血管性认知损害(vascular cognitive impairment,VCI)是仅次于阿尔茨海默病的导致认知损害和痴呆的疾病,包括了从轻度认知损害到痴呆的整个阶段[1]。在长期伴有高血压、糖尿病等脑血管危险因素的中老年人群中,颅内外单侧或双侧的脑供血动脉硬化和渐进性狭窄可造成大脑半球或全脑持续加重的低灌注,从而导致慢性脑低灌注性认知损害的发生和进展,并可伴随反复脑卒中的发生最终导致血管性痴呆。炎症反应被认为是认知损害和痴呆发生过程中的重要环节,慢性脑组织缺血、缺氧可引发持续炎症反应并导致神经损伤[2]。p75神经营养素受体(p75 neurotrophin receptor,p75NTR)属于肿瘤坏死因子(tumor necrosis factor,TNF)受体超家族,与炎症反应及神经凋亡等密切相关。已有研究显示在缺血性脑卒中、阿尔茨海默病患者血清中,被酶降解脱落的p75神经营养素受体细胞外段(p75 neurotrophin receptor-extracellular domain,p75NTR-ECD)含量增高且与疾病的严重程度有关,提示p75NTR可能在神经系统疾病中发挥重要作用[3]。但是,对于p75NTR及p75NTR-ECD在慢性脑低灌注认知损害中的作用及其与炎性因子的关系尚不清楚。基于此,本研究对慢性脑低灌注认知损害患者的血清p75NTR-ECD及炎性因子水平进行测定并探讨二者的相关性。
1 资料和方法 1.1 研究对象参照2011年美国心脏协会/美国卒中协会(American Heart Association/American Stroke Association,AHA/ASA)《血管性认知损害和痴呆科学声明共识》[4]及2016年中国轻度VCI诊断指南[5],选择海军军医大学(第二军医大学)长海医院2018年8月至12月收治的、符合以下纳入和排除标准的慢性脑低灌注血管性认知损害(chronic cerebral hypoperfusion-vascular cognitive impairment,CCH-VCI)患者。纳入标准:(1)主观或知情者报告患者存在≥1个客观检查证实的认知领域损害,采用简易智能精神状态检查(mini-mental state examination,MMSE)和蒙特利尔认知评估(Montreal cognitive assessment,MoCA)量表进行评估;(2)存在脑供血动脉狭窄并造成大脑半球或全脑低灌注的影像学或血流动力学证据;(3)慢性进展,无脑卒中或仅有轻度脑卒中发生且无波动性认知下降;(4)患者或家属签署知情同意书。排除标准:(1)存在抑郁或精神疾病;(2)遗传性痴呆;(3)具有明显波动性的脑卒中后认知损害;(4)其他退行性疾病如阿尔茨海默病、帕金森病、额颞叶痴呆、路易体痴呆等;(5)伴有感染或重要脏器功能障碍者。同时收集相同年龄段(40~85岁)、拥有正常独立生活能力的中老年健康人(健康对照)和缺血性脑卒中(发病2周内,无伴发感染)患者作为对照组。所有入组对象均签署知情同意书,本研究经海军军医大学(第二军医大学)长海医院伦理委员会审批。
1.2 资料搜集与血清处理收集3组研究对象的临床资料,包括性别、年龄、受教育年限,以及高血压、糖尿病、高血脂、吸烟、每天饮酒史等脑血管危险因素。收集晨空腹时肘静脉血,室温下静置30 min后以4 000 r/min(离心半径为10 cm)离心15 min,分装血清于-20 ℃保存。
1.3 检测方法使用双抗体夹心法酶联免疫吸附试验(enzyme linked immunosorbent assay,ELISA)检测各项血清指标。血清p75NTR-ECD ELISA检测试剂盒购自美国Raybiotech公司,血清TNF-α、白细胞介素(interleukin,IL)-1β、IL-6 ELISA检测试剂盒均购自中国Excell Biology公司。
1.4 统计学处理采用SPSS 18.0软件进行统计学分析。呈正态分布的计量资料以x±s表示,3组间比较采用单因素方差分析;呈偏态分布的计量资料以中位数(下四分位数,上四分位数)表示,3组间比较采用Kruskal-Wallis H检验,两组间比较采用Mann-Whitney U检验;计数资料以例数和百分数表示,组间比较采用Pearson χ2检验。相关性分析采用Spearman非参数相关分析。检验水准(α)为0.05。
2 结果 2.1 3组研究对象基本资料共纳入研究对象104例,其中CCH-VCI组和缺血性脑卒中组分别为34例,健康对照组36例。所有纳入对象中位年龄为65.0(59.0,71.8)岁,女性比例为32.7%(34/104)。3组在年龄、性别、受教育年限及糖尿病、高血脂、吸烟和饮酒等脑血管危险因素方面差异均无统计学意义(P均>0.05)。在高血压患者比例方面,3组间差异有统计学意义(χ2=13.159,P=0.001),健康对照组高血压患者比例低于CCH-VCI组(χ2=10.084,P=0.001)和缺血性脑卒中组(χ2=8.504,P=0.004),但CCH-VCI组和缺血性脑卒中组之间差异无统计学意义(χ2=0.078,P=0.779)。见表 1。
|
|
