2. 复旦大学附属华山医院风湿免疫科, 上海 200040
2. Department of Rheumatology and Immunology, Huashan Hospital, Fudan University, Shanghai 200040, China
痛风是最常见的炎症性关节炎,在大多数发达国家,特别是在北美洲和欧洲,痛风的患病率超过1%[1],美国2007—2008年国家健康与营养调查结果显示,美国约有3.9%的成年人(年龄≥20岁)有痛风发作[2]。此外,痛风还与代谢综合征、心血管疾病和肾脏疾病有关[3]。因此,防治痛风有重大的社会经济学意义[4]。典型的急性痛风炎症是由关节内单钠尿酸盐(monosodium urate,MSU)晶体沉积引起的急性自限性炎症[5]。MSU晶体通过Toll样受体(Toll-like receptor,TLR)2和TLR4以及CD14在单核细胞/巨噬细胞表面表达识别,提供细胞外和细胞内的危险信号,启动单核细胞/巨噬细胞对MSU晶体的吞噬[6]。由MSU晶体刺激的单核细胞可极化为促炎性M1型巨噬细胞,其通过完全功能性吞噬MSU晶体引发炎症,促进细胞质NACHT-LRR-PYD结构域蛋白3炎性体的组装,从而释放肿瘤坏死因子α(tumor necrosis factor α,TNF-α)和白细胞介素1β(interleukin 1β,IL-1β)等高促炎性细胞因子,并促进外周血中性粒细胞流入炎症部位[7]。
白藜芦醇是一种含有芪类结构的非黄酮类多酚化合物,广泛存在于自然界植物中,研究表明其具有抗氧化、抗菌消炎、抗血小板聚集作用[8-9]。多项研究证明白藜芦醇还具有治疗痛风的作用,但其具体机制尚未阐明[10-11]。白藜芦醇是沉默信息调节因子1(silent mating type information regulation 1,Sirt1)激动剂,既往研究显示,Sirt1可通过TLR2/Sirt1/核因子κB(nuclear factor κB,NF-κB)途径对IL-1β介导的髓核细胞变性发挥抗炎作用[12]。本研究通过构建小鼠急性痛风性关节炎模型,从巨噬细胞极化角度探究白藜芦醇对痛风的具体作用机制。
1 材料和方法 1.1 主要试剂与仪器白藜芦醇购自美国Selleck公司(货号S1396);MSU购自美国Sigma公司(货号U2625、74542);RPMI 1640培养液、胎牛血清均购自美国Gibco公司(货号12633012、10099);TRIzol试剂盒、反转录PCR试剂盒、SYBR实时荧光定量(qPCR)试剂盒均购自日本TaKaRa公司,ViiATM 7 qPCR系统购自美国Applied Biosystems公司;巨噬细胞M1极化标志物诱导型一氧化氮合酶(inducible nitric oxide synthase,iNOS)兔源多克隆抗体、甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GAPDH)兔源多克隆抗体均购自英国Abcam公司(货号ab15323、ab181602);异硫氰酸荧光素(fluorescein isothiocyanate,FITC)标记的巨噬细胞M2极化标志物F4/80抗体、藻红蛋白(phycoerythrin,PE)标记的巨噬细胞M2极化标志物CD163兔源多克隆抗体均购自美国CST公司(货号52267、93498)。
1.2 MSU晶体的配制将1 g尿酸及0.5 g NaOH加入100 mL双蒸水中,置于水浴锅中80℃加热20 min后可得澄清液体,4℃过夜,冷却后晶体析出,继续采用滴定法(稀盐酸)将溶液pH调整至约7.2,可见大量晶体析出。偏振光显微镜下观察可见所得晶体呈长度为5~25 μm的针尖棒状,具有双折光性。将含MSU晶体的溶液1 500×g离心3 min,弃上清,所得晶体高温干燥后分装并称质量。常温保存。实验时用磷酸盐缓冲液或生理盐水稀释至所需浓度后进行高温、高压灭菌。
