胰腺癌是我国常见的消化系统恶性肿瘤,在我国肿瘤死因中位居第6位[1]。据估计,胰腺癌的发病率将继续上升,到2020年将成为第二大致死肿瘤病种[2]。胰腺导管腺癌(pancreatic ductal adenocarcinoma,PDAC)是胰腺癌中最常见的病理类型,具有进展快、侵袭性强、预后差的特点,目前临床上虽采取手术切除、化学治疗等多种治疗手段,但PDAC患者的5年生存率仍低于5%[3]。因此探明PDAC的发生和进展机制、寻找合适的生物分子靶点对于丰富胰腺癌的治疗策略、改善患者预后意义重大。
PDAC是肿瘤间质最丰富的实体肿瘤之一,在部分病例PDAC组织中间质成分甚至远多于肿瘤成分[4]。PDAC间质包括肿瘤相关成纤维细胞(cancer-associated fibroblast,CAF)、免疫细胞、神经、血管、细胞外基质和可溶性蛋白(如细胞因子和生长因子)等[5]。PDAC中大量增殖活化的CAF近年来备受关注,2015年Lo等[6]发现成纤维细胞激活蛋白(fibroblast activation protein,FAP)表达阳性的CAF可通过诱导T淋巴细胞凋亡与低反应性、抑制免疫细胞对肿瘤细胞恶性表型的识别、招募免疫抑制细胞等途径在PDAC中发挥免疫抑制功能;2017年Deng等[7]报道CAF能够通过分泌基质细胞衍生因子1(stromal cell-derived factor 1,SDF-1)募集免疫抑制细胞,抑制免疫细胞对肿瘤细胞的杀伤作用,帮助胰腺癌细胞逃避宿主免疫监视。髓源性抑制细胞(myeloid-derived suppressor cell,MDSC)是被招募到肿瘤间质微环境中最重要的免疫抑制细胞群[8],也是在肿瘤、感染等病理条件下骨髓细胞分化受阻形成的具有免疫抑制功能的异质细胞群[9]。招募到肿瘤部位的MDSC能够通过分泌精氨酸酶1(arginase 1,Arg-1)耗竭淋巴细胞所需营养物质[10],并可通过诱导氧化应激等方式抑制T淋巴细胞和自然杀伤细胞的功能[11]。
本课题组在前期研究中发现,中性粒细胞样MDSC(neutrophil-like MDSC,nMDSC)在胰腺癌患者外周血及肿瘤组织中明显增多,根据CD13分子表达差异分为CD13高表达的nMDSC(CD13hi-nMDSC)和CD13低表达的nMDSC(CD13low-nMDSC),其中CD13hi-nMDSC是发挥免疫抑制功能的主要细胞群[12]。虽然有研究证实肿瘤间质分泌的细胞因子可能与MDSC的分化、迁移有关,但在PDAC中CAF参与MDSC分化的相关机制目前尚不明确。本研究旨在研究胰腺癌中CAF对MDSC的作用机制。
1 材料和方法 1.1 细胞与试剂5例PDAC患者胰腺癌组织及5名健康志愿者外周血样本均来自于海军军医大学(第二军医大学)长海医院,人包皮成纤维细胞(human foreskin fibroblast,HFF)由上海富衡生物细胞库提供。含10%胎牛血清(fetal bovine serum, FBS)的RPMI 1640培养液购自美国Gibco公司,透明质酸酶、DNA酶Ⅰ、胶原蛋白酶购自美国Sigma公司,RNAfast200 RNA提取试剂盒购自上海飞捷生物技术有限公司,PrimeScript RT reagent Kit with gDNA Eraser、TB Green Premix Ex TaqⅡ购自日本TaKaRa公司,淋巴细胞分离液购自挪威Axis-Shield公司,α-平滑肌肌动蛋白(α-smooth muscle actin,α-SMA)抗体、波形蛋白(vimentin)抗体购自武汉博士德生物工程有限公司,流式抗体、人白细胞DR抗原(human leukocyte DR antigen, HLA-DR)、CD11b、CD33、CD13、CD14、CD15、FAPa以及人重组白细胞介素6(interleukin 6,IL-6)、单核细胞趋化蛋白1(monocyte chemotactic protein 1,MCP-1)、SDF-1酶联免疫吸附试验(enzyme-linked immunosorbent assay,ELISA)试剂盒购自美国Biolegend公司,MCP-1、SDF-1、IL-6和IL-6中和抗体购自美国R & D公司,Janus激酶2(Janus kinase 2,JAK2)/信号转导与转录激活因子(signal transducer and activator of transcription,STAT)3抑制剂FLLL32购自美国Selleck公司。引物由生工生物工程(上海)股份有限公司合成。本研究经海军军医大学(第二军医大学)长海医院伦理委员会审批,所有患者均签署知情同意书。
