2. 解放军疾病预防控制中心, 北京 100071;
3. 海军军医大学(第二军医大学)基础医学院病原生物学教研室, 上海 200433
2. Center for Disease Control and Prevention of PLA, Beijing 100071, China;
3. Department of Pathogen Biology, College of Basic Medical Sciences, Naval Medical University(Second Military Medical University), Shanghai 200433, Chinas
白纹伊蚊(Aedes albopictus)在全球广泛分布[1-2],可传播登革热、基孔肯雅热和寨卡病毒病等多种蚊媒传染病[3-5]。世界卫生组织估计全球约有25亿人口受登革热威胁,每年超过1亿人感染登革病毒[6]。近年,我国的登革热疫情形势严峻,2014年仅广东省的登革热病例就超过45 000例[7]。化学杀虫剂是主要的蚊媒控制措施,拟除虫菊酯类(以下简称菊酯类)是应用广泛、用量最多的杀虫剂,已导致我国多地的白纹伊蚊种群对其产生不同程度的抗性,包括山东、上海、浙江、海南、广东等多个省(市/自治区)[8]。部分地区的白纹伊蚊抗性水平已十分显著,如海口市花卉市场的白纹伊蚊对溴氰菊酯的抗性倍数高达436倍、对氯菊酯的抗性倍数达182倍[9-10]。
研究显示代谢解毒酶活性增加可加速杀虫剂的代谢,是蚊虫抗性产生的重要机制之一,称为代谢抗性[11]。代谢抗性与多种代谢解毒酶有关,如谷胱甘肽S-转移酶(glutathione S-transferase,GST)、多功能氧化酶(mixed function oxidase,MFO)和非特异性酯酶(nonspecific esterase,NSE)等[12-14]。GST是多功能解毒酶系,在昆虫中参与许多内外源有毒物质的代谢,已证实GST参与多种蚊虫对有机磷类、菊酯类和有机氯类等杀虫剂的抗性产生[15]。MFO由细胞色素P450(cytochrome P450,CYP450)介导,是参与各类杀虫剂以及其他内外源化合物代谢的主要解毒酶系,可使杀虫剂活性降低甚至失去杀虫活性。CYP450氧化酶系属亚铁血红素-硫醇盐蛋白超家族,对菊酯类杀虫剂产生抗性的个体较敏感个体的CYP450活性和含量均有不同程度的增加[14]。
击倒抗性(knockdown resistance,kdr)是由于杀虫剂的作用靶点出现突变而导致蚊虫对其敏感性下降。研究证实,蚊虫神经细胞膜上电压门控钠通道(voltage-gated sodium channel,VGSC)编码基因突变,引起钠通道构型变化,导致对双对氯苯基三氯乙烷(dichlorodiphenyltrichloroethane,DDT)和菊酯类杀虫剂产生抗药性[16-17]。本课题组前期研究结果显示,kdr基因的F1534S突变与白纹伊蚊对菊酯类杀虫剂的抗性呈正相关,而I1532T突变与白纹伊蚊对溴氰菊酯的抗性呈负相关[18]。
目前白纹伊蚊对菊酯类杀虫剂抗性与代谢解毒酶活性的关系尚不清楚,多种机制对抗性产生的关系也不甚明确。本研究测定了白纹伊蚊现场抗性种群和敏感品系的GST和MFO活性,分析其活性变化与抗药性表型的相关性,并综合考量代谢抗性与kdr机制在抗性形成中的贡献,为阐明白纹伊蚊对菊酯类杀虫剂抗性产生和发展的分子机制积累数据。