1.3 小鼠急性痛风性关节炎模型建立 1.3.1 分组及造模方案C57BL/6小鼠购自北京维通利华实验动物技术有限公司[实验动物生产许可证号为SCXK(京)2016-0006,使用许可证号为SYXK(京)2017-0033],共26只,雄性,6~8周龄,平均体质量为(20.00±1.04)g。选取18只进行造模,随机平均分为3组,每组6只,分别称质量,并标记各组。造模前先用游标卡尺测量并记录每只小鼠左右足掌的厚度,其足掌厚度基本一致,无显著差异。假手术组:用50 μL无菌生理盐水注射小鼠右侧后肢踝关节。造模+溶剂对照组:造模前7 d连续每天将5% DMSO 0.25 mL注射小鼠腹腔;造模时用50 μL无菌MSU晶体混悬液(含1 mg MSU晶体)注射小鼠右侧后肢踝关节,同时用50 μL无菌生理盐水注射左侧后肢踝关节。造模+白藜芦醇组:造模前7 d连续每天腹腔注射白藜芦醇(0.2 mL,用5% DMSO溶解),每次注射白藜芦醇20 mg/kg体质量;造模方法同造模+溶剂对照组。
1.3.2 小鼠双侧足掌厚度测定及关节滑膜组织H-E染色用MSU晶体混悬液注射于小鼠右侧后肢踝关节后,关节炎症的高峰通常出现于注射后8 h,用游标卡尺测量小鼠双侧后肢的足掌厚度,记录并比较。在炎症高峰期处死小鼠,取右侧后肢足掌关节滑膜组织用4%多聚甲醛溶液固定,脱钙后行H-E染色,观测中性粒细胞浸润面积。
1.4 小鼠腹腔原代巨噬细胞提取与培养另选取8只小鼠进行腹腔巨噬细胞的分离、提取。每只小鼠提取细胞前2~4 d腹腔注射2 mL 6%淀粉肉汤(将牛肉膏0.3 g、蛋白胨1.0 g及氯化钠0.5 g溶于蒸馏水,加热后加入可溶性淀粉6 g,溶解后高压灭菌,4℃保存。加热过程中不停搅拌,防止结成团块)。然后将小鼠引颈处死,分离并培养小鼠腹腔巨噬细胞。细胞培养于含10%胎牛血清的RPMI 1640培养液中,在5% CO2、37℃条件下培养。
调整细胞密度为1×106/mL,并接种于6孔板中,每孔约3 mL,待细胞贴壁后除去未贴壁细胞。各组细胞均以MSU晶体(100 μg/mL)刺激,白藜芦醇干预组提前1 h加入白藜芦醇(100 μmol/L),对照组未加白藜芦醇干预,检测3、6 h后巨噬细胞M1极化标志物iNOS的蛋白表达及炎性因子TNF-α、IL-1β 的mRNA表达。
1.5 蛋白质印迹法检测小鼠腹腔原代巨噬细胞中iNOS蛋白的表达用RIPA裂解液提取细胞总蛋白质,BCA法定量各组蛋白。各取等量样品经10%聚丙烯酰胺凝胶电泳分离后电转移至PVDF膜上,5%脱脂奶粉室温摇床上封闭2 h,加入iNOS、GAPDH一抗抗体(1︰1 000稀释),4℃孵育过夜,加入二抗于37℃温箱孵育1 h,增强化学发光显色,用凝胶成像系统分析处理。以目的蛋白与内参蛋白光密度值的比值作为目的蛋白的相对表达量。实验重复3次。
1.6 qPCR检测小鼠腹腔原代巨噬细胞中TNF-α、IL-1β mRNA的表达按照TRIzol试剂盒说明书从细胞中提取总RNA,将-70℃冻存组织在液氮中充分研磨后依次加入TRIzol、氯仿、异丙醇,离心取上清后以260 nm处的光密度值定量RNA浓度,并于-80℃冰箱保存。用反转录试剂盒在20 μL体系中反转录成cDNA,以此cDNA为模板,用SYBR Green Ⅰ嵌合荧光法行qPCR。TNF-α引物序列:上游5′-CTA CTG AAC TTC GGG GTG AT-3′,下游5′-GGG CCA TGA AAA CTG GTG TG-3′;IL-1β引物序列:上游5′-GAA CAC GGC AGT GGC TTT AAC-3′,下游5′-TGC TTA GCT CTG TCT GCT TTG C-3′。反应条件:95℃预变性30 s;95 ℃变性5 s,60℃退火20 s,72℃延伸1 min,40个循环。引物由生工生物工程(上海)股份有限公司设计并合成。所有数据均采用2–ΔΔCt法进行相对定量分析,实验重复3次。
