2. 海军军医大学(第二军医大学)基础医学院生理学教研室, 上海 200433;
3. 解放军东部战区海军医院重症医学科, 舟山 316000
2. Department of Physiology, College of Basic Medical Sciences, Naval Medical University(Second Military Medical University), Shanghai 200433, China;
3. Intensive Care Unit, Naval Hospital of PLA Eastern Theater Command, Zhoushan 316000, Zhejiang, Chinas
缺血性心脏病是临床最常见的疾病之一。心脏缺血/再灌注(ischemia/reperfusion,I/R)损伤会导致心肌细胞死亡、心脏泵血功能下降,最终发展为心力衰竭[1]。目前认为,心脏I/R过程中能生成大量活性氧(reactive oxygen species,ROS),进而激活氧化应激,加重心脏损伤[2]。心肌细胞内存在大量线粒体,线粒体不仅发挥能量供应作用,其电子传递系统也是ROS的主要来源[3]。当心脏I/R损伤时,线粒体产生的过氧化物导致线粒体应激,破坏线粒体的结构和功能,使线粒体通透性增加、ATP供应减少并释放凋亡相关蛋白,促进心肌细胞功能异常和凋亡[4-6]。
在众多心血管活性物质中,组织激肽释放酶(kallikrein,KLK)的作用日益受到关注。KLK是广泛存在于细胞中的一种蛋白酶,其经典作用是分解激肽原生成激肽类物质,后者通过与其受体结合在组织局部发挥广泛的生物学作用[7]。KLK包括15个亚型(KLK1~15),不同亚型KLK在结构和功能上非常相似[8]。目前对心血管系统中KLK的研究主要集中在KLK1,研究发现KLK1具有抗心脏I/R损伤作用,其机制与抗心肌细胞凋亡、促进心脏血管生成、抑制心肌细胞肥大和纤维化等有关[9-14]。但关于KLK1抗心脏I/R损伤的作用是否与其调节心肌细胞线粒体功能有关尚未见报道。本研究旨在探讨心脏I/R损伤中KLK1对线粒体功能的影响及相关分子机制。
1 材料和方法 1.1 实验动物成年雄性SD大鼠(8周龄,250~300 g)和新生SD大鼠(出生1~2 d)均购自海军军医大学(第二军医大学)实验动物中心[动物生产许可证号:SCXK(沪)2017-0002]。本研究中动物实验经海军军医大学(第二军医大学)动物伦理委员会批准。
1.2 大鼠心脏KLK1基因重组腺病毒感染效果鉴定参照文献[15]的方法进行KLK1基因重组腺病毒的构建、扩增、纯化、鉴定。将16只大鼠随机分为重组腺病毒组和对照组(n=8),重组腺病毒组大鼠通过在心脏左心室前壁注射纯化KLK1基因重组腺病毒[1×1011 PFU,PFU为噬菌斑形成单位(plaque forming unit)]实现KLK1过表达,对照组大鼠注射对照病毒(1×1011 PFU);共5个注射部位,每个部位注射50 μL。1周后处死大鼠取心脏组织,参照文献[16]方法提取心脏组织蛋白。通过蛋白质印迹法检测KLK1蛋白表达量。KLK1一抗购自英国Abcam公司,稀释比例为1:2 000;内参照β-actin一抗购自美国Sigma公司,稀释比例为1:10 000。以KLK1与β-actin表达量的比值计算KLK1相对表达量,验证感染效果。
1.3 大鼠心脏I/R损伤模型制备将24只大鼠随机分为假手术组、模型对照组和KLK1过表达组(n=8)。模型对照组和KLK1过表达组大鼠分别注射对照病毒和KLK1基因重组腺病毒,1周后制备心脏I/R损伤模型。心脏I/R损伤通过结扎冠状动脉左前降支1 h、再灌注2 h的方法实现[16],假手术组不进行结扎和再灌注操作。
1.4 大鼠心肌梗死区面积检测大鼠心脏再灌注结束后,再次结扎冠状动脉,并由股静脉注射2%伊文思蓝染料(0.4 mL/100 g体质量)以明确心肌缺血范围。非缺血区由于灌注了含有染料的血液而染成蓝色,未被染色的区域则为心肌缺血区。随后,从大鼠胸腔取出心脏,并从心尖至心房切成5个2 mm厚的横切片,通过TTC染色(37 ℃,15 min)观察心肌梗死区面积,未梗死心肌组织染成红色,而梗死心肌组织呈苍白色。以心肌梗死区面积与心肌缺血区面积的比值计算心肌相对梗死区面积。
