2. 海军军医大学(第二军医大学)基础医学院医学遗传学教研室, 上海 200433;
3. 上海中医药大学附属第七人民医院呼吸科, 上海 200137
2. Department of Medical Genetics, College of Basic Medical Sciences, Naval Medical University(Second Military Medical University), Shanghai 200433, China;
3. Department of Respiratory Medicine, Seventh People's Hospital of Shanghai, Shanghai University of Traditional Chinese Medicine, Shanghai 200137, China
肺癌是全球发病率和致死率最高的恶性肿瘤之一。依据世界卫生组织调查数据,仅在2016年全球就新增约180万例肺癌患者,并超过150万例患者死亡[1]。按照临床病理类型分类,肺癌可分为小细胞肺癌和非小细胞肺癌(non-small cell lung cancer,NSCLC)两大类,其中NSCLC是最常见的肺癌分型,约占肺癌的85%[2]。2016年的报道显示,虽然外科手术的发展和免疫治疗的出现为肿瘤患者带来了福音,但肺癌预后仍不尽如人意,5年生存率仅约为11%[2]。因此,正确认识肺癌发生、发展过程及分子机制对于肺癌的早期诊断、制定合理的靶向药物治疗方案、提高患者生存率至关重要。
长链非编码RNA(long non-coding RNA,lncRNA)是一类转录本长度超过200 nt、不能编码蛋白质的RNA分子[3]。LncRNA-H19是一种被广泛研究的印记基因,具有重要的生物学功能[4]。He等[5]的研究报道,lncRNA-H19可通过竞争性结合let-7调控滋养层细胞的成球黏附,参与子宫内膜修复。在胚胎造血干细胞发育过程中,lncRNA-H19可通过抑制S-腺苷同型半胱氨酸水解酶的活性,促进内皮细胞向间质细胞分化[6]。另外,乳腺癌、肝癌及神经胶质瘤等与lncRNA-H19表达异常增加有关[7-9]。然而,目前lncRNA-H19在NSCLC中的表达及其作用鲜见报道。本研究通过检测lncRNA-H19在NSCLC组织和人NSCLC细胞系中的表达,探讨lncRNA-H19在NSCLC发生、发展中的作用和机制。
1 材料和方法 1.1 组织样本收集2015年10月至2016年5月海军军医大学(第二军医大学)长海医院确诊的20例NSCLC患者手术切除的NSCLC组织及相应的癌旁正常组织(距离肿瘤组织≥5 cm),其中男15例、女5例,平均年龄为(39±7)岁,术前均未接受化学治疗。获取组织标本后立即浸入液氮冷冻后保存于-80℃。本研究遵循海军军医大学(第二军医大学)生物医学研究伦理委员会规定并通过审批,患者均知情同意。
1.2 细胞培养与转染人NSCLC细胞系A549、NCI-H1299,人正常肺上皮细胞系BEAS-2B均由中国科学院上海细胞库提供。培养条件:使用含10%胎牛血清及100 µg/mL青霉素、链霉素双抗的DMEM高糖培养液,置于37℃、5% CO2 的培养箱中培养。按照Lipo3000转染试剂说明书进行细胞转染,转染48 h后检测转染效率。
1.3 实验试剂DMEM高糖培养液、胰酶、磷酸盐缓冲液(phosphate buffer saline,PBS)及胎牛血清均购自美国Gibco公司;引物、lncRNA-H19过表达质粒、微RNA(microRNA,miRNA)-760模拟剂、双荧光质粒均由生工生物工程(上海)股份有限公司代为设计并合成;Lipo3000转染试剂购自美国ThermoFisher公司;RNA抽提、反转录及实时荧光定量PCR(qPCR)试剂盒均购自日本TaKaRa公司;双荧光素酶报告基因检测试剂盒购自美国Promega公司;CCK-8检测试剂盒购自广州锐博生物技术有限公司;nanog抗体、甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GAPDH),抗体、荧光二抗均购自英国Abcam公司。