1.7 流式细胞术检测小鼠腹腔原代巨噬细胞M2极化标志物对照组及白藜芦醇干预组均以MSU晶体(100 μg/mL)刺激,干预组提前1 h加入白藜芦醇(100 μmol/L),对照组未加白藜芦醇干预,0 h与6 h分别检测M2型巨噬细胞比例。分组处理培养的腹腔原代巨噬细胞经胰酶消化5 min后用细胞刮轻轻刮下来,磷酸盐缓冲液洗涤3次,调整细胞密度为1×105/mL,然后加入F4/80-FITC和CD163-PE抗体各3 μL,4℃避光染色30 min,上流式细胞仪检测。
1.8 统计学处理应用SPSS 19.0软件进行统计学分析,采用GraphPad Prism 5.0软件绘图。数据用x±s表示。两组间比较采用为独立样本t检验;多组间比较首先进行单因素方差分析,再采用LSD-t检验进行两两比较。检验水准(α)为0.05。
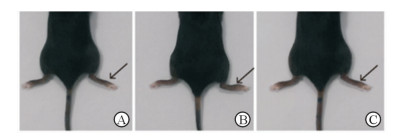
2 结果 2.1 白藜芦醇抑制MSU晶体刺激的小鼠足掌急性炎症造模后8 h处死小鼠,观察各组小鼠足掌肿胀程度并测量小鼠右侧足掌厚度(图 1)。造模+溶剂对照组小鼠足掌肿胀明显,足掌厚度为(2.49±0.12)mm,与假手术组[(1.81±0.03)mm]比较差异有统计学意义(P<0.01),证明小鼠足掌急性炎症模型构建成功。而在造模前给予白藜芦醇干预,可以显著降低模型小鼠足掌肿胀程度[(1.98±0.02)mm vs(2.49±0.12)mm,P<0.01],表明白藜芦醇对MSU晶体刺激的小鼠足掌急性炎症有预防作用。小鼠足掌关节滑膜组织H-E染色后中性粒细胞胞质透亮,细胞核呈深蓝色,且为多形性。结果显示白藜芦醇干预降低了小鼠关节滑膜组织中中性粒细胞的浸润面积(图 2),与小鼠足掌肿胀程度结果相似,提示白藜芦醇可以有效预防急性炎症发生。
|
图 1 各组小鼠足掌肿胀程度观察 Fig 1 Observation on paw swelling degree of mouse in each group A: Sham group; B: Model+solvent control group; C: Model+resveratrol group. Arrows indicate the sites for establishing acute gouty arthritis model |

|
图 2 各组小鼠足掌关节滑膜组织H-E染色观察 Fig 2 H-E staining of synovial tissue of feet and metacarpal joints of mouse in each group A: Sham group; B: Model+solvent control group; C: Model+resveratrol group. Arrows indicate neutrophil infiltration. Original magnification: ×200 |
2.2 白藜芦醇降低小鼠腹腔原代巨噬细胞M1
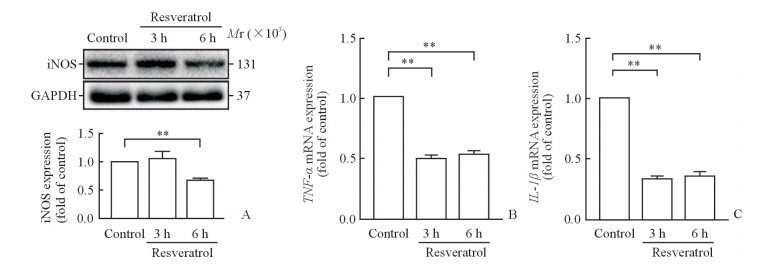
极化标志物iNOS和炎性因子TNF-α、IL-1β mRNA的表达蛋白质印迹分析结果显示,iNOS在白藜芦醇干预后3 h时无明显变化,而在6 h时明显降低(P<0.01,图 3A),说明白藜芦醇可以抑制巨噬细胞M1极化,这一抑制作用在6 h时较明显。qPCR结果则显示白藜芦醇干预后3 h TNF-α mRNA(图 3B)和IL-1β mRNA(图 3C)表达与对照组相比均明显降低(P均<0.01),说明白藜芦醇可以在转录水平明显降低炎性因子的表达。而白藜芦醇干预后3 h与6 h的TNF-αmRNA或IL-1β mRNA表达差异无统计学意义(P均>0.05),说明白藜芦醇对炎性因子的抑制在3 h时即可发挥明显作用。
|