1 材料和方法 1.1 试虫白纹伊蚊现场种群于2017年8月至9月分别采自山东省济南市千佛山公园(JN)、浙江省杭州市上茅家埠(HZ)、上海市宝山区宝山六村(BS)、上海市杨浦区共青森林公园(YP)和海南省海口市美兰区居民区(HK),上述5个现场种群均为对溴氰菊酯和氯菊酯已产生抗性的种群[18]。在孳生地采集幼虫或蛹带回养虫室,常规方法饲养至成蚊,以羽化后3~5 d的F0代未吸血雌蚊作为未接触过杀虫剂的试虫,将对杀虫剂(溴氰菊酯、氯菊酯)敏感性的个体作为接触杀虫剂后的试虫。白纹伊蚊敏感品系(JS)于2004年引自江苏省寄生虫病防治研究所,连续14年饲养于海军军医大学(第二军医大学)海军医学系热带病学教研室,期间未暴露于任何杀虫剂。
1.2 主要试剂BCA蛋白含量测定试剂盒、L-还原型谷胱甘肽(glutathione,GSH)、1-氯-2, 4-二硝基苯(1-chloro-2, 4-dinitrobenzene,CDNB)购自美国Sigma-Aldrich公司,3, 3’, 5, 5’-四甲基联苯胺(3, 3’, 5, 5’-tetramethylbenzidine,TMBZ)购自美国Amresco公司,细胞色素C(cytochrome C,Cyt-C)标准品购自中国药品生物制品检定所,丙酮、乙醚购自国药集团化学试剂有限公司。
1.3 酶活性测定参照文献[19-20]方法,用蚊虫头部测定酶活性。冰上取单只雌蚊头部,加入150 μL ddH2O,手动研磨约20 s,4 ℃ 12 000×g离心2 min,取上清作为酶原备用。每组样品不少于40只雌蚊,每个样品设置3个复孔测试;同一批比较的样品,同一批次进行测定。
1.3.1 蛋白质含量取25 μL酶原、200 μL BCA溶液加入酶标板,37 ℃反应30 min,室温放置5 min,测定562 nm波长处的光密度(D)值(D562),按蛋白质含量测定试剂盒说明建立标准曲线,计算样品的蛋白质含量(mg/mL)。
1.3.2 GST活性取10 μL酶原、200 μL GSH/CDNB工作液(125 μL 63 mmol/L CDNB溶液加入2.5 mL 10 mmol/L GSH溶液中)加入酶标板,立即测定340 nm波长处的D值(D340);室温放置20 min,再次测定D340[21]。用CDNB的消光系数(5.76 mmol/L)将D值转换成酶活力值[21],GST活力根据以下公式计算:GST [nmol/(min·mg)]=[(DT20-DT0)×稀释倍数×1 000]/[20 min×5.76 mmol/L×蛋白质含量(mg/mL)]。其中,DT20为20 min时测定的D340,DT0为0 min时测定的D340。
1.3.3 MFO活性取20 μL酶原、80 μL 0.625 mol/L pH 7.2的磷酸钾溶液、200 μL TMBZ工作液(0.01 g TMBZ溶于5 mL甲醇和15 mL 0.25 mol/L pH 5.0的醋酸钠缓冲液)和25 μL 3% H2O2加入酶标板,混匀后室温放置2 h后测定650 nm波长处的D值(D650)。用Cyt-C标准品建立标准曲线,计算体系中Cyt-C的含量,MFO酶活力根据以下公式计算:MFO(nmol/mg)=[Cyt-C(μg)×106]/[13 000 g/mol×样本体积(μg)×蛋白质含量(mg/mL)]。
1.4 白纹伊蚊现场种群酶活性比较取白纹伊蚊BS和HK种群未接触杀虫剂和接触杀虫剂后的试虫测定其GST和MFO活性,比较接触杀虫剂后酶活性的变化;取白纹伊蚊现场种群接触杀虫剂后的试虫测定其GST和MFO活性,并与敏感品系的酶活性基线进行比较。
1.5 白纹伊蚊对菊酯类杀虫剂抗性产生的分子机制贡献率基于GST和MFO的酶活性数据,以及相同试虫的kdr突变(I1532和F1534)数据(kdr突变数据详见文献[18]),采用分类回归树方法(classification and regression tree,CART)分析GST和MFO、kdr的I1532和F1534突变对白纹伊蚊菊酯类杀虫剂抗性产生的相对贡献率,对计算的数据进行标化后制图[22]。