1.2 原代CAF的分离培养收集5例PDAC患者术后新鲜肿瘤组织标本,使用眼科剪将组织剪碎,用透明质酸酶(0.1 mg/mL)、DNA酶Ⅰ(0.1 mg/mL)、胶原蛋白酶(1 mg/mL)配制的消化酶体系消化1 h,70 μm细胞滤器过滤,离心,去上清,将沉淀重悬后放入5% CO2培养箱中培养,24 h后轻柔换液,48 h后再次换液,用磷酸盐缓冲液(phosphate buffer saline,PBS)漂洗2~3次,去掉未贴壁的细胞和细胞碎片。当细胞融合度达到80%~90%时,以1:1比例传代,12 h后换液,利用差速贴壁的原理将贴壁晚的胰腺癌细胞、内皮细胞等非成纤维细胞除去,传代培养3~5代后细胞形态趋于一致。
1.3 原代CAF表型鉴定采用细胞免疫荧光技术和流式细胞术鉴定原代CAF及HFF的表型。细胞免疫荧光鉴定:使用胰酶将CAF或HFF消化成单个细胞,离心,重悬,将悬液滴加在载玻片中央,置于5% CO2培养箱中培养3 h,固定、破膜,然后滴加α-SMA抗体(1:200)、波形蛋白抗体(1:200),置于湿盒内4 ℃过夜,用PBS漂洗2~3次,加入二抗,用DAPI复染细胞核,封片拍照。流式细胞术鉴定:使用胰酶将CAF或HFF消化成单个细胞,离心,重悬,加入PE标记的抗人FAPa抗体(10 μL/106个细胞),4 ℃避光孵育30 min,上机检测,计算FAPa阳性细胞群比例。
1.4 CAF中相关细胞因子表达检测采用实时荧光定量PCR法检测CAF和HFF中细胞因子mRNA的表达。收集1×106个细胞,提取总RNA并检测RNA纯度,反转录合成cDNA。以cDNA为模板、β-actin为内参照基因进行实时荧光定量PCR,反应体系为20 μL,反应条件:95 ℃ 30 s;95 ℃ 10 s,60 ℃ 10 s,72 ℃ 10 s,40个循环;引物序列见表 1。采用2-ΔΔCt法计算目的基因的相对表达量。
|
|
表 1 用于实时荧光定量PCR反应的细胞因子引物序列 Tab 1 Primer sequences of each cytokine for quantitative real-time PCR |
采用ELISA检测CAF培养上清中相关细胞因子蛋白的表达。将CAF和HFF 2种细胞分别以1×106/mL的密度加入10 cm培养皿中,在培养皿中加入含1% FBS的DMEM培养液培养细胞;分别收集2种细胞培养24 h和48 h的培养上清,用0.22 μm滤膜过滤去除杂质及细胞碎片后,在微孔板中加入实验样品(培养上清)及标准品,室温孵育2 h;洗板,加入检测抗体,室温孵育1 h;再次洗板后加入辣根过氧化物酶标记的链霉亲和素溶液反应30 min;洗板后加入显色底物TMB反应15 min,然后加入终止溶液,立刻用酶标仪检测450 nm及570 nm处的光密度值。
1.5 CAF对MDSC分化的影响及细胞因子在分化中的作用采用Ficoll密度梯度离心法分离健康志愿者外周血单个核细胞(peripheral blood mononuclear cell,PBMC)并计数,在6孔板内加入2×106个细胞/孔。将CAF和HFF培养至70%~80%融合后,将培养液换成含1% FBS的DMEM培养液,培养48 h后收集培养上清。分别采用6种培养体系培养PBMC,即单纯培养液(对照组)、含20% CAF培养上清的培养液、含20% HFF培养上清的培养液、含10 μg/mL人重组IL-6蛋白的培养液、含10 μg/mL人重组SDF-1蛋白的培养液、含10 μg/mL人重组MCP-1蛋白的培养液,分别培养7 d。在上述培养体系中加入IL-6中和抗体,以不加IL-6中和抗体的培养液为对照,将PBMC培养7 d。在上述培养体系中加入JAK2/STAT3抑制剂FLLL32,以二甲基亚砜(dimethyl sulfoxide,DMSO)作为对照,培养7 d。培养上清、细胞因子和培养液每2~3 d更换1次。用流式细胞术鉴定PBMC的表型变化,检测指标为HLA-DR、CD11b、CD33、CD13、CD14和CD15。
