移植肾功能延迟恢复(delayed graft function,DGF)是肾移植术后的常见并发症[1]。移植肾穿刺活组织检查是诊断DGF的金标准,但其属于有创性检查,特异度不高且并发症多,临床应用有限[2]。目前常用术后7 d血肌酐>400 μmol/L作为DGF的诊断指标[1]。但血肌酐易受体质量、年龄、性别和肌肉代谢等其他因素的影响,诊断特异度不高[3]。肾缺血再灌注损伤引起的炎症和氧化损伤是导致DGF的危险因素[4]。研究表明,血小板可以参与机体炎症反应过程[5]。因此,本研究拟通过动态监测同种异体肾移植患者术后5个临床常规检测的血小板参数——血小板计数(platelet count,PLT)、大型血小板比值(platelet-large cell ratio,P-LCR)、平均血小板体积(mean platelet volume,MPV)、血小板分布宽度(platelet volume distribution width,PDW)和血小板比容(platelet hematocrit,PCT)的动态变化,探讨血小板参数与肾移植术后发生DGF的关系。
1 资料和方法 1.1 研究对象本研究共纳入2016年7月至2018年5月在海军军医大学(第二军医大学)长海医院器官移植科接受肾移植手术的患者109例,根据术后是否发生DGF分为DGF组(n=41)和非DGF组(non-DGF组,n=68)。DGF诊断标准:患者肾移植术后7 d内需要血液透析治疗,或者虽未经过血液透析治疗,但在术后7 d血肌酐仍>400 μmol/L [4, 6]。纳入标准:(1)供者和受者ABO血型相符;(2)群体反应性抗体(panel reactive antibody,PRA)均≤ 10%,淋巴细胞毒交叉配合试验死细胞比例≤ 10%,人类白细胞抗原(human leukocyte antigen,HLA)配型错配数为1~5个,热缺血时间3~10 min,冷缺血时间6~24 h;(3)所有受者均采用口服他克莫司(FK506)+霉酚酸酯+醋酸泼尼松的三联抗排斥药物方案。排除标准:(1)术前7 d内使用过影响血小板聚集功能药物的患者;(2)输注过全血或血液制品者;(3)术后出现各种感染或其他并发症的患者;(4)观察时间内失访;(5)亲属供肾活体肾移植受者;(6)临床资料收集不全者。本研究经海军军医大学(第二军医大学)长海医院伦理委员会审批。
1.2 血液标本采集及检测方法用EDTA抗凝管分别采集患者术前和术后1、3、7、14 d的外周血各2 mL,1 h内在Sysmex XN-9000血液分析仪(日本希森美康公司)上使用同品牌配套进口试剂进行检测,获取PLT、P-LCR、MPV、PDW和PCT等数据。
1.3 统计学处理采用SPSS 22.0软件进行统计学分析。符合正态分布的计量资料以x ± s表示,两组间比较采用独立样本t检验;计数资料以例数和百分数表示。采用受试者工作特征(receiver operating characteristic,ROC)曲线判断血小板参数预测DGF的效能,并计算灵敏度和特异度,选取约登指数(约登指数=灵敏度+特异度-1)最大时所对应的值为最佳截断值。检验水准(α)为0.05。
2 结果 2.1 血小板参数在肾移植术后的变化趋势纳入研究的109例患者中男70例、女39例,年龄为15~70岁,平均年龄为(40.0 ± 12.4)岁。供者均为尸体供肾,受者均为首次接受肾移植。由表 1可见,肾移植术后DGF组和non-DGF组患者的PLT均迅速降低,至术后3 d时达到最低值,随后两组PLT均缓慢升高,在术后14 d时基本恢复到术前水平;DGF组PLT在术后14 d内始终低于对照组,其中术后7 d时两组差异有统计学意义(P<0.05)。肾移植术后DGF组和non-DGF组患者的MPV均呈先升高后降低趋势,两组分别于术后7 d和术后3 d达到最高值;DGF组MPV在术后14 d内始终高于对照组,其中术后7 d时两组差异均有统计学意义(P均<0.05)。肾移植术后DGF组和non-DGF组患者的PCT呈先降低后升高的趋势,在术后3 d时最低;术后7 d时DGF组低于non-DGF组,差异有统计学意义(P<0.05)。肾移植术后DGF组与non-DGF组PDW呈先升高后降低趋势,两组PDW均在术后7 d时达到最大,且术后7 d时DGF组PDW大于non-DGF组,差异有统计学意义(P<0.05)。肾移植术后DGF组与non-DGF组P-LCR呈先升高后降低趋势,两组P-LCR均在术后7 d时达到最大,但术后7 d时两组P-LCR差异无统计学意义(P=0.184)。