表 1 3组研究对象的基本特征比较 Tab 1 Comparison of basic characteristics of patients among three groups |
2.2 3组研究对象血清p75NTR-ECD、TNF-α、IL-1β、IL-6水平比较
ELISA检测结果(图 1)显示,CCH-VCI组血清p75NTR-ECD水平[(544.36(440.88,628.50)pg/mL]高于健康对照组[276.49(262.59,313.87)pg/mL]和缺血性脑卒中组[366.87(337.09,450.43)pg/mL],差异均有统计学意义(U=87.500、335.500,P均<0.05)。健康对照组血清TNF-α水平[73.71(56.94,79.81)pg/mL]低于缺血性脑卒中组[218.67(143.76,281.28)pg/mL]和CCH-VCI组[196.02(141.20,280.35)pg/mL],差异均有统计学意义(U=4.000、31.000,P均<0.05]。健康对照组血清IL-1β水平[42.98(34.52,51.34)pg/mL]低于缺血性脑卒中组[76.87(59.10,99.91)pg/mL]和CCH-VCI组[68.23(60.79,91.94)pg/mL],差异均有统计学意义(U=132.000、106.000,P均<0.05]。健康对照组血清IL-6水平[14.97(11.76,21.19)pg/mL]低于缺血性脑卒中组[64.45(43.13,86.76)pg/mL]和CCH-VCI组[51.04(40.24,65.26)pg/mL],差异均有统计学意义(U=13.000、48.000,P均<0.05)。
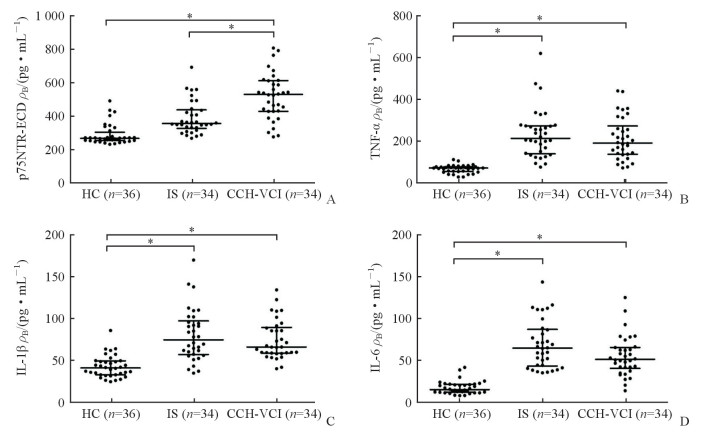
|
图 1 3组研究对象血清p75NTR-ECD、TNF-α、IL-1β、IL-6水平比较 Fig 1 Comparison of serum levels of p75NTR-ECD, TNF-α, IL-1β and IL-6 among three groups A: The serum p75NTR-ECD levels in the three groups; B: The serum TNF-α levels in the three groups; C: The serum IL-1β levels in the three groups; D: The serum IL-6 levels in the three groups. p75NTR-ECD: p75 neurotrophin receptor-extracellular domain; TNF-α: Tumor necrosis factor α; IL: Interleukin; HC: Healthy control; IS: Ischemic stroke; CCH-VCI: Chronic cerebral hypoperfusion-vascular cognitive impairment. *P < 0.05 |
2.3 CCH-VCI患者血清p75NTR-ECD水平与TNF-α、IL-1β、IL-6水平的相关性分析
Spearman相关分析结果(图 2)显示,CCH-VCI患者血清p75NTR-ECD水平与TNF-α水平存在相关性(r=0.391,P=0.022),与IL-1β和IL-6无明显相关性(r=0.032、0.164,P=0.855、0.355)。

|
图 2 慢性脑低灌注性认知损害患者血清p75NTR-ECD水平与TNF-α、IL-1β、IL-6水平Spearman相关性分析 Fig 2 Spearman correlation analysis of serum p75NTR-ECD with TNF-α, IL-1β and IL-6 in patients with chronic cerebral hypoperfusion-vascular cognitive impairment A: The correlation analysis of p75NTR-ECD with TNF-α; B: The correlation analysis of p75NTR-ECD with IL-1β; C: The correlation analysis of p75NTR-ECD with IL-6. p75NTR-ECD: p75 neurotrophin receptor-extracellular domain; TNF-α: Tumor necrosis factor α; IL: Interleukin |
3 讨论
