近年来,异基因造血干细胞移植(allogenetic hematopoietic stem cell transplantation,allo-HSCT)已成为治疗血液病的重要方法,甚至是治愈某些血液病的唯一方法,急性移植物抗宿主病(acute graft versus host disease,aGVHD)是其治疗后主要并发症之一,严重时可导致患者死亡[1]。共刺激分子CD28超家族成员,包括CD28、可诱导共刺激分子(inducible costimulator,ICOS)、程序性死亡蛋白1(programmed death 1,PD-1)和细胞毒性T淋巴细胞相关抗原4(cytotoxic T lymphocyte-associated antigen 4,CTLA-4),可与共刺激分子配体CD80/86、可诱导共刺激分子配体(inducible costimulator ligand,ICOSL)、程序性死亡蛋白配体1(programmed death-ligand 1,PD-L1)结合产生第二信号,诱导T淋巴细胞活化。虽然目前已知部分共刺激分子对aGVHD具有调节效应[2-3],但其在allo-HSCT治疗后的动态变化规律及各分子间的相互作用尚不完全明确。本研究以aGVHD小鼠模型为研究对象,探讨共刺激分子表达的动态变化及其与aGVHD的关系。
1 材料和方法 1.1 实验分组供体为5只约8周龄的C57BL/6J(H2KD-H2KB+)雄性小鼠,受体为30只约8周龄的CB6F1(H2KD+H2KB+)雌性小鼠。所有小鼠均购自北京维通利华实验动物技术有限公司[实验动物生产许可证号:SCXK(京)2016-0006、SCXK(京)2016-0011,实验动物使用许可证号:SYXK(京)2017-0033]。受体小鼠置于透气纸盒中一次性接受137Cs γ射线照射,总剂量为9 Gy。将受体小鼠随机分为3组,每组10只:(1)单纯照射组,受体小鼠接受γ射线照射后不注射任何细胞;(2)骨髓移植组,受体小鼠接受γ射线照射后4~6 h经尾静脉注射5×106个供体来源去T淋巴细胞的骨髓有核细胞;(3)aGVHD组,受体小鼠接受γ射线照射后4~6 h经尾静脉同时注射5×106个供体来源去T淋巴细胞的骨髓有核细胞和3×107个供体来源脾细胞。
1.2 H-E染色移植后21 d骨髓移植组和aGVHD组各取2只小鼠获得脾、小肠、肝、肺组织标本。采用4%多聚甲醛溶液固定、石蜡包埋、切片,然后行H-E染色观察炎症反应。H-E染色方法:石蜡切片经脱蜡、水化处理后,于37 ℃烤箱中烘烤1 h取出,自来水润洗1次;苏木精染液浸染3~5 min,自来水冲洗1~2 min;1%盐酸乙醇分化数秒;1%氨水溶液返蓝数秒,然后水洗1~2 min;伊红染液浸染1~3 min;无水乙醇脱水5 min,二甲苯透明5 min。中性树胶封片,于光学显微镜下观察形态病理学变化。
1.3 外周血流式细胞术骨髓移植组和aGVHD组各取4只小鼠,分别在移植后7、14、21、28 d采集面静脉血,采用美国BD公司Arai Ⅱ流式细胞仪及其配套软件行免疫荧光分析。采用分装后的CD3-APC-H7、CD4-V510、CD8-PE、ICOS-APC、CTLA-4-PE-CY7、PD-1-FITC、CD28-V421抗体(均购自美国BD公司)检测CD4+ T淋巴细胞及CD8+ T淋巴细胞表面ICOS、PD-1、CD28及CTLA-4抗原表达。采用分装后的CD3-APC-H7、CD4-V510、CD8-PE、γ干扰素(interferon γ,IFN-γ)、白细胞介素(interleukin,IL)-4、IL-4、IL-17抗体(均购自美国BD公司)检测CD4+ T淋巴细胞内IFN-γ、IL-4、IL-17的表达水平,分别研究CD4+ T淋巴细胞中辅助性T细胞(T-helper cell,Th)1、Th2、Th17亚群的分布。
1.4 3, 3’-二氨基联苯胺(3, 3’-diaminobenzidine,DAB)染色取小鼠结肠组织标本制作石蜡切片,进行免疫组织化学染色,染色方法为DAB染色,所用抗体均购自生工生物工程(上海)股份有限公司。观察共刺激分子配体CD80、ICOSL、PD-L1表达情况。