1.6 统计学处理应用SPSS 20.0软件进行数据分析,用GraphPad Prism 7软件绘图。将所有的酶活性数据进行正态分布检验。数据以x±s表示,酶活性基线比较用单因素方差分析;各种群的酶活性比较用两独立样本t检验,如方差不齐则采用t’检验。检验水准(α)为0.05。
2 结果 2.1 白纹伊蚊的酶活性检测结果白纹伊蚊敏感品系的GST和MFO活性分别为(552.323 1±149.103 8)nmol/(min • mg)和(0.209 3±0.063 5)nmol/mg;现场种群BS和HK的GST活性分别为(315.103 8±103.301 6)nmol/(min • mg)和(409.162 8±108.060 9)nmol/(min • mg),MFO活性分别为(0.113 6±0.046 3)nmol/mg和(0.178 2±0.058 5)nmol/mg。白纹伊蚊实验室敏感品系的GST和MFO活性均高于现场BS和HK抗性种群(P均<0.01)。
2.2 白纹伊蚊现场种群接触杀虫剂后酶活性与未接触杀虫剂的基线比较白纹伊蚊BS现场种群接触溴氰菊酯后的GST和MFO活性与未接触杀虫剂的基线比较,差异均无统计学意义(P>0.05);接触氯菊酯后GST活性升高(P均<0.05)、MFO活性也升高(P<0.01),见图 1。
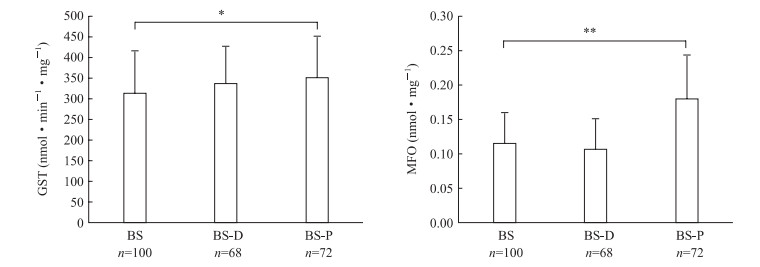
|
图 1 白纹伊蚊BS现场种群接触杀虫剂后的酶活性与未接触杀虫剂的基线比较 Fig 1 Comparison of enzyme activities of Aedes albopictus BS field population before and after exposure to insecticides GST: Glutathione S-transferase; BS: Baoshan Sixth Village, Baoshan District, Shanghai; D: Deltamethrin; P: Permethrin; MFO: Mixed function oxidase; Cyt-C: Cytochrome C. *P < 0.05, **P < 0.01. x±s |
与白纹伊蚊HK现场种群未接触杀虫剂的基线比较,接触溴氰菊酯后GST活性差异无统计学意义(P>0.05)、MFO活性升高(P<0.01);接触氯菊酯后GST和MFO活性差异均无统计学意义(P均>0.05),见图 2。
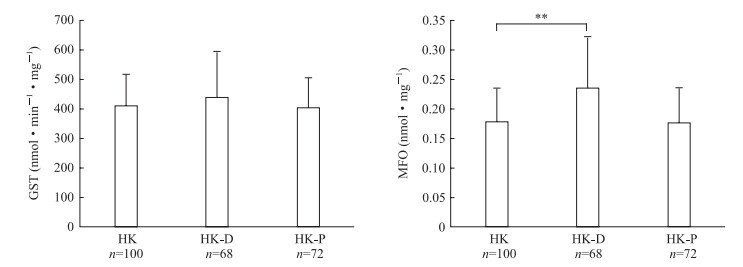
|
图 2 白纹伊蚊HK现场种群接触杀虫剂后的酶活性与未接触杀虫剂的基线比较 Fig 2 Comparison of enzyme activities of Aedes albopictus HK field population before and after exposure to insecticides GST: Glutathione S-transferase; HK: Meilan District Residential Area, Haikou City, Hannan Province; D: Deltamethrin; P: Permethrin; MFO: Mixed function oxidase; Cyt-C: Cytochrome C. **P < 0.01. x±s |
2.3 白纹伊蚊现场种群接触杀虫剂后酶活性与敏感品系基线的比较
见图 3,白纹伊蚊5个现场种群接触溴氰菊酯后,除YP种群GST活性与敏感品系基线比较差异有统计学意义(P<0.05)外,其他种群GST活性与敏感品系基线比较差异均无统计学意义(P均>0.05);JN、YP和HK种群的MFO活性高于敏感品系基线(P<0.05,P<0.01),而HZ和BS种群MFO活性低于敏感品系基线,但差异均无统计学意义(P均>0.05)。