1.5 大鼠心脏缺血危险区细胞凋亡检测取大鼠心脏缺血危险区的心肌组织,用多聚甲醛溶液固定,石蜡包埋切片,并进行TUNEL染色[16],同时对全部细胞核进行DAPI染色。显微镜下,凋亡细胞核被染成红色,全部细胞核被染成蓝色,以凋亡细胞核占全部细胞核的比值(TUNEL/DAPI)计算心肌细胞凋亡率。
1.6 大鼠心脏线粒体分离及线粒体功能检测取大鼠心脏缺血危险区心肌组织,采用线粒体分离试剂盒(上海碧云天生物技术公司)分离线粒体。(1)线粒体过氧化物生成量检测:将MitoSOX(终浓度为5 μmol/L)以二甲基亚砜(dimethyl sulfoxide,DMSO)溶解后加入线粒体悬液,30 min后以100 μL HEPES缓冲液(10 mmol/L HEPES,pH 7.4,150 mmol/L NaCl,5 mmol/L KCl,1 mmol/L MgCl2,1.8 mmol/L CaCl2)重悬,检测荧光值。(2)线粒体膜电位检测:向线粒体悬液内加入2 μmol/L JC-1,孵育15 min,检测荧光值,以绿色荧光与红色荧光的比值估计线粒体的损伤程度。(3)线粒体ATP生成量检测:向线粒体悬液中加入蛋白提取液并匀浆,离心(10 000×g,10 min)取上清,使用ATP生物发光试剂盒(上海碧云天生物技术公司)检测上清液中ATP浓度[17]。
1.7 新生大鼠心肌细胞培养与处理从新生SD大鼠(出生1~2 d)心室组织分离心肌细胞[17]。在组织裂解液中将心室剪成小块(1 mm3)。随后在37 ℃水浴摇床中以组织消化液处理心脏组织块,每次10 min,并收集消化后的上清液(细胞悬液);对未被消化的组织块进行下一轮消化,重复4~5次。将收集的细胞悬液离心(3 000×g,5 min)取沉淀;随后以细胞培养液重悬,并移至培养瓶中培养1 h(37 ℃),使非心肌细胞贴壁。取培养瓶中的上清,即为纯化的心肌细胞。以1×105/cm2的密度将心肌细胞接种于培养板进行后续实验。组织裂解液(pH 7.35)成分:116 mmol/L NaCl,20 mmol/L HEPES,0.8 mmol/L Na2HPO4,5.6 mmol/L葡萄糖,5.4 mmol/L KCl,0.8 mmol/L MgSO4;组织消化液成分:裂解液、0.1%胰蛋白酶、0.05% Ⅱ型胶原酶;细胞培养液成分:10%胎牛血清,DMEM培养液,15 mmol/L HEPES,0.1 mmol/L BrdU。
1.8 KLK1过表达对大鼠心肌细胞缺氧/复氧(hypoxia/reoxygenation,H/R)损伤后线粒体功能的影响及受体机制分析新生大鼠心肌细胞培养48 h后,加入KLK1基因重组腺病毒(感染复数为10)感染24 h实现KLK1 过表达[15];对照组细胞感染对照病毒。病毒感染完成后,诱导大鼠心肌细胞H/R损伤。在低氧培养箱(94% N2,5% CO2,1% O2)中培养24 h以实现缺氧,随后在正常培养条件下培养2 h进行复氧。通过MTT法检测细胞活力[18]。为探讨H/R处理后KLK1调节心肌细胞线粒体功能的受体机制,在细胞缺氧开始时加入缓激肽1型受体(bradykinin receptor type 1,B1R)阻断剂R715或缓激肽2型受体(bradykinin receptor type 2,B2R)阻断剂HOE140,观察线粒体功能的变化。R715和HOE140购自美国Tocris公司,工作浓度为10-6 mol/L。
1.9 统计学处理应用SPSS 13.0软件进行统计学分析。数据用x±s表示。多组数据之间的比较采用单因素方差分析,多重比较采用最小显著差异(least significant difference,LSD)检验;两组数据之间的比较采用独立样本t检验。检验水准(α)为0.05。
2 结果 2.1 KLK1 过表达减轻大鼠心脏I/R损伤通过重组腺病毒心脏局部注射的方式实现了大鼠心脏KLK1蛋白过表达,结果见图 1。
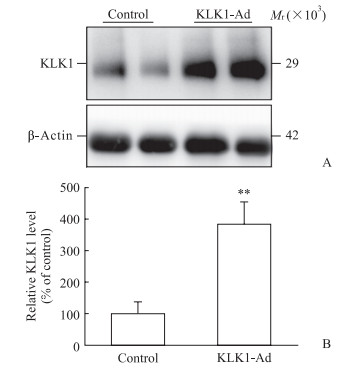
|
