[10]。然而这2种瓣膜系统发生上述心律失常的概率不同。Urena等[11]分析了202例置入球扩式瓣膜系统患者的资料,术前心电图均显示无传导阻滞或起搏器植入,61例(30.2%)患者于住院期间发现左束支传导阻滞,其中52例(85.2%)患者恢复正常传导功能,30例(49.2%)在出院后7 d内恢复,22例(36.1%)于术后长期随访时恢复,这一结果与其他研究结果[12-14]一致。另一项纳入91例置入自膨胀瓣膜系统患者研究中,并没有排除术前存在传导阻滞及有起搏器植入的患者,49例(54%)的患者新发左束支传导阻滞或在6个月的随访中发生延迟性左束支传导阻滞[10]。此外也有研究提示,自膨胀瓣膜系统可能导致较球扩式瓣膜系统更严重的机械损伤,以新发的左束支传导阻滞最为常见[15]。本研究中自膨胀瓣膜系统导致新发左束支传导阻滞的总发生率为50%(10/20),低于国外文献报道的发生率(59%)[10]。新发房室传导阻滞及心室间传导阻滞常发生在TAVR术后的48 h内,并在术后30 d内恢复正常传导。研究显示约22%的患者在球扩式瓣膜置入术后或外科瓣膜置换术后出现房室传导阻滞,这些患者成为永久性房室传导阻滞并需植入心脏永久起搏器的风险是其他患者的5倍[16]。然而大多数的完全性房室传导阻滞及新发左束支传导阻滞在术后初期恢复正常传导。例如在Bjerre Thygesen等[17]的一项研究中,234名受试者中46例(19.7%)患者在自膨胀瓣膜置入术后发生Ⅱ度和(或)Ⅲ度房室传导阻滞且有起搏器植入的绝对适应证,然而半数患者在手术24 h后恢复了正常传导。本研究中10%(2/20)的自膨胀瓣膜置入患者发生Ⅲ度房室传导阻滞,但均于术后7~12 d恢复正常房室传导;右束支传导阻滞在Venus-A瓣膜和J-Valve瓣膜置入后均有发生,发生率分别为17%(2/12)及25%(2/8);37%(3/8)的J-Valve瓣膜置入患者在围手术期出现室性心律失常,这可能与术中经心尖路径操作导致心肌损伤有关。本研究中无一例患者需要植入心脏永久起搏器。
综上所述,TAVR无疑是心血管外科高手术风险的高龄主动脉瓣狭窄患者绝佳的治疗选择。但开展TAVR是一个较为复杂的过程,围手术期的临床治疗及远期的随访观察亦是循序渐进的过程,因此TAVR术后心律失常的发生时间窗、追踪心律失常的转归是需要临床医师密切关注的环节,从而避免过度治疗,完善医疗程序,将TAVR更好地应用于患者。
| [1] |
中华医学会心血管病学分会结构性心脏病学组, 中国医师协会心血管内科医师分会结构性心脏病专业委员会. 中国经导管主动脉瓣置换术临床路径专家共识[J]. 中国介入心脏病学杂志, 2018, 26: 661-668. DOI:10.3969/j.issn.1004-8812.2018.12.001 |
| [2] |
World Medical Association General Assembly. World Medical Association Declaration of Helsinki:ethical principles for medical research involving human subjects[J]. J Int Bioethique, 2004, 15: 124-129. DOI:10.3917/jib.151.0124 |
| [3] |
LEON M B, SMITH C R, MACK M J, MAKKAR R R, SVENSSON L G, KODALI S K, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients[J]. N Engl J Med, 2016, 374: 1609-1620. DOI:10.1056/NEJMoa1514616 |
| [4] |
ZHAO Z G, JILAIHAWI H, FENG Y, CHEN M. Transcatheter aortic valve implantation in bicuspid anatomy[J]. Nat Rev Cardiol, 2015, 12: 123-128. DOI:10.1038/nrcardio.2014.161 |
| [5] |
MYLOTTE D, LEFEVRE T, SØNDERGAARD L, WATANABE Y, MODINE T, DVIR D, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease[J]. J Am Coll Cardiol, 2014, 64: 2330-2339. DOI:10.1016/j.jacc.2014.09.039 |
| [6] |
PERLMAN G Y, BLANKE P, DVIR D, PACHE G, MODINE T, BARBANTI M, et al. Bicuspid aortic valve stenosis:favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study[J]. JACC Cardiovasc Interv, 2016, 9: 817-824. DOI:10.1016/j.jcin.2016.01.002 |
| [7] |
YOUSEF A, SIMARD T, WEBB J, RODÉS-CABAU J, COSTOPOULOS C, KOCHMAN J, et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve:a patient level multi-center analysis[J]. Int J Cardiol, 2015, 189: 282-288. DOI:10.1016/j.ijcard.2015.04.066 |
| [8] |