|
|
表 1 肾移植术后两组患者血小板参数的动态比较 Tab 1 Comparison of platelet parameters between 2 groups after renal transplantation |
2.2 血小板参数对肾移植术后DGF的预测价值
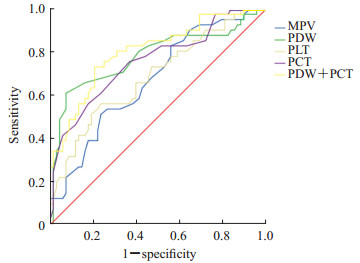
选择DGF组和non-DGF组肾移植术后7 d差异有统计学意义的4项血小板参数(MPV、PDW、PLT和PCT)进行ROC曲线分析,结果(图 1、表 2)显示PDW和PCT对DGF有中度预测效能,ROC曲线下面积(area under curve,AUC)分别为0.781和0.758,95%置信区间(confidence interval,CI)分别为0.683~0.880和0.662~0.853,最佳截断值分别为16.75 fL和0.155%。以最佳截断值为标准时二者诊断DGF的特异度分别为92.6%和63.2%,灵敏度分别为61.0%和75.6%。肾移植术后7 d PCT和PDW联合检测诊断DGF的AUC为0.805,95% CI为0.718~0.891,灵敏度为73.2%,特异度为79.4%。上述结果提示PDW和PCT对DGF有早期诊断价值,但PDW的特异度更高,PCT的灵敏度稍高于PDW,当患者术后7 d PDW>16.75 fL、PCT<0.155%时发生DGF的风险增大,且PCT和PDW联合检测的诊断效能优于单独检测。
|
图 1 血小板参数预测肾移植术后DGF的ROC曲线 Fig 1 ROC curve of DGF predicted by platelet parameters after renal transplantation DGF: Delayed graft function; ROC: Receiver operating characteristic; MPV: Mean platelet volume; PDW: Platelet volume distribution width; PLT: Platelet count; PCT: Platelet hematocrit |
|
|
表 2 血小板参数预测肾移植术后DGF的价值比较 Tab 2 Comparison of platelet parameters in predicting DGF after renal transplantation |
3 讨论
DGF是肾移植术后同种异体移植物存活的主要障碍。发生DGF的患者术后早期住院时间延长,生活质量下降,长期预后较差,包括长期同种异体移植肾功能差、排斥率高[7-9]、移植物功能丢失和患者死亡等[10]。目前还没有有效治疗DGF的方法,但早期诊断和治疗干预可能会提高移植物和患者的生存率[11]。DGF损伤的发病机制目前仍未完全清楚,可能是多因素联合作用导致。研究表明,缺血再灌注损伤可能会导致细胞损伤和死亡,并且加重氧化应激反应,氧化应激失调可在移植的器官内诱发一系列炎症反应引起细胞损伤,从而导致移植肾受损[12-15]。有研究证实,血小板的产物5-羟基二十碳四烯酸(5-hydroxyeicosatetraenoic acid,5-HETE)、12-羟基二十碳四烯酸(12-hydroxyeicosatetraenoic acid,12-HETE)和15-羟基二十碳四烯酸(15-hydroxyeicosatetraenoic acid,15-HETE)在人体内的含量与移植术后肾功能恢复情况有关[16-17]。血小板的另一产物血栓烷B2(thromboxane B2,TXB2)也可以作为肾功能恢复相关的移植前监测指标[17]。这些研究表明,肾移植术后血小板表现出不同的活化水平,是导致移植术后肾功能恢复差异的原因之一。