1.6 统计学处理应用SPSS 20.0软件进行统计学分析。计量资料以x±s表示,组间比较采用独立样本t检验。检验水准(α)为0.05。
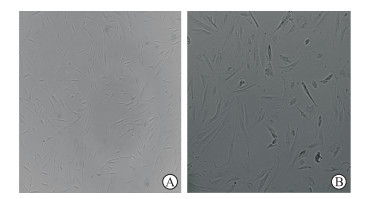
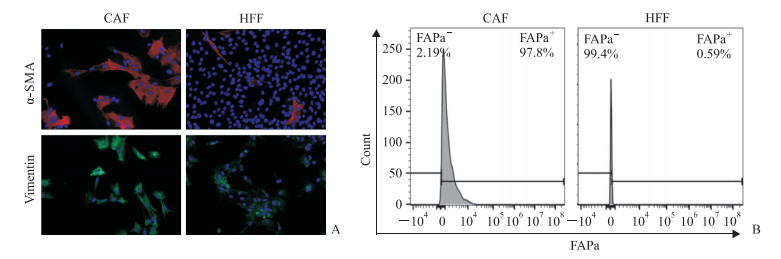
2 结果 2.1 PDAC肿瘤组织分离的原代CAF纯度及表型鉴定从PDAC肿瘤组织中分离的CAF纯化3~5代后,光学显微镜下可见细胞形态稳定,呈均一的长梭状成纤维样结构,未见内皮细胞等杂细胞(图 1)。采用细胞免疫荧光技术检测CAF中α-SMA和波形蛋白的表达情况,结果显示CAF表达α-SMA和波形蛋白,而HFF仅表达波形蛋白(图 2A)。采用流式细胞术检测CAF中另一活化标志物FAPa的表达情况,结果显示分离的原代CAF中超过90%的细胞FAPa表达阳性,而HFF基本为FAPa阴性细胞群(图 2B)。可见分离的原代CAF纯度高,均表达活化标志物α-SMA和FAPa,符合实验要求。
|
图 1 原代分离的CAF在显微镜下的细胞形态 Fig 1 Morphology of primary isolated cancer-associated fibroblasts (CAFs) under microscope Original magnification: ×200 (A), ×400 (B) |
|
图 2 原代分离的CAF表型鉴定 Fig 2 Phenotypic identification of primary isolated CAFs A: The expression levels of α-SMA and vimentin in CAFs and HFFs (control) were analyzed by immunofluorescence technique. The slide was stained with 4', 6-diamidino-2-phenylindole (DAPI, blue), vimentin antibody (green), and α-SMA antibody (red); B: The expression levels of FAPa on the surface of CAFs and HFFs were analyzed by flow cytometry. CAF: Cancer-associated fibroblast; α-SMA: α-Smooth muscle actin; HFF: Human foreskin fibroblast; FAPa: Fibroblast activation protein a. Original magnification: ×200(A) |
2.2 PDAC肿瘤组织分离的原代CAF中细胞因子的表达
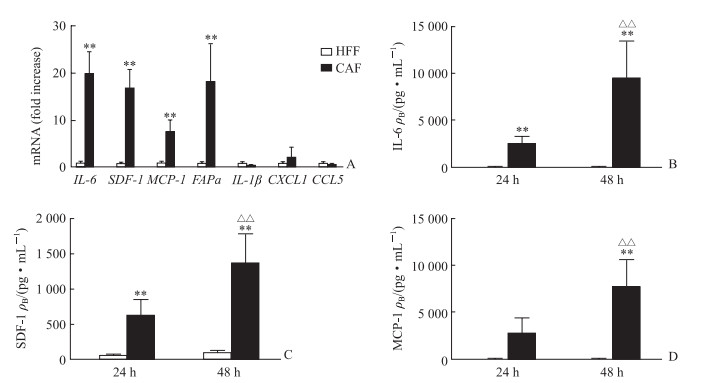
从5例PDAC患者肿瘤组织中分离获得5株CAF,通过实时荧光定量PCR检测相关细胞因子mRNA表达水平,筛选CAF与对照细胞HFF表达差异大的细胞因子,结果显示IL-6、SDF-1、MCP-1这3个因子在CAF中的表达量均高于HFF(P均<0.01,图 3A)。用ELISA检测原代分离的CAF和对照细胞HFF培养24 h、48 h的培养上清中IL-6、SFD-1、MCP-1的表达量,结果显示CAF培养上清中IL-6、SDF-1、MCP-1的表达量均高于HFF培养上清(P均<0.01),而且培养上清中IL-6、SDF-1、MCP-1的表达量均随着培养时间的延长而升高(P均<0.01,图 3B~3D)。