ZHU D, CHEN Y, ZHANG J, HU J, GUO Y. Transapical implantation of a new second-generation transcatheter heart valve in patients with pure aortic regurgitation:a preliminary report[J]. Interact Cardiovasc Thorac Surg, 2015, 20: 860-862. DOI:10.1093/icvts/ivv049 |
| [9] |
MANGIERI A, MONTALTO C, PAGNESI M, LANZILLO G, DEMIR O, TESTA L, et al. TAVI and post procedural cardiac conduction abnormalities[J]. Front Cardiovasc Med, 2018, 5: 85. DOI:10.3389/fcvm.2018.00085 |
| [10] |
PIAZZA N, NUIS R J, TZIKAS A, OTTEN A, ONUMA Y, GARCÍA-GARCÍA H, et al. Persistent conduction abnormalities and requirements for pacemaking six months after transcatheter aortic valve implantation[J]. EuroIntervention, 2010, 6: 475-484. DOI:10.4244/EIJ30V6I4A80 |
| [11] |
URENA M, MOK M, SERRA V, DUMONT E, NOMBELA-FRANCO L, DELAROCHELLIÈRE R, et al. Predictive factors and long-term clinical consequences of persistent left bundle branch block following transcatheter aortic valve implantation with a balloon-expandable valve[J]. J Am Coll Cardiol, 2012, 60: 1743-1752. DOI:10.1016/j.jacc.2012.07.035 |
| [12] |
URENA M, WEBB J G, CHEEMA A, SERRA V, TOGGWEILER S, BARBANTI M, et al. Impact of new-onset persistent left bundle branch block on late clinical outcomes in patients undergoing transcatheter aortic valve implantation with a balloon-expandable valve[J]. JACC Cardiovasc Interv, 2014, 7: 128-136. |
| [13] |
GUTIÉRREZ M, RODÉS-CABAU J, BAGUR R, DOYLE D, DELAROCHELLIÈRE R, BERGERON S, et al. Electrocardiographic changes and clinical outcomes after transapical aortic valve implantation[J]. Am Heart J, 2009, 158: 302-308. DOI:10.1016/j.ahj.2009.05.029 |
| [14] |
GODIN M, ELTCHANINOFF H, FURUTA A, TRON C, ANSELME F, BEJAR K, et al. Frequency of conduction disturbances after transcatheter implantation of an Edwards Sapien aortic valve prosthesis[J]. Am J Cardiol, 2010, 106: 707-712. DOI:10.1016/j.amjcard.2010.04.029 |
| [15] |
NUIS R J, VAN MIEGHEM N M, SCHULTZ C J, TZIKAS A, VAN DER BOON R M, MAUGENEST A M, et al. Timing and potential mechanisms of new conduction abnormalities during the implantation of the Medtronic CoreValve System in patients with aortic stenosis[J]. Eur Heart J, 2011, 32: 2067-2074. DOI:10.1093/eurheartj/ehr110 |
| [16] |
BLEIZIFFER S, RUGE H, HÖRER J, HUTTER A, GEISBÜSCH S, BROCKMANN G, et al. Predictors for new-onset complete heart block after transcatheter aortic valve implantation[J]. JACC Cardiovasc Interv, 2010, 3: 524-530. DOI:10.1016/j.jcin.2010.01.017 |
| [17] |
BJERRE THYGESEN J, LOH P H, CHOLTEESUPACHAI J, FRANZEN O, SØNDERGAARD L. Reevaluation of the indications for permanent pacemaker implantation after transcatheter aortic valve implantation[J]. J Invasive Cardiol, 2014, 26: 94-99. |
 2019, Vol. 40
2019, Vol. 40