本研究通过观察5项临床常规检测的血小板参数(PLT、MPV、PCT、PDW和P-LCR)在肾移植术前、术后的动态变化,分析血小板与DGF的关系。结果显示,肾移植受者的PLT和PCT在移植术后降低,然后缓慢恢复至术前水平;MPV、PDW和P-LCR在肾移植术后均有所增高,后缓慢降低至正常水平。术后早期PLT和PCT降低,可能因为肾移植是有创手术,早期血小板参与创伤的止血和凝血过程,从而造成血小板的破坏增多。MPV、PDW和P-LCR增加,可能是因为移植术后尿毒症潴留产物对骨髓的抑制作用下降,骨髓造血功能逐渐恢复正常,造血干细胞可正常分化成熟,巨核系细胞正常增殖,产生较多年轻的大血小板。本研究还发现,肾移植术后发生DGF的患者,PLT始终低于未发生DGF的患者,术后7 d两组间差异有统计学意义(P<0.05);MPV始终高于未发生DGF的患者,术后7 d两组间差异有统计学意义(P<0.05);PCT在术后7 d时低于未发生DGF的患者,两组间差异有统计学意义(P<0.05)。该结果提示DGF患者在骨髓造血干细胞分化成熟过程中出现血小板活化过程,血小板破坏增多,消耗增加,进一步佐证了血小板参与移植肾炎症反应和损伤再修复过程。
本研究采用ROC曲线评估血小板参数对DGF的预测价值。结果显示,肾移植术后7 d PDW和PCT均对DGF有中等程度的诊断效能(AUC为0.7~0.9),但PDW特异度高于PCT,PCT灵敏度稍高于PDW,PCT和PDW联合检测的诊断效能优于单独检测。上述结果提示当肾移植患者术后7 d发现PDW>16.75 fL、PCT<0.155%时应警惕DGF的发生。
综上所述,同种异体肾移植患者血小板参数的动态变化与DGF的发生有一定的关系,术后7 d的PCT和PDW对预测DGF具有一定的临床价值,当术后7 d PDW>16.75 fL、PCT<0.155%时患者发生DGF的风险增大。相比病理活组织检查,血液标本易于获取,患者更易于接受,因此血小板参数有望成为肾移植术后早期预测DGF的无创性标志物。
| [1] |
MALLON D H, SUMMERS D M, BRADLEY J A, PETTIGREW G J. Defining delayed graft function after renal transplantation:simplest is best[J]. Transplantation, 2013, 96: 885-889. DOI:10.1097/TP.0b013e3182a19348 |
| [2] |
RANJAN P, NADA R, JHA V, SAKHUJA V, JOSHI K. The role of C4d immunostaining in the evaluation of the causes of renal allograft dysfunction[J]. Nephrol Dial Transplant, 2008, 23: 1735-1741. DOI:10.1093/ndt/gfm843 |
| [3] |
MAIER H T, ASHRAF M I, DENECKE C, WEISS S, AUGUSTIN F, MESSNER F, et al. Prediction of delayed graft function and long-term graft survival by serum and urinary neutrophil gelatinase-associated lipocalin during the early postoperative phase after kidney transplantation[J/OL]. PLoS One, 2018, 13: e0189932. doi: 10.1371/journal.pone.0189932.