DAB染色方法:石蜡切片经60 ℃恒温箱中烘烤及二甲苯浸泡脱蜡,于乙醇中浸泡水化,并用蒸馏水浸泡清洗3次。柠檬酸三钠修复抗原,在高压锅中保压后自然冷却至室温。蒸馏水浸泡清洗2次,免疫染色洗涤液洗3次。使用免疫组织化学油性笔圈出组织样本位置,滴加0.3%过氧化氢甲醇溶液,洗涤并封闭。加入一抗稀释液后置于4 ℃冰箱孵育过夜。第2天室温下复温并洗涤4次,加入酶标二抗。洗涤后DAB染液显色5~15 min,充分水洗,苏木精复染,脱色、封片,于光学显微镜下观察免疫病理学变化情况。
小鼠结肠组织上皮细胞膜呈棕黄色为DAB染色阳性,分别计算CD80、ICOSL、PD-L1阳性表达率:阳性表达率(%)=阳性切片数/切片总数×100%,每张切片随机选择3个高倍镜视野,每个视野下阳性细胞数占比均≥50%则判定该切片为阳性。
1.5 统计学处理应用SPSS 20.0软件及GraphPad Prism 6软件进行统计学分析。呈正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验,各组不同时间点同一因变量重复测量数据的比较采用方差分析;呈偏态分布的计量资料以中位数(范围)表示。采用log-rank检验进行生存分析。检验水准(α)为0.05。
2 结果 2.1 各组受体小鼠log-rank生存分析单纯照射组受体小鼠均在γ射线照射后19 d内死亡。aGVHD组小鼠在γ射线照射后出现aGVHD症状的时间为14(11~18)d,生存时间为22(13~30)d;骨髓移植组小鼠在γ射线照射后均未出现aGVHD症状,30 d内均存活。见图 1。
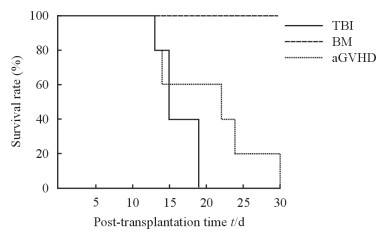
|
图 1 各组小鼠接受γ射线照射后log-rank生存曲线 Fig 1 Log-rank survival curve of mice in each group after γ-ray irradiation TBI: Total body irradiation; BM: Bone marrow transplantation; aGVHD: Acute graft versus host disease. n=10 |
2.2 移植后受体小鼠一般情况与形态病理学变化
骨髓移植组受体小鼠在照射后1周内出现短暂性轻度腹泻。aGVHD组小鼠接受移植后10 d左右出现弓背、脱毛、腹泻等典型aGVHD表现;移植后21 d脾、小肠、肝、肺组织均可见明显的炎症细胞浸润,组织结构部分破坏,出现典型aGVHD病理表现。与骨髓移植组小鼠相比,aGVHD组小鼠移植后21 d脾脏组织结构紊乱,皮质、髓质交界模糊,出现大片细胞坏死,并可见大量炎症细胞浸润(图 2A);小肠组织腺体明显减少,绒毛缩短或断裂,腺体下方大量炎症细胞浸润(图 2B);肝组织汇管区大量炎症细胞浸润(图 2C);肺组织部分肺泡腔及间质炎症细胞浸润(图 2D)。
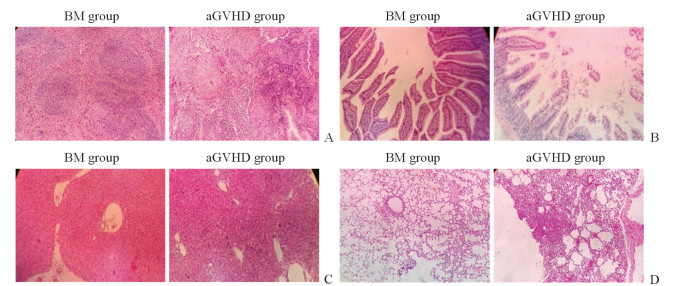
|
图 2 移植后21 d小鼠的各脏器组织病理学表现 Fig 2 Pathological features of mouse organs at 21 d after transplantation A: Spleen; B: Intestine; C: Liver; D: Lung. BM: Bone marrow transplantation; aGVHD: Acute graft versus host disease. H-E staining. Original magnification: ×40 |
2.3 移植后共刺激分子表达情况
如表 1、表 2所示,aGVHD组小鼠外周血CD4+ T淋巴细胞表面CTLA-4表达在移植后7 d开始即呈下降趋势,CD8+ T淋巴细胞表面CTLA-4表达在移植后14 d开始也呈下降趋势,在移植后28 d时均低于骨髓移植组(t=202.23、339.41,P均=0.01)。aGVHD组小鼠外周血CD4+ T淋巴细胞表面ICOS表达在移植后21 d开始上升,CD8+ T淋巴细胞表面ICOS表达在移植后7 d开始即呈上升趋势(F=845.02,P=0.01),在28 d时均高于骨髓移植组(t=108.89、152.74,P均=0.01)。aGVHD组小鼠外周血CD4+和CD8+ T淋巴细胞表面PD-1表达在移植后7 d开始均呈下降趋势,CD28表达均呈上升趋势,但PD-1仅在CD8+ T淋巴细胞表面表达差异有统计学意义(F=53.73,P=0.02),CD28仅在CD4+ T淋巴细胞表面表达差异有统计学意义(F=210.46,P=0.01),在移植后28 d时二者表达水平与骨髓移植组比较差异均有统计学意义(PD-1:t=130.11、149.90,P均=0.01;CD28:t=13.62、465.28,P=0.03、0.01)。