1.4 RNA抽提与qPCR检测依据TRIzol试剂说明书提取组织和细胞总RNA,通过反转录获得cDNA,反应体系为10 µL:2 μL 5×反应混合液、1 μL RNA,7 μL H2O;反应条件:37℃ 15 min、85℃ 15 s。以cDNA为模板进行qPCR,反应体系为20 μL:10 μL SYBR,1 μL cDNA,1 μL引物,8 μL H2O;反应条件:95℃ 10 min;95℃ 30 s,60℃ 15 s,72℃ 20 s,40个循环;72℃ 10 min。引物序列:lncRNA-H9上游5′-GGC AAG AAG CGG GTC TGT-3′,下游5′-GTG CAG CAT ATT CAT TTC CAA G-3′;nanog上游5′-TTT GTG GGC CTG AAG AAA ACT-3′,下游5′-AGG GCT GTC CTG AAT AAG CAG-3′;GAPDH上游5′-GGA GCG AGA TCC CTC CAA AAT-3′,下游5′-GGC TGT TGT CAT ACT TCT CAT GG-3′。以GAPDH为内参照基因,采用2-ΔΔCt法计算RNA的相对表达量。
1.5 细胞功能学实验 1.5.1 CCK-8法检测细胞增殖取对数生长期A549细胞,稀释至细胞密度为3×104/mL的细胞悬液,并以每孔100 µL接种于96孔板。待细胞贴壁后,分别在培养0、24、48、72、96 h时加入10 µL CCK-8试剂,再培养2 h后用酶标仪检测450 nm处光密度值。
1.5.2 Transwell法检测细胞迁移取对数生长期A549细胞,用无血清培养液稀释至细胞密度为1×105/mL的细胞悬液,取400 µL接种于Transwell小室内,小室外添加400 µL完全培养液。连续培养24 h后取出小室,用预热后的PBS轻轻润洗,浸泡于4%多聚甲醛溶液中固定20 min。再次取出小室,用蒸馏水洗涤后自然晾干,加入结晶紫中染色液染色30 min。洗去结晶紫染液后在显微镜下观察。
1.6 双荧光素酶报告基因实验采用美国Promega公司的双荧光素酶报告基因检测系统,依据实验说明进行操作,计算萤火虫荧光值与海肾荧光值的比值,评估报告基因在细胞中的相对活力。
1.7 蛋白质印迹法用细胞刮收集细胞,用含有蛋白酶抑制剂的RIPA细胞裂解液裂解,100℃煮沸10 min变性后冰上冷却,然后保存于-20℃。配制十二烷基硫酸钠-聚丙烯酰胺凝胶,取20 µL蛋白质样本进行电泳。转膜后用快速封闭液封闭1 h,加入按1:1 000稀释的nanog抗体、GAPDH抗体稀释液室温孵育2 h,用吐温磷酸盐缓冲液(phosphate buffer saline-Tween,PBST)洗膜3次,再加入二抗稀释液室温孵育1 h,用PBST洗膜3次。用Odyssey近红外扫描仪扫描。
1.8 统计学处理应用SPSS 17.0软件进行统计学分析。呈正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验;呈偏态分布的计量资料以中位数表示,两组间比较采用非参数秩和检验。检验水准(α)为0.05。
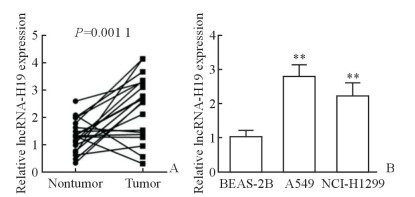
2 结果 2.1 LncRNA-H19在NSCLC组织及细胞中表达增加qPCR检测结果显示,lncRNA-H19在NSCLC组织中的表达高于其在癌旁正常组织中的表达,差异有统计学意义(P=0.001 1,图 1A);lncRNA-H19在人NSCLC细胞系A549和NCI-H1299细胞中的表达均高于其在人正常肺上皮细胞系BEAS-2B细胞中的表达,差异均有统计学意义(P均<0.01,图 1B)。
|
图 1 qPCR检测NSCLC组织和细胞系中lncRNA-H19的表达 Fig 1 Expression of lncRNA-H19 in NSCLC tissues and cell lines detected by qPCR A: Relative expression of lncRNA-H19 in NSCLC tissues and nontumor tissues. n=20. B: Relative expression of lncRNA-H19 in human NSCLC cell lines (A549 and NCI-H1299) and human normal lung cell line (BEAS-2B). **P < 0.01 vs BEAS-2B. n=3, x±s.>NSCLC: Non-small cell lung cancer; lncRNA: Long non-coding RNA |