|
| [4] |
PERICO N, CATTANEO D, SAYEGH M H, REMUZZI G. Delayed graft function in kidney transplantation[J]. Lancet, 2004, 364: 1814-1827. DOI:10.1016/S0140-6736(04)17406-0 |
| [5] |
KAYA M G, YARLIOGLUES M, GUNEBAKMAZ O, GUNTURK E, INANC T, DOGAN A, et al. Platelet activation and inflammatory response in patients with non-dipper hypertension[J]. Atherosclerosis, 2010, 209: 278-282. DOI:10.1016/j.atherosclerosis.2009.09.010 |
| [6] |
SOLA R, ALARCÓN A, JIMÉNEZ C, OSUNA A. The influence of delayed graft function[J]. Nephrol Dial Transplant, 2004, 19(Suppl 3): ii32-ii37. |
| [7] |
BOOM H, MALLAT M J, DE FIJTER J W, ZWINDERMAN A H, PAUL L C. Delayed graft function influences renal function, but not survival[J]. Kid Int, 2001, 58: 859-866. |
| [8] |
YARLAGADDA S G, COCA S G, FORMICA R N Jr, POGGIO E D, PARIKH C R. Association between delayed graft function and allograft and patient survival:a systematic review and meta-analysis[J]. Nephrol Dial Transplant, 2009, 24: 1039-1047. |
| [9] |
WU W K, FAMURE O, LI Y, KIM S J. Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation[J]. Kid Int, 2015, 88: 851-858. DOI:10.1038/ki.2015.190 |
| [10] |
NARAYANAN R, CARDELLA C J, CATTRAN D C, COLE E H, TINCKAM K J, SCHIFF J, et al. Delayed graft function and the risk of death with graft function in living donor kidney transplant recipients[J]. Am J Kid Dis, 2010, 56: 961-970. DOI:10.1053/j.ajkd.2010.06.024 |
| [11] |
MOGULLA M, BHATTACHARJYA S, CLAYTON P A. Risk factors for and outcomes of delayed graft function in living donor kidney transplantation-a retrospective study[J/OL]. Transpl Int, 2019. doi: 10.1111/tri.13472.
|
| [12] |
SALVADORI M, ROSSO G, BERTONI E. Update on ischemia-reperfusion injury in kidney transplantation:pathogenesis and treatment[J]. World J Transplant, 2015, 5: 52-67. DOI:10.5500/wjt.v5.i2.52 |
| [13] |
LEE D M, JACKSON K W, KNOWLTON N, WAGES J, ALAUPOVIC P, SAMUELSSON O, et al. Oxidative stress and inflammation in renal patients and healthy subjects[J/OL]. PLoS One, 2011, 6: e22360. doi: 10.1371/journal.pone.0022360.
|
| [14] |
SHAPIRO M D, BAGLEY J, LATZ J, GODWIN J G, GE X, TULLIUS S G, et al. MicroRNA expression data reveals a signature of kidney damage following ischemia reperfusion injury[J/OL]. PLoS One, 2011, 6: e23011. doi: 10.1371/journal.pone.0023011.
|
| [15] |
HARIHARAN N, ZHAI P, SADOSHIMA J. Oxidative stress stimulates autophagic flux during ischemia/reperfusion[J]. Antioxid Redox Signal, 2011, 14: 2179-2190. DOI:10.1089/ars.2010.3488 |
| [16] |
DOLEGOWSKA B, BLOGOWSKI W, SAFRANOW K, DOMANSKI L, JAKUBOWSKA K, OLSZEWSKA M. Lipoxygenase-derived hydroxyeicosatetraenoic acids-novel perioperative markers of early post-transplant allograft function?[J]. Nephrol Dial Transplant, 2010, 25: 4061-4067. DOI:10.1093/ndt/gfq320 |
| [17] |
DOŁEGOWSKA B, BŁOGOWSKI W, DOMAŃSKI L. Dynamics of thromboxane level changes during early phase of allograft reperfusion[J]. Clin Transplant, 2009, 23: 716-722. DOI:10.1111/j.1399-0012.2009.00983.x |
 2019, Vol. 40
2019, Vol. 40