|
|
表 1 移植后各时间点小鼠CD4+ T淋巴细胞表面共刺激分子表达 Tab 1 Expression of costimulators on CD4+ T lymphocytes in mice at different time points after transplantation |
|
|
表 2 移植后各时间点小鼠CD8+ T淋巴细胞表面共刺激分子表达 Tab 2 Expression of costimulators on CD8+ T lymphocytes in mice at different time points after transplantation |
2.4 移植后aGVHD发生靶组织中共刺激分子配体的表达
取移植后21 d小鼠结肠组织行DAB染色,结果显示,骨髓移植组小鼠结肠组织CD80、ICOSL、PD-L1表达均为阴性,aGVHD组结肠组织CD80、ICOSL、PD-L1阳性表达率分别为40%、80%、80%。见图 3。
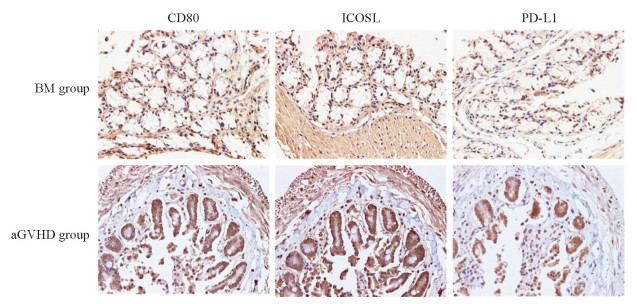
|
图 3 移植后21 d小鼠结肠组织中CD80、ICOSL、PD-L1表达 Fig 3 Expression of CD80, ICOSL and PD-L1 in colon tissues of mice 21 d after transplantation BM: Bone marrow transplantation; aGVHD: Acute graft versus host disease; ICOSL: Inducible costimulator ligand; PD-L1: Programmed death-ligand 1. 3, 3'-diaminobenzidine (DAB) staining. Original magnification: ×400 |
2.5 移植后CD4+ T淋巴细胞内IFN-γ、IL-4、IL-17表达
移植后21 d,aGVHD组小鼠CD4+ T淋巴细胞内IFN-γ、IL-17表达均较骨髓移植组增加[(18.000±1.803)% vs(11.900±2.476)%、(2.500±0.360)% vs(0.667±0.322)%],而IL-4表达减少[(5.600±1.308)% vs(11.200±3.161)%],差异均有统计学意义(t=2.836、3.450、6.574,P=0.026、0.003、0.047)。
3 讨论aGVHD的病理生理过程包括3个阶段:(1)预处理(包括化学治疗和放射治疗)导致患者组织、上皮细胞损伤,释放相关炎性因子IL-1、IL-6、肿瘤坏死因子α(tumor necrosis factor α,TNF-α)等形成炎症;(2)受体抗原提呈细胞黏附分子、共刺激分子和抗原表达增加,使供体来源的T淋巴细胞活化继续分泌IFN-γ、IL-2等炎性因子,从而导致Th向Th1极化;(3)细胞毒性T淋巴细胞、细胞因子和天然免疫细胞共同攻击aGVHD靶器官,引起相关靶细胞死亡,靶器官出现aGVHD反应。在整个过程中,共刺激分子发挥了重要作用。发生aGVHD时,供体T淋巴细胞抗原受体与受体靶组织抗原提呈细胞表面抗原肽主要组织相容性复合物结合后产生第一信号。同时,T淋巴细胞与抗原提呈细胞上多个共刺激分子结合产生第二信号,活化供体T淋巴细胞。本研究探讨了allo-HSCT后小鼠T淋巴细胞表面4种共刺激分子的表达和3种T淋巴细胞亚群在CD4+ T淋巴细胞中的分布情况,结果显示它们均与移植后aGVHD有关,并且移植后共刺激分子的表达水平随着时间的推移而改变。