2.2 过表达lncRNA-H19促进NSCLC细胞的增殖与迁移
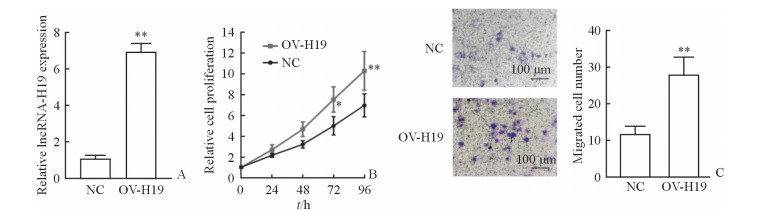
qPCR结果(图 2A)显示,在A549细胞中过表达lncRNA-H19后,细胞中lncRNA-H19表达增加,与对照组相比差异有统计学意义(P<0.01),说明过表达细胞模型构建成功。CCK-8实验结果(图 2B)显示,过表达lncRNA-H19可以提高A549细胞的增殖能力,在细胞培养72、96 h时与对照组相比差异均有统计学意义(P<0.05,P<0.01)。Transwell实验结果(图 2C)显示,过表达lncRNA-H19可以提高A549细胞的迁移能力,与对照组相比差异有统计学意义(P<0.01)。
|
图 2 过表达lncRNA-H19促进人NSCLC细胞系A549的增殖和迁移 Fig 2 Overexpression of lncRNA-H19 improving proliferation and migration of human NSCLC cell line A549 A: Overexpression of lncRNA-H19 was confirmed by qPCR; B: CCK-8 analysis revealed that overexpression of lncRNA-H19 dramatically enhanced the proliferation ability of A549 cells; C: Transwell assay showed that overexpression of lncRNA-H19 dramatically improved the migration ability of A549 cells. lncRNA: Long non-coding RNA; NSCLC: Non-small cell lung cancer; NC: Negative control; OV-H19: Overexpression of lncRNA-H19; CCK-8: Cell counting kit-8. *P < 0.05, **P < 0.01 vs NC group. n=3, x±s |
2.3 LncRNA H19特异性吸附miRNA-760并调控其靶基因nanog的表达
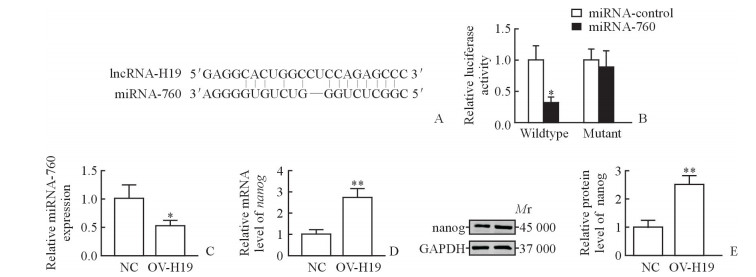
在线数据库starBase v3.0(http://starbase.sysu.edu.cn/)预测分析结果(图 3A)显示,lncRNA-H19能特异性吸附miRNA-760。双荧光素酶报告基因检测结果(图 3B)显示,野生型lncRNA-H19与miRNA-760同时转染细胞后荧光度值下降(P<0.05),表明lncRNA-H19与miRNA-760之间存在特异性结合。qPCR检测结果(图 3C)显示,过表达lncRNA-H19可抑制A549细胞中miRNA-760的表达,与对照组相比差异有统计学意义(P<0.05)。qPCR(图 3D)和蛋白质印迹分析检测结果(图 3E)显示,过表达lncRNA-H19促进A549细胞中nanog基因在mRNA和蛋白质水平的表达,差异均有统计学意义(P均<0.01)。
|
图 3 LncRNA-H19特异性结合miRNA-760并调控miRNA-760和nanog基因的表达 Fig 3 LncRNA-H19 directly bound to miRNA-760 and regulated the expression of miRNA-760 and nanog gene A: The binding sites between lncRNA-H19 and miRNA-760 were predicted by starBase v3.0; B: Duel-luciferase reporter analysis revealed that lncRNA-H19 bound to