图 3 各组小鼠腹腔原代巨噬细胞中iNOS蛋白及TNF-α、IL-1β mRNA的表达 Fig 3 Expression of iNOS protein and TNF-α and IL-1β mRNA in primary macrophages from mouse abdominal cavity in each group A: Expression of iNOS protein detected by Western blotting; B: Expression of TNF-α mRNA detected by qPCR; C: Expression of IL-1β mRNA detected by qPCR. iNOS: Inducible nitric oxide synthase; TNF-α: Tumor necrosis factor α; IL-1β: Interleukin 1β. **P < 0.01. n=3, x±s |
2.3 白藜芦醇促进小鼠腹腔原代巨噬细胞M2极化
流式细胞术检测M2型巨噬细胞标志物F4/80和CD163结果显示,白藜芦醇干预后F4/80+细胞比例较高;与对照组相比,白藜芦醇干预后F4/80+CD163+细胞比例在6 h时明显上调(P<0.01,图 4)。

|
图 4 流式细胞术检测小鼠腹腔原代巨噬细胞M2极化 Fig 4 M2-polarization of primary macrophages from mouse abdominal cavity detected by flow cytometry A: Control group; B: Resveratrol group; C: Quantitatively analysis of M2-polarized macrophage (F4/80+CD163+). **P < 0.01. n = 3, x±s |
3 讨论
巨噬细胞根据其分泌的细胞因子和细胞膜表面黏附分子的不同分为不同的亚型,不同亚型巨噬细胞的功能状态具有连续性,目前关注较多的是经典激活型(M1型)和替代激活型(M2型)。M1型巨噬细胞激活后释放促炎性细胞因子,促进炎症反应的持续;而M2型巨噬细胞激活后释放抑制炎症的因子,抑制炎症反应[13-15]。抗炎性M2型巨噬细胞可以抑制急性MSU晶体诱导的炎症并抑制caspase-1活化和IL-1β的产生[15]。在痛风炎症发展中,巨噬细胞从M1到M2的极化可能有助于急性炎症的自限性,而极化障碍则导致急性炎症慢性化[16]。由此可见巨噬细胞极化在痛风急性炎症调节中起关键作用,但目前这一极化调节的具体机制尚不清楚。
本研究从巨噬细胞极化出发,探究了白藜芦醇预防小鼠急性痛风性关节炎的机制。首先通过MSU晶体刺激成功构建小鼠急性痛风性关节炎模型,并且证明白藜芦醇可以有效缓解模型小鼠的炎症反应。为进一步探究其机制,我们分离、提取了小鼠腹腔原代巨噬细胞,分别体外给予MSU晶体和MSU晶体+白藜芦醇干预后,检测了炎症指标TNF-α和IL-1β的mRNA表达水平,发现白藜芦醇同时降低了这2项指标的表达。蛋白质印迹分析结果显示白藜芦醇干预组巨噬细胞M1极化标志物iNOS的表达水平降低,而流式细胞术结果显示M2极化标志物F4/80+CD163+细胞比例上升,说明白藜芦醇促进了M2极化的发生。由此我们认为,白藜芦醇可能通过促进M2极化,抑制炎性因子TNF-α与IL-1β的产生,从而对小鼠急性痛风性关节炎起到预防作用。
白藜芦醇是Sirt1激活剂,Sirt1是依赖于烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide,NAD)的第三类组蛋白去乙酰化酶(histone deacetylase,HDAC),广泛存在于机体成熟组织中[17]。Sirt1作为抗炎的明星分子,主要作用机制之一是抑制NF-κB的亚基P65第310位赖氨酸乙酰化,从而抑制NF-κB核移位,起到广泛的抗炎作用[18]。已有研究显示,Sirt1具有抗动脉粥样硬化、抗炎和抗衰老的作用[19-20]。进一步的研究表明,Sirt1通过调控巨噬细胞从M1向M2极化从而发挥炎症抑制作用[21]。巨噬细胞敲除Sirt1后,发生M1极化,从而促进应激相关炎症[22]。而Sirt1髓系敲除的小鼠腹腔巨噬细胞M2型标志物精氨酸酶1(arginase 1,Arg1)表达明显下降[23]。因此我们认为白藜芦醇可能是通过激活Sirt1促进巨噬细胞M2极化,抑制炎性因子产生,缓解小鼠急性痛风性关节炎。