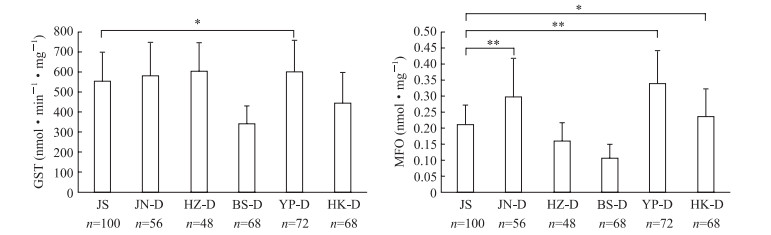
|
图 3 白纹伊蚊现场种群接触溴氰菊酯后的酶活性与敏感品系基线比较 Fig 3 Comparison of enzyme activities of Aedes albopictus between field population exposure to deltamethrin (D) and the susceptible strain GST: Glutathione S-transferase; JS: The Aedes albopictus susceptible strain; JN: Qianfoshan Park, Jinan City, Shandong Province; HZ: Shangmaojiabu, Hangzhou City, Zhejiang Province; BS: Baoshan Sixth Village, Baoshan District, Shanghai; YP: Gongqing Forest Park, Yangpu District, Shanghai; HK: Meilan District Residential Area, Haikou City, Hannan Province; MFO: Mixed function oxidase. *P < 0.05, **P < 0.01. x±s |
见图 4,白纹伊蚊5个现场种群接触氯菊酯后,GST活性与敏感品系的基线比较均下降,但差异均无统计学意义(P均>0.05);MFO活性除JN种群升高外(P<0.05),其他种群均呈下降趋势,但差异均无统计学意义(P均>0.05)。
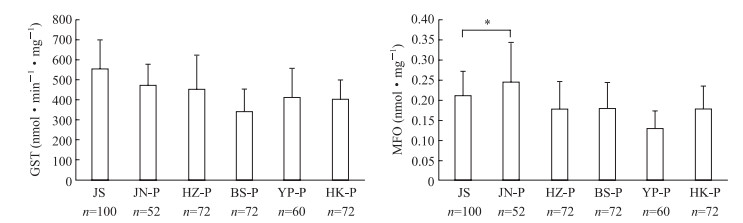
|
图 4 白纹伊蚊现场种群接触氯菊酯后的酶活性与敏感品系基线比较 Fig 4 Comparison of enzyme activities of Aedes albopictus between field population exposure GST: Glutathione S-transferase; JS: The Aedes albopictus susceptible strain; JN: Qianfoshan Park, Jinan City, Shandong Province; HZ: Shangmaojiabu, Hangzhou City, Zhejiang Province; BS: Baoshan Sixth Village, Baoshan District, Shanghai; YP: Gongqing Forest Park, Yangpu District, Shanghai; HK: Meilan District Residential Area, Haikou City, Hannan Province; MFO: Mixed function oxidase. *P < 0.05. x±s |
2.4 代谢解毒酶活性和kdr突变对抗性产生的贡献率
将所有接触过杀虫剂的生物测定个体合并后进行CART分析,结果显示白纹伊蚊对溴氰菊酯的抗性形成中,GST活性和kdr F1534突变的贡献率较大,其次是MFO活性,kdr I1532突变贡献率最小;白纹伊蚊对氯菊酯的抗性形成中,kdr F1534突变的贡献率最大,其次是GST和MFO活性,kdr I1532突变无贡献(图 5)。
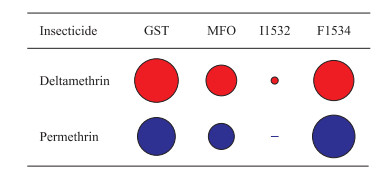
|