miRNA-760 specifically; C: qPCR analysis revealed that overexpression of lncRNA-H19 significantly decreased the relative expression of miRNA-760 in human NSCLC cell line A549; D: qPCR analysis revealed that overexpression of lncRNA-H19 significantly decreased the relative mRNA expression of nanog in A549 cells; E: Western blotting analysis revealed that overexpression of lncRNA-H19 significantly decreased the relative protein expression of nanog in A549 cells. lncRNA: Long non-coding RNA; miRNA: microRNA; NSCLC: Non-small cell lung cancer; NC: Negative control; OV-H19: Overexpression of lncRNA-H19; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. *P < 0.05, **P < 0.01 vs miRNA-control in Fig 3B, vs NC in Fig 3C-3E. n=3, x±s |
2.4 过表达miRNA-760抑制lncRNA-H19对NSCLC细胞增殖和迁移的促进作用
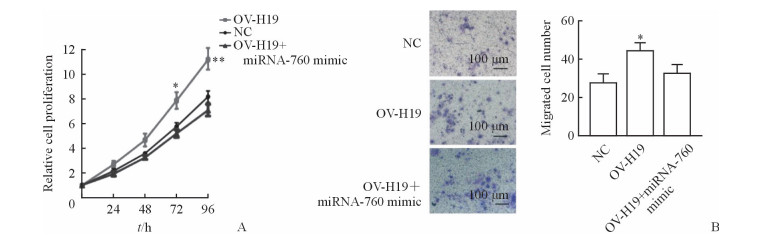
CCK-8实验结果(图 4A)显示,在过表达lncRNA-H19的A549细胞中转染miRNA-760模拟剂后,lncRNA-H19对A549细胞的促增殖作用被抑制,在细胞培养72、96 h时与过表达组相比差异均有统计学意义(P<0.05、P<0.01)。Transwell实验结果(图 4B)显示,过表达miRNA-760可以抑制lncRNA-H19对A549细胞迁移的促进作用,与lncRNA-H19过表达组相比差异有统计学意义(P<0.05)。
|
图 4 MiRNA-760模拟剂处理抑制lncRNA-H19对人NSCLC细胞系A549细胞增殖和迁移的促进作用 Fig 4 MiRNA-760 mimic reserved lncRNA-H19-induced proliferation and migration of human NSCLC cell line A549 A: Treatment of miRNA-760 mimic dampened the proliferation ability of A549 cells that was enhanced by overexpression of lncRNA-H19; B: Treatment of miRNA-760 mimic dampened the migration ability of A549 cells that was enhanced by overexpression of lncRNA-H19. miRNA: microRNA; lnRNA: Long non-coding RNA; NSCLC: Non-small cell lung cancer; NC: Negative control; OV-H19: Overexpression of lncRNA-H19. *P < 0.05, **P < 0.01 vs OV-H19+miRNA-760 mimic. n=3, x±s |
3 讨论