| [1] |
KUO C F, GRAINGE M J, ZHANG W, DOHERTY M. Global epidemiology of gout: prevalence, incidence and risk factors[J]. Nat Rev Rheumatol, 2015, 11: 649-662. DOI:10.1038/nrrheum.2015.91 |
| [2] |
SMITH E, HOY D, CROSS M, MERRIMAN T R, VOS T, BUCHBINDER R, et al. The global burden of gout: estimates from the Global Burden of Disease 2010 study[J]. Ann Rheum Dis, 2014, 73: 1470-1476. DOI:10.1136/annrheumdis-2013-204647 |
| [3] |
BEVIS M, BLAGOJEVIC-BUCKNALL M, MALLEN C, HIDER S, RODDY E. Comorbidity clusters in people with gout: an observational cohort study with linked medical record review[J]. Rheumatology (Oxford), 2018, 57: 1358-1363. DOI:10.1093/rheumatology/key096 |
| [4] |
CHOI H K, MOUNT D B, REGINATO A M, American College of Physicians, American Physiological Society. Pathogenesis of gout[J]. Ann Intern Med, 2005, 143: 499-516. DOI:10.7326/0003-4819-143-7-200510040-00009 |
| [5] |
MARTIN W J, GRAINGER R, HARRISON A, HARPER J L. Differences in MSU-induced superoxide responses by neutrophils from gout subjects compared to healthy controls and a role for environmental inflammatory cytokines and hyperuricemia in neutrophil function and survival[J]. J Rheumatol, 2010, 37: 1228-1235. DOI:10.3899/jrheum.091080 |
| [6] |
LIU-BRYAN R, PRITZKER K, FIRESTEIN G S, TERKELTAUB R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation[J]. J Immunol, 2005, 174: 5016-5023. DOI:10.4049/jimmunol.174.8.5016 |
| [7] |
LIU Y F, TU S H, CHEN Z, WANG Y, HU Y H, DONG H. Effects of modified simiao decoction on IL-1β and TNF α secretion in monocytic THP-1 cells with monosodium urate crystals-induced inflammation[J/OL]. Evid Based Complement Alternat Med, 2014, 2014: 406816. doi: 10.1155/2014/406816.