|
图 3 原代分离的CAF中相关细胞因子的表达 Fig 3 Expression of cytokines in primary isolated CAFs A: Expression levels of cytokines in CAFs and HFFs were detected by quantitative real-time PCR; B-D: Expression levels of IL-6, SDF-1 and MCP-1 in CAFs and HFFs culture supernatant at different time points by enzyme-like immunosorbent assay, respectively. CAF: Cancer-associated fibroblast; HFF: Human foreskin fibroblast; IL: Interleukin; SDF-1: Stromal cell-derived factor 1; MCP-1: Monocyte chemotactic protein 1; FAPa: Fibroblast activation protein a; CXCL1: Chemokine ligand 1; CCL5: Chemokine 5. **P < 0.01 vs HFF; △△P < 0.01 vs 24 h. n=5, x±s |
2.3 CAF培养上清促进PBMC分化为CD13hi-nMDSC
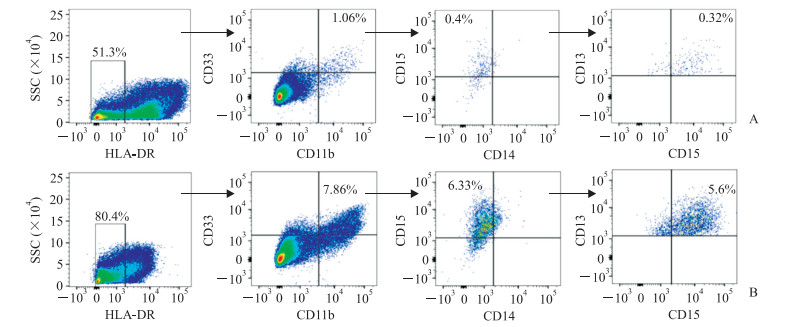
流式细胞术分析结果显示,用CAF培养上清培养的健康志愿者PBMC中HLA-DR-CD11b+CD33+MDSC比例高于与对照细胞HFF培养上清培养的PBMC,而且CD13hi-nMDSC的数量也多于对照组(图 4A、图 4B)。
|
图 4 流式细胞术分析PBMC分化情况 Fig 4 Differentiation of PBMCs detected by flow cytometry A: The differentiation of PBMCs cultured with HFFs culture supernatant for 7 d; B: The differentiation of PBMCs cultured with CAFs culture supernatant for 7 d. PBMC: Peripheral blood mononuclear cell; HFF: Human foreskin fibroblast; CAF: Cancer-associated fibroblast; HLA-DR: Human leukocyte DR antigen |
为了探讨这些因子在MDSC分化中的作用,在培养体系中分别加入人重组IL-6、SDF-1、MCP-1蛋白,结果显示单独加入人重组IL-6蛋白可以诱导PBMC向CD13hi-nMDSC分化(P<0.01),单独加入人重组SDF-1或MCP-1蛋白不能诱导CD13hi-nMDSC亚群的增加,提示CAF分泌的细胞因子IL-6可能参与介导PBMC分化为CD13hi-nMDSC(图 5)。
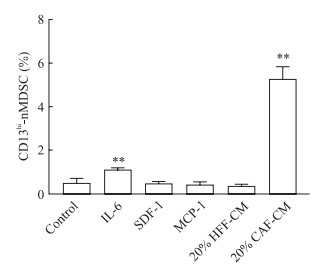
|
图 5 培养7 d后PBMC中分化的CD13hi-nMDSC亚群比例 Fig 5 Percentage of CD13hi-nMDSCs subpopulation differentiated from PBMCs after 7 d of culture PBMC: Peripheral blood mononuclear cell; CD13hi-nMDSC: CD13-high expression neutrophil-like myeloid-derived suppressor cell; IL-6: Interleukin 6; SDF-1: Stromal cell-derived factor 1; MCP-1: Monocyte chemotactic protein 1; HFF: Human foreskin fibroblast; CAF: Cancer-associated fibroblast; CM: Conditional medium. **P < 0.01 vs control group. n=5, x±s |