图 5 代谢解毒酶活性和kdr突变在白纹伊蚊对菊酯类杀虫剂抗性产生中的贡献率 Fig 5 Contribution rates of metabolic detoxification enzyme activity and kdr mutations to resistance formation of Aedes albopictus against pyrethroids kdr: Knockdown resistance; GST: Glutathione S-transferase; MFO: Mixed function oxidase |
分别分析各白纹伊蚊现场种群酶活性和kdr突变在溴氰菊酯和氯菊酯抗性产生中的贡献率,因个别种群个体数未达到软件计算的最低数量要求,因此针对溴氰菊酯抗性种群JN、BS、HZ、HK以及氯菊酯抗性种群BS、HZ、YP、HK进行了分析。结果(图 6)显示,除HK种群外,其他种群酶活性变化均起主要的贡献,kdr突变的作用较小;在HK种群中,kdr F1534突变是氯菊酯抗性形成的主要机制,其在溴氰菊酯抗性形成中也有一定的贡献。
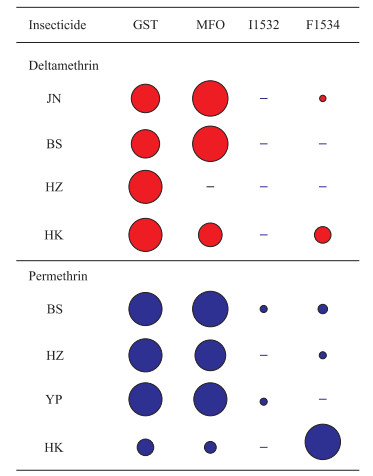
|
图 6 代谢解毒酶活性和kdr突变在白纹伊蚊不同抗性种群菊酯类杀虫剂抗性产生中的贡献率 Fig 6 Contribution rates of metabolic detoxification enzyme activity and kdr mutations to resistance formation of different Aedes albopictus resistant populations against pyrethroids kdr: Knockdown resistance; GST: Glutathione S-transferase; MFO: Mixed function oxidases; JN: Qianfoshan Park, Jinan City, Shandong Province; BS: Baoshan Sixth Village, Baoshan District, Shanghai; HZ: Shangmaojiabu, Hangzhou City, Zhejiang Province; HK: Meilan District Residential Area, Haikou City, Hannan Province; YP: Gongqing Forest Park, Yangpu District, Shanghai |
3 讨论
本研究结果显示,白纹伊蚊敏感品系代谢解毒酶GST和MFO活性基线水平高于现场抗性种群,接触杀虫剂后现场种群的酶活性与其基线相比呈升高趋势,但与敏感品系基线相比酶活性变化却无明显规律,提示代谢解毒酶活性变化与菊酯类杀虫剂抗性的相关性并不密切,白纹伊蚊对菊酯类杀虫剂抗性的产生有多个机制参与,酶活性与kdr突变均发挥了重要作用,但在不同种群其贡献率存在差异。
代谢抗性是蚊虫对菊酯类杀虫剂抗性产生的重要机制之一[15]。研究表明,中华按蚊(Anopheles sinensis)、催命按蚊(Anopheles funestus)等对菊酯类杀虫剂的抗性与GST和MFO活性呈正相关[22-23]。而白纹伊蚊的菊酯类抗性产生机制中,代谢解毒酶活性也起到一定作用[24-26]。李袁飒等[24]发现,白纹伊蚊的GST活性与溴氰菊酯抗性呈正相关;而吴家红等[25]认为CYP450单加氧酶活性升高是溴氰菊酯抗性产生的主要机制,而GST活性变化不是其主要机制。上述研究中白纹伊蚊采集地区和敏感品系均不同,抗性和酶活性测定的蚊虫阶段也不同,这可能是导致结论不同的重要原因。本研究发现,不同白纹伊蚊抗性种群的酶活性基线有显著差异,敏感种群基线反而高于部分现场种群,该现象与通常理解的抗性越高酶活性越高的观念相悖。然而,本研究经多次重复实验均观察到相同的结果,提示各种群的酶活性基线水平存在差异,这可能与白纹伊蚊种群的遗传背景、杀虫剂接触历史和气候条件等因素有关。因此,以敏感种群基线作为参照的GST和MFO活性比较方法不适合作为检测白纹伊蚊种群对菊酯类杀虫剂抗性的单因素标志。