|
| [8] |
SHEU S Y, CHEN W S, SUN J S, LIN F H, WU T. Biological characterization of oxidized hyaluronic acid/resveratrol hydrogel for cartilage tissue engineering[J]. J Biomed Mater Res A, 2013, 101: 3457-3466. DOI:10.1002/jbm.a.34653 |
| [9] |
PARK E J, PEZZUTO J M. The pharmacology of resveratrol in animals and humans[J]. Biochim Biophys Acta, 2015, 1852: 1071-1113. DOI:10.1016/j.bbadis.2015.01.014 |
| [10] |
YANG Q B, HE Y L, ZHONG X W, XIE W G, ZHOU J G. Resveratrol ameliorates gouty inflammation via upregulation of sirtuin 1 to promote autophagy in gout patients[J]. Inflammopharmacology, 2019, 27: 47-56. DOI:10.1007/s10787-018-00555-4 |
| [11] |
LI H, OU G, HE Y, REN L, YANG X, ZENG M. Resveratrol attenuates the MSU crystal-induced inflammatory response through the inhibition of TAK1 activity[J]. Int Immunopharmacol, 2019, 67: 62-68. DOI:10.1016/j.intimp.2018.12.004 |
| [12] |
SHEN J, FANG J, HAO J, ZHONG X, WANG D, REN H, et al. SIRT1 inhibits the catabolic effect of IL-1β through TLR2/SIRT1/NF-κB pathway in human degenerative nucleus pulposus cells[J]. Pain Physician, 2016, 19: E215-E226. |
| [13] |
MARTINEZ F O, GORDON S. The M1 and M2 paradigm of macrophage activation: time for reassessment[J/OL]. F1000Prime Rep, 2014, 6: 13. doi: 10.12703/P6-13.
|
| [14] |
MILLS C D. M1 and M2 macrophages: oracles of health and disease[J]. Crit Rev Immunol, 2012, 32: 463-488. DOI:10.1615/CritRevImmunol.v32.i6.10 |
| [15] |
PELEGRIN P, SURPRENANT A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1β release through pyrophosphates[J]. EMBO J, 2009, 28: 2114-2127. DOI:10.1038/emboj.2009.163 |
| [16] |
ITALIANI P, BORASCHI D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation[J/OL]. Front Immunol, 2014, 5: 514. doi: 10.3389/fimmu.2014.00514.
|
| [17] |
VAZIRI H, DESSAIN S K, NG EATON E, IMAI S I, FRYE R A, PANDITA T K, et al. hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase[J]. Cell, 2001, 107: 149-159. DOI:10.1016/S0092-8674(01)00527-X |
| [18] |
GAO J, ZHOU R, YOU X, LUO F, HE H, CHANG X, et al. Salidroside suppresses inflammation in a D-galactose-induced rat model of Alzheimer's disease via SIRT1/NF-κB pathway[J]. Metab Brain Dis, 2016, 31: 771-778. DOI:10.1007/s11011-016-9813-2 |
| [19] |
GORENNE I, KUMAR S, GRAY K, FIGG N, YU H, MERCER J, et al. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis[J]. Circulation, 2013, 127: 386-396. DOI:10.1161/CIRCULATIONAHA.112.124404 |
| [20] |
HUBBARD B P, SINCLAIR D A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases[J]. Trends Pharmacol Sci, 2014, 35: 146-154. DOI:10.1016/j.tips.2013.12.004 |
| [21] |
ZHANG Z, XU J, LIU Y, WANG T, PEI J, CHENG L, et al. Mouse macrophage specific knockout of SIRT1 influences macrophage polarization and promotes angiotensin Ⅱ-induced abdominal aortic aneurysm formation[J]. J Genet Genomics, 2018, 45: 25-32. DOI:10.1016/j.jgg.2018.01.002 |
| [22] |
PARK S Y, LEE S W, LEE S Y, HONG K W, BAE S S, KIM K, et al. SIRT1/adenosine monophosphate-activated protein kinase α signaling enhances macrophage polarization to an anti-inflammatory phenotype in rheumatoid arthritis[J/OL]. Front Immunol, 2017, 8: 1135. doi: 10.3389/fimmu.2017.01135.
|
| [23] |
HAH Y S, CHEON Y H, LIM H S, CHO H Y, PARK B H, KA S O, et al. Myeloid deletion of SIRT1 aggravates serum transfer arthritis in mice via nuclear factor-κB activation[J/OL]. PLoS One, 2014, 9: e87733. doi: 10.1371/journal.pone.0087733.
|
 2019, Vol. 40
2019, Vol. 40