本研究利用qPCR法检测了20对NSCLC组织和相应癌旁正常组织中lncRNA-H19的表达情况,发现lncRNA-H19在癌组织中的表达水平高于癌旁正常组织;同时,相较人正常肺上皮细胞,人NSCLC细胞系中lncRNA-H19的表达也增加。结果提示lncRNA-H19与NSCLC的发生、发展密切相关。为了进一步明确lncRNA-H19在NSCLC发生、发展过程中的作用,本实验构建了lncRNA-H19过表达的人NSCLC细胞系,并通过CCK-8法和Transwell实验评估lncRNA-H19对NSCLC细胞增殖和迁移的影响,结果显示,lncRNA-H19过表达可以提高人NSCLC细胞的增殖和迁移能力,表明lncRNA-H19对促进NSCLC的发生、发展有重要作用。
竞争性内源RNA(competing endogenous RNA,ceRNA)假说认为,非编码RNA可以通过竞争性结合内源性miRNA,阻断miRNA与其下游靶分子间的相互作用,间接调控mRNA的降解,发挥生物学作用[10]。Wang等[11]研究发现,LINC00336通过特异性吸附miRNA-6852参与胱硫醚β合成酶的调控,从而促进肺癌的发生、发展。相似的,也有报道称lncRNA-PVT1通过竞争性结合miRNA-365参与肝癌的形成[12]。因此,我们推测lncRNA-H19在NSCLC中的促癌作用可能也依赖于ceRNA机制。为了深入探讨lncRNA-H19在NSCLC发生、发展中的作用机制,我们利用生物信息学分析预测lncRNA-H19可能吸附的miRNA。结果显示,lncRNA-H19与抑癌基因miRNA-760[13]之间存在结合位点。双荧光素酶报告基因实验证实,lncRNA-H19确实可以特异性地吸附miRNA-760。进一步qPCR检测和蛋白质印迹实验表明,lncRNA-H19过表达可以降低miRNA-760的表达水平,同时促进miRNA-760下游靶基因nanog的表达。为了证实lncRNA-H19在NSCLC中的促癌作用依赖于其特异性吸附miRNA-760,我们用miRNA-760模拟剂处理过表达lncRNA-H19的A549细胞后,采用CCK-8实验和Transwell实验分别检测细胞的增殖和迁移能力,结果显示,miRNA-760模拟剂可以抑制lncRNA-H19的促增殖作用和促迁移作用。以上实验结果均表明,lncRNA-H19可通过特异性地吸附miRNA-760调控原癌基因nanog的表达,从而促进NSCLC的发生和发展。
综上所述,lncRNA-H19在NSCLC的发生、发展中发挥着重要作用,其可通过特异性吸附miRNA-760调控nanog基因的表达促进NSCLC细胞的增殖和迁移,本研究结论为NSCLC的治疗提供了新的干预靶点。近年来许多研究表明,在肿瘤组织中特异性表达的lncRNA是一种潜在肿瘤标志物,如NSCLC患者血浆中lncRNA-UCA1的表达较正常人增加[14],而lncRNA-H19在NSCLC患者血浆中的表达水平及其临床意义仍待进一步探究。同时,虽然本研究在细胞水平证实了lncRNA-H19在NSCLC细胞增殖和迁移中的作用,但干扰lncRNA-H19是否可以作为NSCLC的治疗策略仍需通过动物实验进一步明确。
| [1] |
GARRISON G W. Lung cancer screening[J]. Cancer Cytopathol, 2016, 124: 533-534. DOI:10.1002/cncy.21751 |
| [2] |
MINARI R, BORDI P, TISEO M. Third-generation epidermal growth factor receptor-tyrosine kinase inhibitors in T790M-positive non-small cell lung cancer: review on emerged mechanisms of resistance[J]. Transl Lung Cancer Res, 2016, 5: 695-708. DOI:10.21037/tlcr.2016.12.02 |
| [3] |
DHANOA J K, SETHI R S, VERMA R, ARORA J S, MUKHOPADHYAY C S. Long non-coding RNA: its evolutionary relics and biological implications in mammals: a review[J/OL]. J Anim Sci Technol, 2018, 60: 25. doi: 10.1186/s40781-018-0183-7.