受体在接受致死性γ射线照射后,抗原提呈细胞可通过抗原提呈激活自身反应性T淋巴细胞,进而引发aGVHD[4-6]。CTLA-4和CD28可竞争性结合B7分子活化淋巴细胞功能抗原1等分子,从而阻止T淋巴细胞活化炎性因子风暴[7]。T淋巴细胞与aGVHD的发生有关,而CTLA-4可能通过调节T淋巴细胞活动调控aGVHD的严重程度[2-3]。本实验中,aGVHD组小鼠CD4+ T淋巴细胞和CD8+ T淋巴细胞表面CTLA-4表达水平在aGVHD启动后14~28 d呈逐渐下降趋势,且在28 d时低于骨髓移植组,提示CTLA-4对aGVHD有抑制作用,其表达水平可能和aGVHD严重程度有关。
本研究中,aGVHD组小鼠在移植后21 d开始CD4+ T淋巴细胞和CD8+ T淋巴细胞表面正性共刺激分子ICOS的表达均逐渐增加,说明随着时间的推移aGVHD病情逐渐加重;而骨髓移植组小鼠仅在γ射线照射后出现短暂辐照所致的靶组织损伤及炎性因子释放,在移植后缓慢恢复正常,故ICOS表达逐渐降低。研究表明,ICOS-免疫球蛋白融合蛋白能阻断CD4+ T淋巴细胞增殖并促进其凋亡,转入ICOS-免疫球蛋白融合蛋白的骨髓间充质干细胞干预可显著减轻aGVHD小鼠的症状,验证了ICOS对aGVHD的正性推动作用[8]。
PD-1与CTLA-4同为共抑制分子,在移植后CD4+ T淋巴细胞及CD8+ T淋巴细胞表面表达均呈下降趋势。而由于CD28与CTLA-4竞争性结合CD80/CD86,移植后aGVHD组小鼠CD4+ T淋巴细胞及CD8+ T淋巴细胞表面的CTLA-4表达下降有助于CD28与其配体结合,因此移植后aGVHD组CD4+ T淋巴细胞及CD8+ T淋巴细胞表面CD28的表达水平逐渐升高。
由H-E染色结果可知,移植后aGVHD小鼠小肠组织中可见明显的aGVHD表现,如炎症细胞浸润、组织结构破坏等。DAB染色结果显示,相比骨髓移植组,移植后aGVHD组小鼠CD80、ICOSL、PD-L1表达均增加,提示共刺激分子与其配体结合产生第二信号增多,T淋巴细胞活化增强,促进了aGVHD的发生、发展,这进一步验证了ICOS对aGVHD的促进作用。
既往研究报道,CTLA-4表达上调时IFN-γ、IL-17、IL-22等细胞因子表达下调,IL-4表达明显上调[9-12]。ICOS与其配体结合后IFN-γ、IL-5、IL-13、IL-10表达均增加,而IL-2表达未见明显变化[13]。PD-1和PD-L1结合可抑制CD4+ T淋巴细胞向Th1和Th17分化及炎性因子的释放[14]。在本研究中,移植后aGVHD组小鼠CD4+ T淋巴细胞和CD8+ T淋巴细胞表面CTLA-4、PD-1表达较骨髓移植组减少,ICOS、CD28表达增加,第二信号增加,从而促使效应T细胞及Th活化,IFN-γ、IL-17表达增加,IL-4表达减少,最终导致Th向Th1极化,使aGVHD进行性加重。
综上所述,发生aGVHD时正性共刺激分子CD28和ICOS表达均逐渐增加,负性共刺激分子CTLA-4和PD-1表达均逐渐减少,Th向Th1极化。此外,CTLA-4、ICOS、PD-1均可能通过调节Th1、Th2、Th17等T淋巴细胞亚群分布调控aGVHD的发生、发展。
| [1] |
WINGARD J R, MAJHAIL N S, BRAZAUSKAS R, WANG Z, SOBOCINSKI K A, JACOBSOHN D, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation[J]. J Clin Oncol, 2011, 29: 2230-2239. DOI:10.1200/JCO.2010.33.7212 |
| [2] |
ZHENG J, LIU Y, LIU Y, LIU M, XIANG Z, LAM K T, et al. Human CD8+ regulatory T cells inhibit GVHD and preserve general immunity in humanized mice[J/OL]. Sci Transl Med, 2013, 5: 168ra9. doi: 10.1126/scitranslmed.3004943.