溴氰菊酯和氯菊酯分属2种类型的拟除虫菊酯,前者属Ⅱ型拟除虫菊酯(不含α-氰基),后者是Ⅰ型拟除虫菊酯(含α-氰基)。研究表明,2种类型的拟除虫菊酯的中毒症状大相径庭[27]。Ⅰ型主要影响交感神经,导致钠离子通道延长开放,造成神经细胞轴突持续放电,产生全身抽搐、微颤、过度兴奋等震颤现象;而Ⅱ型多作用于运动神经,导致钠离子通道延长开放,造成膜去极化过度,使运动失调或流涎,出现痉挛、麻痹等[28]。不同类型菊酯类杀虫剂对蚊虫的作用机制不同,其抗性产生的机制也有差异。多项研究表明,蚊虫对杀虫剂产生抗性是多种机制共同作用的结果[11, 29],本研究结果显示白纹伊蚊也是如此。代谢抗性机制与kdr机制在2种类型菊酯类杀虫剂抗性产生中的贡献率不同,对溴氰菊酯(Ⅱ型)抗性产生的贡献率顺序是GST>kdr(F1534)>MFO>kdr(I1532),而对氯菊酯(Ⅰ型)抗性产生的贡献率顺序为kdr(F1534)>GST>MFO。本研究提示在氯菊酯抗性产生的机制中,kdr起主要作用,与既往研究结果[30-31]一致;而对溴氰菊酯的抗性则是代谢机制与kdr机制共同作用的结果。
代谢抗性与kdr可能是白纹伊蚊对菊酯类杀虫剂抗性产生和发展中相互协同的2种机制,如白纹伊蚊HK种群的抗性水平高于其他种群[18],kdr突变在该种群中的贡献率尤为突出。海口市全年温暖潮湿,白纹伊蚊HK种群每年可繁殖多代,种群数量大,推测HK种群在接触菊酯类杀虫剂的初期,酶活性升高是对杀虫剂抗性产生的主要机制;而在杀虫剂选择压力下,kdr突变的个体被筛选,经过数代积累突变的个体逐渐在种群占据优势,此时kdr机制为主要作用,种群中的酶活性较初期无明显升高,甚至反而有所下降。其他种群尚未出现上述特点,可能与杀虫剂使用、种群数量等有关。然而,当前的研究结果还不足以完全支持该结论,未来还需要长时间连续观察更多的种群进一步验证。
志谢 本课题在研究过程中,得到诸多老师的支持与帮助,在此表示诚挚的谢意!浙江省疾病预防控制中心姚立农老师,中国疾病预防控制中心寄生虫病防治所张丽老师,海南省海口市疾病预防控制中心林怡、陈学文和蔡芳老师等协助采集现场标本;美国疾病预防控制中心顾卫东教授协助CART数据分析。
| [1] |
BONIZZONI M, GASPERI G, CHEN X, JAMES A A. The invasive mosquito species Aedes albopictus:current knowledge and future perspectives[J]. Trends Parasitol, 2013, 29: 460-468. DOI:10.1016/j.pt.2013.07.003 |
| [2] |
杨舒然, 刘起勇. 白纹伊蚊的全球分布及扩散趋势[J]. 中国媒介生物学及控制杂志, 2013, 24: 1-4. |
| [3] |
GUO C, ZHOU Z, WEN Z, LIU Y, ZENG C, XIAO D, et al. Global epidemiology of dengue outbreaks in 1990-2015: a systematic review and meta-analysis[J/OL]. Front Cell Infect Microbiol, 2017, 7: 317. doi: 10.3389/fcimb.2017.00317.