2.4 CAF通过IL-6/STAT3通路介导PBMC向CD13hi-nMDSC分化
为探究CAF培养上清中细胞因子参与CD13hi-nMDSC分化的具体作用机制,在培养体系中加入针对IL-6的中和抗体,结果显示加入IL-6中和抗体能够减少由CAF培养上清诱导的分化(图 6A,P < 0.05),表明IL-6参与介导CD13hi-nMDSC的分化。为进一步验证IL-6/STAT3通路是否参与CD13hi-nMDSC的分化过程,在培养体系中加入STAT3抑制剂FLLL32,结果显示加入FLLL32能有效抑制IL-6及CAF培养上清诱导的CD13hi-nMDSC分化(图 6B,P < 0.05)。表明CAF分泌的IL-6能通过STAT3通路介导PBMC向CD13hi-nMDSC分化。
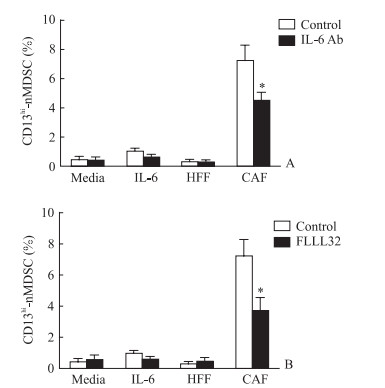
|
图 6 IL-6中和抗体与STAT3抑制剂FLLL32对PBMC分化为CD13hi-nMDSC的影响 Fig 6 Effects of IL-6 neutralizing antibody (Ab) and STAT3 blocker FLLL32 on proportion of CD13hi-nMDSCs differentiated from PBMCs IL-6: Interleukin 6; STAT3: Signal transducer and activator of transcription 3; PBMC: Peripheral blood mononuclear cell; CD13hi-nMDSC: CD13-high expression neutrophil-like myeloid-derived suppressor cell. *P < 0.05 vs control group. n=5, x±s |
3 讨论
PDAC是常见的进展迅速、恶性程度高、死亡率高的恶性肿瘤。PDAC患者确诊时多处于晚期,尽管目前医疗水平已取得较大的进步,但仍有高达94%的患者在确诊5年内死亡[13]。因此探寻PDAC的发生、发展机制,对寻找PDAC治疗靶点、改善患者预后意义重大。近年来,胰腺癌肿瘤微环境在肿瘤发生、发展中的作用引起研究者的关注[14]。Rakhra等[15]将肿瘤微环境比作肿瘤细胞赖以生存和增殖的“土壤”,能够帮助肿瘤细胞逃避免疫系统的攻击,弱化抗肿瘤治疗效果。MDSC是肿瘤微环境中最重要的免疫抑制细胞群,在食管癌、肝癌、乳腺癌等肿瘤中的表达量较高,并且其表达量与肿瘤患者预后、免疫治疗效果密切相关[16-18]。本课题组在前期研究中发现在胰腺癌中MDSC以nMDSC升高为主,尤其是CD13hi-nMDSC亚群在肿瘤组织内大量扩增聚集,CD13hi-nMDSC分泌大量的Arg-1抑制T淋巴细胞增殖,是促进免疫抑制微环境形成的主要细胞群[12]。
CAF是一群处于活化状态的成纤维样细胞群,来源多样,主要包括胰腺星状细胞、肿瘤微环境中固有的成纤维细胞、上皮细胞、间充质干细胞和骨髓来源的干细胞[19]。有研究发现在胰腺癌等肿瘤组织内星状细胞、CAF等间质成分与肿瘤患者MDSC分化密切相关,CAF分泌的细胞因子、趋化蛋白等能够促进未成熟的免疫细胞向MDSC分化并促进MDSC的募集[20];同时也有研究证实,在肝癌中CAF可以通过SDF-1α/趋化因子受体4(C-X-C chemokine receptor 4, CXCR4)途径吸引外周血中的单核细胞,并通过IL-6介导的STAT3通路诱导单核细胞分化成CD14+HLA-DR- MDSC[7];这些MDSC能够抑制T淋巴细胞增殖并改变T淋巴细胞的表型和功能,如诱导调节性T细胞和T淋巴细胞的凋亡、上调IL-10表达、下调干扰素γ表达等[21]。上述研究表明CAF与肿瘤患者体内MDSC的增殖活化有关,但是PDAC中MDSC尤其是CD13hi-nMDSC分化的机制尚不清楚。