|
| [3] |
YOO J S, LEE Y J, YOON J W, HYUNG K E, HWANG K W. CTLA-4-Tg/CD-28-KO mice exhibit reduced T cell proliferation in vivo compared to CD-28-KO mice in a graft-versus-host disease model[J]. Korean J Physiol Pharmacol, 2012, 16: 349-353. DOI:10.4196/kjpp.2012.16.5.349 |
| [4] |
ZHANG Y, SHLOMCHIK W D, JOE G, LOUBOUTIN J P, ZHU J, RIVERA A, et al. APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease[J]. J Immunol, 2002, 169: 7111-7118. DOI:10.4049/jimmunol.169.12.7111 |
| [5] |
SHLOMCHIK W D, COUZENS M S, TANG C B, MCNIFF J, ROBERT M E, LIU J, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells[J]. Science, 1999, 285: 412-415. DOI:10.1126/science.285.5426.412 |
| [6] |
DUFFNER U A, MAEDA Y, COOKE K R, REDDY P, ORDEMANN R, LIU C, et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease[J]. J Immunol, 2004, 172: 7393-7398. DOI:10.4049/jimmunol.172.12.7393 |
| [7] |
DILLON T J, CAREY K D, WETZEL S A, PARKER D C, STORK P J. Regulation of the small GTPase Rap1 and extracellular signal-regulated kinases by the costimulatory molecule CTLA-4[J]. Mol Cell Biol, 2005, 25: 4117-4128. DOI:10.1128/MCB.25.10.4117-4128.2005 |
| [8] |
XU H, LI X, LIU D, LI J, ZHANG X, CHEN X, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility[J]. Nature, 2013, 496: 523-527. DOI:10.1038/nature12058 |
| [9] |
ZHU F, ZHONG X M, QIAO J, LIU Q, SUN H Y, CHEN W, et al. Cytotoxic T lymphocyte antigen-4 down-regulates T helper 1 cells by increasing expression of signal transducer and activator of transcription 3 in acute graft-versus-host disease[J]. Biol Blood Marrow Transplant, 2016, 22: 212-219. DOI:10.1016/j.bbmt.2015.11.003 |
| [10] |
TSUCHIYAMA J, YOSHINO T, SAITO T, FURUKAWA T, ITO K, FUSE I, et al. Cutaneous lymphocyte antigen-positive T cells may predict the development of acute GVHD:alterations and differences of CLA+ T-and NK-cell fractions[J]. Bone Marrow Transplant, 2009, 43: 863-873. DOI:10.1038/bmt.2008.392 |
| [11] |
BETTS B C, SAGATYS E M, VEERAPATHRAN A, LLOYD M C, BEATO F, LAWRENCE H R, et al. CD4+ T cell STAT3 phosphorylation precedes acute GVHD, and subsequent Th17 tissue invasion correlates with GVHD severity and therapeutic response[J]. J Leukoc Biol, 2015, 97: 807-819. DOI:10.1189/jlb.5A1114-532RR |
| [12] |
ZHAO K, ZHAO D, HUANG D, YIN L, CHEN C, PAN B, et al. Interleukin-22 aggravates murine acute graft-versus-host disease by expanding effector T cell and reducing regulatory T cell[J]. J Leukoc Biol, 2015, 97: 807-819. DOI:10.1189/jlb.5A1114-532RR |
| [13] |
王斌, 杨建民. 可诱导共刺激分子在造血干细胞移植中的作用[J]. 白血病·淋巴瘤, 2007, 16: 76-78. DOI:10.3760/cma.j.issn.1009-9921.2007.01.028 |
| [14] |
KARIM R, JORDANOVA E S, PIERSMA S J, KENTER G G, CHEN L, BOER J M, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma[J]. Clin Cancer Res, 2009, 15: 6341-6347. DOI:10.1158/1078-0432.CCR-09-1652 |
 2019, Vol. 40
2019, Vol. 40