|
| [4] |
CHU M, YUAN W, WU S, WANG Z, MAO L, TIAN T, et al. Quantitative assessment of polymorphisms in H19 lncRNA and cancer risk: a meta-analysis of 13, 392 cases and 18, 893 controls[J]. Oncotarget, 2016, 7: 78631-78639. |
| [5] |
HE D, ZENG H, CHEN J, XIAO L, ZHAO Y, LIU N. H19 regulates trophoblastic spheroid adhesion by competitively binding to let-7[J/OL]. Reproduction, 2019 Feb 1. pii: REP-18-0339.R2. doi: 10.1530/REP-18-0339.
|
| [6] |
ZHOU J, XU J, ZHANG L, LIU S, MA Y, WEN X, et al. Combined single-cell profiling of lncRNAs and functional screening reveals that H19 is pivotal for embryonic hematopoietic stem cell development[J/OL]. Cell Stem Cell, 2019, 24: 285-298.e5. doi: 10.1016/j.stem.2018.11.023.
|
| [7] |
GAO H, HAO G, SUN Y, LI L, WANG Y. Long noncoding RNA H19 mediated the chemosensitivity of breast cancer cells via Wnt pathway and EMT process[J]. Onco Targets Ther, 2018, 11: 8001-8012. DOI:10.2147/OTT.S172379 |
| [8] |
WEI L Q, LI L, LU C, LIU J, CHEN Y, WU H. Involvement of H19/miR-326 axis in hepatocellular carcinoma development through modulating TWIST1[J]. J Cell Physiol, 2019, 234: 5153-5162. DOI:10.1002/jcp.27319 |
| [9] |
HU Q, YIN J, ZENG A, JIN X, ZHANG Z, YAN W, et al. H19 functions as a competing endogenous RNA to regulate EMT by sponging miR-130a-3p in glioma[J]. Cell Physiol Biochem, 2018, 50: 233-245. DOI:10.1159/000494002 |
| [10] |
ZHANG J, LIU L, LI J, LE T D. LncmiRSRN: identification and analysis of long non-coding RNA related miRNA sponge regulatory network in human cancer[J]. Bioinformatics, 2018, 34: 4232-4240. DOI:10.1093/bioinformatics/bty525 |
| [11] |
WANG M, MAO C, OUYANG L, LIU Y, LAI W, LIU N, et al. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA[J/OL]. Cell Death Differ, 2019 Feb 20. doi: 10.1038/s41418-019-0304-y.
|
| [12] |
YANG L, PENG X, JIN H, LIU J. Long non-coding RNA PVT1 promotes autophagy as ceRNA to target ATG3 by sponging microRNA-365 in hepatocellular carcinoma[J]. Gene, 2019, 697: 94-102. DOI:10.1016/j.gene.2019.02.036 |
| [13] |
HAN M L, WANG F, GU Y T, PEI X H, GE X, GUO G C, et al. MicroR-760 suppresses cancer stem cell subpopulation and breast cancer cell proliferation and metastasis: by down-regulating NANOG[J]. Biomed Pharmacother, 2016, 80: 304-310. DOI:10.1016/j.biopha.2016.03.024 |
| [14] |
WANG H M, LU J H, CHEN W Y, GU A Q. Upregulated lncRNA-UCA1 contributes to progression of lung cancer and is closely related to clinical diagnosis as a predictive biomarker in plasma[J]. Int J Clin Exp Med, 2015, 8: 11824-11830. |
 2019, Vol. 40
2019, Vol. 40