脑缺血低灌注是VCI的一个重要病因,也是CCH-VCI和阿尔茨海默病的共同病理特征之一[6],在无法及时解除脑缺血低灌注的情况下,如何通过防治脑持续低灌注导致的神经功能和结构损伤以避免痴呆已成为目前研究的热点。研究发现血管性痴呆、阿尔茨海默病和脑卒中的神经损伤中存在共同的发病机制,包括氧化应激、炎症反应、神经突触损害及神经凋亡等,其中炎症反应在疾病中发挥着重要作用[7-8]。中枢神经系统内免疫细胞活化引起的炎症反应可帮助机体清除有害成分,并促进组织功能修复,但是持续的、过度的炎症反应将对组织细胞产生损害,促进神经突触损伤和神经细胞凋亡。Schmidt等[9]通过对1 050名日裔美国人进行长达25年的随访,发现非特异性炎性因子高敏C-反应蛋白水平较高者后期出现各类痴呆的风险约是高敏C-反应蛋白水平较低者的3倍。Engelhart等[10]开展的Rotterdam研究同样证明了炎症不仅与外周疾病相关,同时也能够在中枢神经系统疾病出现症状之前发生可被检测的异常改变。
脑组织受到缺氧等损伤后,小胶质细胞、星形胶质细胞活化,它们和一些受损的神经元和少突胶质细胞可释放促炎因子,如TNF-α、IL-6、IL-1β、IL-17A和γ干扰素等[11]。TNF-α是一种早期促炎因子,能够诱导IL-1β、IL-6等次级细胞因子产生,具有激发炎症级联反应的作用。通过颈动脉结扎建立血管性痴呆大鼠模型后,在造模术后数周至数月均可在缺血脑组织内检测到多种炎性因子表达增加,提示炎症反应可能参与了血管性痴呆的形成[12]。Magalhães等[13]通过对轻度认知损害和可疑阿尔茨海默病患者进行血清学测定,发现血清TNF-α水平与认知和功能评价结果呈负相关,提示炎症反应在痴呆前即已存在,并且可能与疾病进展有关,但该研究结果未能提供IL-6与认知损害的证据。Fung等[14]对57例VCI患者进行基因多态性检测研究,结果显示VCI组TNF-α基因T1031C突变频率高于非VCI组,提示在VCI患者中TNF-α具有更高的转录水平。Belkhelfa等[15]通过免疫组织化学法检测发现,死亡24 h内血管性痴呆患者大脑海马中TNF-α和IL-1β含量均较生前健康水平明显升高,提示炎症反应参与了血管性痴呆的发病机制。本研究结果显示,CCH-VCI患者血清TNF-α水平较中老年健康对照者升高,IL-1β和IL-6水平也同样升高,提示CCH-VCI患者存在炎症反应,这种炎症状态可能是促进认知损害的重要因素。同时本研究在缺血性脑卒中患者血清中检测到更高的炎性因子水平,这可能与其处于缺血性脑卒中急性期和应激状态有关。
研究表明,炎性因子的表达受到核因子κB(nuclear factor κB,NF-κB)等信号通路的调控,而p75NTR是介导NF-κB通路的上游信号分子之一[16]。p75NTR是一种Ⅰ型跨膜蛋白受体,由可被酶解脱落的细胞膜内外段和跨膜段氨基酸序列组成,在神经系统特别是胆碱能神经元上表达丰富,其能在协同受体的配合下高亲和力结合多种神经营养素前体,从而通过多种信号转导通路影响神经元的增殖、存活等。基于大鼠脑缺血模型研究显示,其脑组织中p75NTR表达均出现上调,且这种上调与损伤的严重程度有关[17-18]。Wei等[19]通过对大鼠缺血性视网膜损伤的研究也发现了这种与损伤严重程度有关的p75NTR表达上调现象。上述研究均提示p75NTR可能在缺血性神经损伤中发挥重要作用,但其损伤机制仍不明确。本研究结果显示,CCH-VCI患者血清p75NTR-ECD水平升高,提示脑缺血损伤后的p75NTR表达可能增加;本研究结果还显示CCH-VCI组血清p75NTR-ECD水平高于缺血性脑卒中组,由此我们推测持续的大脑半球或全脑损伤后p75NTR的增加程度可能超过了局部脑缺血后其表达水平。Choi和Friedman[20-21]研究发现p75NTR可通过NF-κB和丝裂原活化蛋白激酶p38途径与TNF-α、IL-1β等炎性因子产生相互作用,IL-1β还可易化神经生长因子前体(pro-nerve growth factor,proNGF)-p75NTR介导的促凋亡作用,同时使p75NTR表达上调。这些结果表明,TNF-α等炎性因子与p75NTR相互影响,并可能共同在神经损伤中发挥重要作用。相关性分析发现,CCH-VCI患者血清p75NTR-ECD水平与TNF-α水平存在一定的相关性,也间接提示持续性脑缺血损伤后p75NTR与炎症反应可能存在相关性。但本研究并未发现血清中p75NTR-ECD水平与IL-1β、IL-6水平的相关性,该结果可能与本研究样本量和检测标本类型是血清而非脑脊液有关。
综上所述,本研究结果显示CCH-VCI患者血清中p75NTR-ECD与炎性因子TNF-α、IL-1β、IL-6水平等均升高,提示在慢性脑低灌注损伤后p75NTR表达增加及炎症反应的存在,二者可能存在相互作用并最终共同促进了VCI的发生和进展。即便如此,鉴于临床脑组织样本难以获得的局限性,我们仍然需要通过动物实验研究进一步证实,并需从神经元及相关蛋白分子层面验证上述结果。
| [1] |
O'BRIEN J T, THOMAS A. Vascular dementia[J]. Lancet, 2015, 386: 1698-1706. DOI:10.1016/S0140-6736(15)00463-8 |
| [2] |
GORELICK P B. Role of inflammation in cognitive impairment:results of observational epidemiological studies and clinical trials[J]. Ann N Y Acad Sci, 2010, 1207: 155-162. DOI:10.1111/nyas.2010.1207.issue-1 |