本研究通过实时荧光定量PCR、ELISA筛选出CAF与对照细胞HFF表达差异较大的3种细胞因子IL-6、SDF-1、MCP-1,并发现CAF培养上清能够促进PBMC分化为CD13hi-nMDSC。为进一步探讨CAF培养上清促进MDSC分化的作用机制,我们在培养体系中分别加入人重组IL-6、SDF-1、MCP-1蛋白,结果发现人重组SDF-1、MCP-1蛋白均不能诱导CD13hi-nMDSC亚群的增加,而人重组IL-6蛋白可诱导PBMC向CD13hi-nMDSC分化。目前已有研究证实IL-6在其他疾病中参与MDSC的分化,如Deng等[7]发现肝癌中CAF分泌的IL-6能使PBMC向MDSC分化。为进一步证实IL-6在PDAC中调控MDSC的分化过程,我们在培养体系中加入IL-6中和抗体,结果发现CAF培养上清诱导PBMC的分化减少。以上结果说明,CAF主要通过高表达IL-6介导PBMC分化为CD13hi-nMDSC。
Mace等[20]证实用CAF培养上清培养PBMC可使STAT3快速磷酸化,且其磷酸化水平与IL-6刺激后的细胞相似,而STAT1或STAT5等磷酸化水平均未见增加,说明IL-6可能主要通过STAT3通路促使PBMC向MDSC分化。为验证IL-6/STAT3通路是否参与CD13hi-nMDSC的分化过程,我们在培养体系中加入STAT3抑制剂FLLL32,结果发现加入FLLL32后能有效抑制IL-6及CAF培养上清诱导的CD13hi-nMDSC的分化。说明CAF可以通过IL-6/STAT3通路促进CD13hi-nMDSC这一新亚群的扩增。
CAF是一群来源多样的异质性细胞群,在不同胰腺癌患者中分离的CAF表型特征及细胞因子的表达量有一定的差异性。本研究仅分析了5例PDAC患者肿瘤组织来源的CAF与CD13hi-nMDSC分化的关系,而且尚未研究CD13hi-nMDSC向肿瘤部位聚集的机制,具有一定的局限性。在后续实验中,我们将增加样本量进一步研究CAF与CD13hi-nMDSC分化和迁移的相关机制。
综上所述,PDAC是胰腺癌中最常见的病理类型,具有进展快、侵袭性强、预后差等特点。MDSC在PDAC中通过促进肿瘤免疫抑制微环境的形成,进而在PDAC的发生、发展过程中发挥作用。本研究对PDAC主要成分之一CAF与MDSC分化的关系进行探讨,发现CAF可能通过IL-6/STAT3通路促进PBMC分化成CD13hi-nMDSC。这一发现有助于进一步理解PDAC患者肿瘤免疫逃逸机制,为寻找PDAC新的免疫治疗靶点、探索新的治疗策略提供了新思路。
| [1] |
CHEN W, SUN K, ZHENG R, ZENG H, ZHANG S, XIA C, et al. Cancer incidence and mortality in China, 2014[J]. Chin J Cancer Res, 2018, 30: 1-12. DOI:10.21147/j.issn.1000-9604.2018.01.01 |
| [2] |
SIEGEL R, MA J, ZOU Z, JEMAL A. Cancer statistics, 2014[J]. CA Cancer J Clin, 2014, 64: 9-29. DOI:10.3322/caac.21208 |
| [3] |
AIER I, SEMWAL R, SHARMA A, VARADWAJ P K. A systematic assessment of statistics, risk factors, and underlying features involved in pancreatic cancer[J]. Cancer Epidemiol, 2019, 58: 104-110. DOI:10.1016/j.canep.2018.12.001 |
| [4] |