|
| [4] |
LOUNIBOS L P, KRAMER L D. Invasiveness of Aedes aegypti and Aedes albopictus and vectorial capacity for chikungunya virus[J]. J Infect Dis, 2016, 214(Suppl 5): S453-S458. DOI:10.1093/infdis/jiw285 |
| [5] |
EPELBOIN Y, TALAGA S, EPELBOIN L, DUSFOUR I. Zika virus:an updated review of competent or naturally infected mosquitoes[J]. PLoS Negl Trop Dis, 2017, 11: e0005933. DOI:10.1371/journal.pntd.0005933 |
| [6] |
KULARATNE S A. Dengue fever[J]. BMJ, 2015, 351: h4661. |
| [7] |
LI M T, SUN G Q, YAKOB L, ZHU H P, JIN Z, ZHANG W Y. The driving force for 2014 dengue outbreak in Guangdong, China[J/OL]. PLoS One, 2016, 11: e0166211. doi: 10.1371/journal.pone.0166211.
|
| [8] |
孟凤霞, 王义冠, 冯磊, 刘起勇. 我国登革热疫情防控与媒介伊蚊的综合治理[J]. 中国媒介生物学及控制杂志, 2015, 26: 4-10. DOI:10.11853/j.issn.1003.4692.2015.01.002 |
| [9] |
王晓花, 陈辉莹, 杨新艳, 林怡, 蔡芳, 钟汶兵, 等. 海口市白纹伊蚊对菊酯类杀虫剂的抗药性及击倒抗性基因突变分析[J]. 第二军医大学学报, 2015, 36: 832-838. WANG X H, CHEN H Y, YANG X Y, LIN Y, CAI F, ZHONG W B, et al. Resistance to pyrethroid insecticides and analysis of knockdown resistance (kdr) gene mutations in Aedes albopictus from Haikou City[J]. Acad J Sec Mil Med Univ, 2015, 36: 832-838. |
| [10] |
曾林海, 孙定炜, 赵伟, 李善干, 杨霞. 海南省埃及伊蚊和白纹伊蚊对常用杀虫剂的敏感性测定[J]. 中国媒介生物学及控制杂志, 2010, 21: 148-149. |
| [11] |
SMITH L B, KASAI S, SCOTT J G. Pyrethroid resistance in Aedes aegypti and Aedes albopictus:important mosquito vectors of human diseases[J]. Pestic Biochem Physiol, 2016, 133: 1-12. DOI:10.1016/j.pestbp.2016.03.005 |
| [12] |
HEMINGWAY J, HAWKES N J, MCCARROLL L, RANSON H. The molecular basis of insecticide resistance in mosquitoes[J]. Insect Biochem Mol Biol, 2004, 34: 653-665. DOI:10.1016/j.ibmb.2004.03.018 |
| [13] |
PETHUAN S, JIRAKANJANAKIT N, SAENGTHARATIP S, CHAREONVIRIYAPHAP T, KAEWPA D, RONGNOPARUT P. Biochemical studies of insecticide resistance in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera:Culicidae) in Thailand[J]. Trop Biomed, 2007, 24: 7-15. |
| [14] |
ISHAK I H, RIVERON J M, IBRAHIM S S, STOTT R, LONGBOTTOM J, IRVING H, et al. The cytochrome P450 gene CYP6P12 confers pyrethroid resistance in kdr-free Malaysian populations of the dengue vector Aedes albopictus[J/OL]. Sci Rep, 2016, 6: 24707. doi: 10.1038/srep24707.
|
| [15] |
史琦琪, 程鹏, 公茂庆. 蚊虫抗药性分子机制研究进展[J]. 中国媒介生物学及控制杂志, 2016, 27: 515-519. DOI:10.11853/j.issn.1003.8280.2016.05.028 |
| [16] |
WILLIAMSON M S, MARTINEZ-TORRES D, HICK C A, DEVONSHIRE A L. Identification of mutations in the housefly para-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroid insecticides[J]. Mol Gen Genet, 1996, 252(1/2): 51-60. |
| [17] |
SAAVEDRA-RODRIGUEZ K, URDANETA-MARQUEZ L, RAJATILEKA S, MOULTON M, FLORES A E, FERNANDEZ-SALAS I, et al. A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti[J]. Insect Mol Biol, 2007, 16: 785-798. |
| [18] |
GAO J P, CHEN H M, SHI H, PENG H, MA Y J. Correlation between adult pyrethroid resistance and knockdown resistance (kdr) mutations in Aedes albopictus (Diptera: Culicidae) field populations in China[J/OL]. Infect Dis Poverty, 2018, 7: 86. doi: 10.1186/s40249-018-0471-y.