| [3] |
JIAO S S, BU X L, LIU Y H, WANG Q H, LIU C H, YAO X Q, et al. Differential levels of p75NTR ectodomain in CSF and blood in patients with Alzheimer's disease: a novel diagnostic marker[J/OL]. Transl Psychiatry, 2015, 5: e650. doi: 10.1038/tp.2015.146.
|
| [4] |
GORELICK P B, SCUTERI A, BLACK S E, DECARLI C, GREENBERG S M, IADECOLA C, et al. Vascular contributions to cognitive impairment and dementia:a statement for healthcare professionals from the american heart association/american stroke association[J]. Stroke, 2011, 42: 2672-2713. DOI:10.1161/STR.0b013e3182299496 |
| [5] |
田金洲, 解恒革, 秦斌, 樊东升, 时晶, 王鲁宁. 中国血管性轻度认知损害诊断指南[J]. 中华内科杂志, 2016, 55: 249-256. DOI:10.3760/cma.j.issn.0578-1426.2016.03.022 |
| [6] |
ROMÁN G C. Brain hypoperfusion:a critical factor in vascular dementia[J]. Neurol Res, 2004, 26: 454-458. DOI:10.1179/016164104225017686 |
| [7] |
VIJAYAN M, KUMAR S, BHATTI J S, REDDY P H. Molecular links and biomarkers of stroke, vascular dementia, and Alzheimer's disease[J]. Prog Mol Biol Transl Sci, 2017, 146: 95-126. DOI:10.1016/bs.pmbts.2016.12.014 |
| [8] |
IADECOLA C. The pathobiology of vascular dementia[J]. Neuron, 2013, 80: 844-866. DOI:10.1016/j.neuron.2013.10.008 |
| [9] |
SCHMIDT R, SCHMIDT H, CURB J D, MASAKI K, WHITE L R, LAUNER L J. Early inflammation and dementia:a 25-year follow-up of the Honolulu-Asia aging study[J]. Ann Neurol, 2002, 52: 168-174. DOI:10.1002/(ISSN)1531-8249 |
| [10] |
ENGELHART M J, GEERLINGS M I, MEIJER J, KILIAAN A, RUITENBERG A, VAN SWIETEN J C, et al. Inflammatory proteins in plasma and the risk of dementia:the Rotterdam study[J]. Arch Neurol, 2004, 61: 668-672. DOI:10.1001/archneur.61.5.668 |
| [11] |
KARVE I P, TAYLOR J M, CRACK P J. The contribution of astrocytes and microglia to traumatic brain injury[J]. Br J Pharmacol, 2016, 173: 692-702. DOI:10.1111/bph.v173.4 |
| [12] |
VENKAT P, CHOPP M, CHEN J. Models and mechanisms of vascular dementia[J]. Exp Neurol, 2015, 272: 97-108. DOI:10.1016/j.expneurol.2015.05.006 |
| [13] |