FEIG C, GOPINATHAN A, NEESSE A, CHAN D S, COOK N, TUVESON A. The pancreas cancer microenvironment[J]. Clin Cancer Res, 2012, 18: 4266-4276. DOI:10.1158/1078-0432.CCR-11-3114 |
| [5] |
FARROW B, ALBO D, BERGER D H. The role of the tumor microenvironment in the progression of pancreatic cancer[J]. J Surg Res, 2008, 149: 319-328. DOI:10.1016/j.jss.2007.12.757 |
| [6] |
LO A, WANG L C S, SCHOLLER J, MONSLOW J, AVERY D, EVANS R A, et al. Abstract 3187:Depleting cells expressing fibroblast activation protein disrupts tumor-promoting desmoplasia[J]. Cancer Res, 2015, 75(15 Suppl): 3187. |
| [7] |
DENG Y, CHENG J, FU B, LIU W, CHEN G, ZHANG Q, et al. Hepatic carcinoma-associated fibroblasts enhance immune suppression by facilitating the generation of myeloid-derived suppressor cells[J]. Oncogene, 2017, 36: 1090-1101. DOI:10.1038/onc.2016.273 |
| [8] |
GABITASS R F, ANNELS N E, STOCKEN D D, PANDHA H A, MIDDLETON G W. Elevated myeloidderived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13[J]. Cancer Immunol Immunother, 2011, 60: 1419-1430. DOI:10.1007/s00262-011-1028-0 |
| [9] |
PYZER A R, COLE L, ROSENBLATT J, AVIGAN D E. Myeloid-derived suppressor cells as effectors of immune suppression in cancer[J]. Int J Cancer, 2016, 139: 1915-1926. DOI:10.1002/ijc.v139.9 |
| [10] |
ZHU X, PRIBIS J P, RODRIGUEZ P C, MORRIS S M Jr, VODOVOTZ Y, BILLIAR T R, et al. The central role of arginine catabolism in T-cell dysfunction and increased susceptibility to infection after physical injury[J]. Ann Surg, 2014, 259: 171-178. DOI:10.1097/SLA.0b013e31828611f8 |
| [11] |
CHANG J H, JIANG Y, PILLARISETTY V G. Role of immune cells in pancreatic cancer from bench to clinical application: an updated review[J/OL]. Medicine (Baltimore), 2016, 95: e5541. doi: 10.1097/MD.0000000000005541.