|
| [19] |
BROGDON W G, BARBER A M. Microplate assay of glutathione S-transferase activity for resistance detection in single-mosquito triturates[J]. Comp Biochem Physiol B, 1990, 96: 339-342. DOI:10.1016/0305-0491(90)90385-7 |
| [20] |
POLSON K A, RAWLINS S C, BROGDON W G, CHADEE D D. Characterisation of DDT and pyrethroid resistance in Trinidad and Tobago populations of Aedes aegypt[J]. Bull Entomol Res, 2011, 101: 435-441. DOI:10.1017/S0007485310000702 |
| [21] |
AÏKPON R, SÈZONLIN M, OSSÈ R, AKOGBÉTO M. Evidence of multiple mechanisms providing carbamate and organophosphate resistance in field An. gambiae population from Atacora in Benin[J/OL]. Parasit Vectors, 2014, 7: 568. doi: 10.1186/s13071-014-0568-5.
|
| [22] |
CHANG X, ZHONG D, FANG Q, HARTSEL J, ZHOU G, SHI L, et al. Multiple resistances and complex mechanisms of Anopheles sinensis mosquito: a major obstacle to mosquito-borne diseases control and elimination in China[J/OL]. PLoS Negl Trop Dis, 2014, 8: e2889. doi: 10.1371/journal.pntd.0002889.
|
| [23] |
SANGBA M L, DEKETRAMETE T, WANGO S P, KAZANJI M, AKOGBETO M, NDIATH M O. Insecticide resistance status of the Anopheles funestus population in Central African Republic: a challenge in the war[J/OL]. Parasit Vectors, 2016, 9: 230. doi: 10.1186/s13071-016-1510-9.
|
| [24] |
李袁飒, 孟凤霞, 蔡松武, 刘起勇. 广东省湛江市抗溴氰菊酯白纹伊蚊代谢酶活性及其特征研究[J]. 中国媒介生物学及控制杂志, 2013, 24: 103-107. |
| [25] |
吴家红, 赵彤言, 董言德. 五株白纹伊蚊对溴氰菊酯抗性生物测定和生化检测的研究[J]. 中国人兽共患病杂志, 2005, 21: 851-854. |
| [26] |
NGOAGOUNI C, KAMGANG B, BRENGUES C, YAHOUEDO G, PAUPY C, NAKOUNÉ E, et al. Susceptibility profile and metabolic mechanisms involved in Aedes aegypti and Aedes albopictus resistant to DDT and deltamethrin in the Central African Republic[J/OL]. Parasit Vectors, 2016, 9: 599. doi: 10.1186/s13071-016-1887-5.
|
| [27] |
VERSCHOYLE R D, ALDRIDGE W N. Structureactivity relationships of some pyrethroids in rats[J]. Arch Toxicol, 1980, 45: 325-329. DOI:10.1007/BF00293813 |
| [28] |
NARAHASHI T. Neuronal ion channels as the target sites of insecticides[J]. Pharmacol Toxicol, 1996, 79: 1-14. DOI:10.1111/bcpt.1996.79.issue-1 |
| [29] |
LIU N. Insecticide resistance in mosquitoes:impact, mechanisms, and research directions[J]. Annu Rev Entomol, 2015, 60: 537-559. DOI:10.1146/annurev-ento-010814-020828 |
| [30] |
ISHAK I H, JAAL Z, RANSON H, WONDJI C S. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia[J/OL]. Parasit Vectors, 2015, 8: 181. doi: 10.1186/s13071-015-0797-2.
|
| [31] |
KUSHWAH R B, MALLICK P K, RAVIKUMAR H, DEV V, KAPOOR N, ADAK T P, et al. Status of DDT and pyrethroid resistance in Indian Aedes albopictus and absence of knockdown resistance (kdr) mutation[J]. J Vector Borne Dis, 2015, 52: 95-98. |
 2019, Vol. 40
2019, Vol. 40