MAGALHÃES C A, FERREIRA C N, LOURES C M G, FRAGA V G, CHAVES A C, OLIVEIRA A C R, et al. Leptin, hsCRP, TNF-α and IL-6 levels from normal aging to dementia:relationship with cognitive and functional status[J]. J Clin Neurosci, 2018, 56: 150-155. DOI:10.1016/j.jocn.2018.08.027 |
| [14] |
FUNG H C, CHEN C M, WU Y R, HSU W C, RO L S, LIN J C, et al. Heat shock protein 70 and tumor necrosis factor alpha in Taiwanese patients with dementia[J]. Dement Geriatr Cogn Disord, 2005, 20: 1-7. DOI:10.1159/000085067 |
| [15] |
BELKHELFA M, BEDER N, MOUHOUB D, AMRI M, HAYET R, TIGHILT N, et al. The involvement of neuroinflammation and necroptosis in the hippocampus during vascular dementia[J]. J Neuroimmunol, 2018, 320: 48-57. DOI:10.1016/j.jneuroim.2018.04.004 |
| [16] |
SALMINEN A, HUUSKONEN J, OJALA J, KAUPPINEN A, KAARNIRANTA K, SUURONEN T. Activation of innate immunity system during aging:NF-κB signaling is the molecular culprit of inflamm-aging[J]. Ageing Res Rev, 2008, 7: 83-105. DOI:10.1016/j.arr.2007.09.002 |
| [17] |
IRMADY K, JACKMAN K A, PADOW V A, SHAHANI N, MARTIN L A, CERCHIETTI L, et al. MiR-592 regulates the induction and cell death-promoting activity of p75NTR in neuronal ischemic injury[J]. J Neurosci, 2014, 34: 3419-3428. DOI:10.1523/JNEUROSCI.1982-13.2014 |
| [18] |
PARK J A, LEE J Y, SATO T A, KOH J Y. Co-induction of p75NTR and p75NTR-associated death executor in neurons after zinc exposure in cortical culture or transient ischemia in the rat[J]. J Neurosci, 2000, 20: 9096-9103. DOI:10.1523/JNEUROSCI.20-24-09096.2000 |
| [19] |
WEI Y, WANG N, LU Q, ZHANG N, ZHENG D, LI J. Enhanced protein expressions of sortilin and p75NTR in retina of rat following elevated intraocular pressure-induced retinal ischemia[J]. Neurosci Lett, 2007, 429(2/3): 169-174. |
| [20] |
CHOI S, FRIEDMAN W J. Inflammatory cytokines IL-1β and TNF-α regulate p75NTR expression in CNS neurons and astrocytes by distinct cell-type-specific signalling mechanisms[J/OL]. ASN Neuro, 2009, 1: e00010. doi: 10.1042/AN20090009.
|
| [21] |
CHOI S, FRIEDMAN W J. Interleukin-1β enhances neuronal vulnerability to proNGF-mediated apoptosis by increasing surface expression of p75NTR and sortillin[J]. Neuroscience, 2014, 257: 11-19. DOI:10.1016/j.neuroscience.2013.10.058 |
 2019, Vol. 40
2019, Vol. 40