|
| [12] |
ZHANG J, XU X, SHI M, CHEN Y, YU D, ZHAO C, et al. CD13hi neutrophil-like myeloid-derived suppressor cells exert immune suppression through arginase 1 expression in pancreatic ductal adenocarcinoma[J/OL]. Oncoimmunology, 2017, 6: e1258504. doi: 10.1080/2162402X.2016.1258504.
|
| [13] |
ROSSI M L, REHMAN A A, GONDI C S. Therapeutic options for the management of pancreatic cancer[J]. World J Gastroenterol, 2014, 20: 11142-11159. DOI:10.3748/wjg.v20.i32.11142 |
| [14] |
KUMAR V, PATEL S, TCYGANOV E, GABRILOVICH D I. The nature of myeloid-derived suppressor cells in the tumor microenvironment[J]. Trends Immunol, 2016, 37: 208-220. DOI:10.1016/j.it.2016.01.004 |
| [15] |
RAKHRA K, BACHIREDDY P, ZABUAWALA T, ZEISER R, XU L, KOPELMAN A, et al. CD4+ T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation[J]. Cancer Cell, 2010, 18: 485-498. DOI:10.1016/j.ccr.2010.10.002 |
| [16] |
GABITASS R F, ANNELS N E, STOCKEN D D, PANDHA H A, MIDDLETON G W. Elevated myeloidderived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13[J]. Cancer Immunol Immunother, 2011, 60: 1419-1430. DOI:10.1007/s00262-011-1028-0 |
| [17] |
OHKI S, SHIBATA M, GONDA K, MACHIDA T, SHIMURA T, NAKAMURA I, et al. Circulating myeloidderived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer[J]. Oncol Rep, 2012, 28: 453-458. DOI:10.3892/or.2012.1812 |
| [18] |
DIAZ-MONTERO C M, SALEM M L, NISHIMURA M I, GARRETT-MAYER E, COLE D J, MONTERO A J. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy[J]. Cancer Immunol Immunother, 2009, 58: 49-59. DOI:10.1007/s00262-008-0523-4 |
| [19] |
FRANCO O E, SHAW A K, STRAND D W, HAYWARD S W. Cancer associated fibroblasts in cancer pathogenesis[J]. Semin Cell Dev Biol, 2010, 21: 33-39. DOI:10.1016/j.semcdb.2009.10.010 |
| [20] |
MACE T A, AMEEN Z, COLLINS A, WOJCIK S, MAIR M, YOUNG G S, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner[J]. Cancer Res, 2013, 73: 3007-3018. DOI:10.1158/0008-5472.CAN-12-4601 |
| [21] |
ZHOU J, NEFEDOVA Y, LEI A, GABRILOVICH D. Neutrophils and PMN-MDSC:their biological role and interaction with stromal cells[J]. Semin Immunol, 2018, 35: 19-28. DOI:10.1016/j.smim.2017.12.004 |
 2019, Vol. 40
2019, Vol. 40