图 1 KLK1重组腺病毒感染后大鼠心脏KLK1蛋白表达结果 Fig 1 Cardiac KLK1 protein expression of rats infected with KLK1-Ad KLK1: Kallikrein 1; KLK1-Ad: KLK1 recombinant adenovirus. **P < 0.01 vs control group. n=8, x±s |
通过结扎冠状动脉左前降支1 h、再灌注2 h的方法造成大鼠急性心脏I/R损伤,并立即检测心脏损伤情况。与I/R组相比,KLK1过表达组大鼠心肌梗死区面积减少、心脏缺血危险区心肌细胞凋亡率降低(P均<0.01,图 2)。提示KLK1过表达具有抗心脏I/R损伤作用。
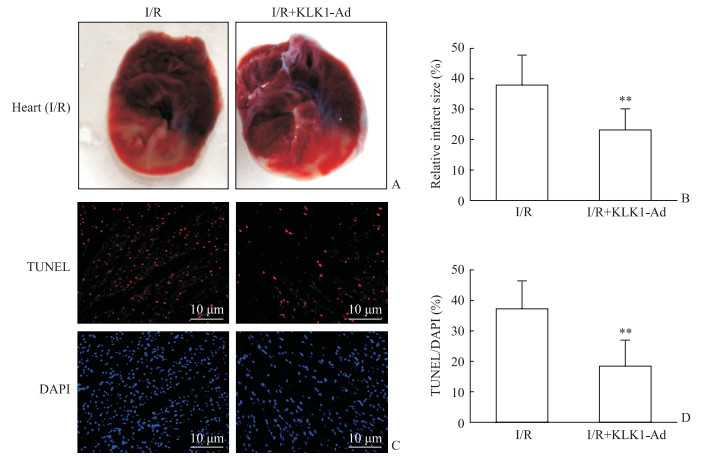
|
图 2 KLK1过表达减轻大鼠心脏I/R损伤 Fig 2 KLK1 overexpression mitigating cardiac I/R injury in vivo in rats A: Representative sections showing the injured heart: remote area (non-ischemic area, stained blue), ischemic risk area (stained red) and infarct area (pale); B: Quantified data of the relative infarct size (infarct area/ischemic area); C: Representative TUNEL staining images showing the apoptotic nuclei (TUNEL positive, red) and total nuclei (DAPI, blue) in ischemic risk area; D: Percentage of TUNEL positive nuclei (TUNEL/DAPI). KLK1: Kallikrein 1; I/R: Ischemia/reperfusion; KLK1-Ad: KLK1 recombinant adenovirus infection; TUNEL: Terminal dexynucleotidyl transferase-mediated dUTP nick end labeling; DAPI: 4', 6-Diamidino-2-phenylindole. **P < 0.01 vs I/R group. n=8, x±s |
2.2 KLK1过表达减轻大鼠心脏I/R损伤后线粒体功能障碍
大鼠心脏I/R损伤后,在心脏缺血危险区分离线粒体,检测线粒体功能相关指标。结果显示,与I/R组相比,KLK1过表达组心脏线粒体过氧化物生成量降低、膜电位增加、ATP生成量增加(P均<0.01,图 3)。提示KLK1过表达能减轻大鼠心脏I/R损伤后线粒体功能障碍。
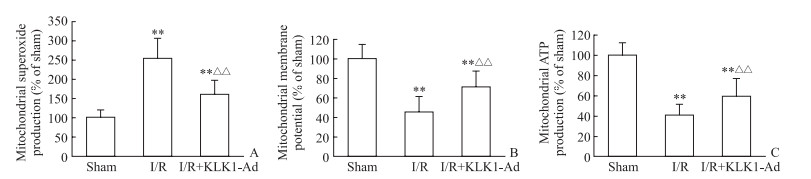
|
图 3 KLK1过表达减轻大鼠心脏I/R损伤后线粒体功能障碍 Fig 3 KLK1 overexpression mitigating mitochondrial dysfunction induced by cardiac I/R injury in vivo in rats A: Mitochondrial superoxide production; B: Mitochondrial membrane potential; C: Mitochondrial ATP production. KLK1: Kallikrein 1; I/R: Ischemia/reperfusion; KLK1-Ad: KLK1 recombinant adenovirus infection; ATP: Adenosine triphosphate. **P < 0.01 vs sham group; △△P < 0.01 vs I/R group. n=8, x±s |
2.3 KLK1过表达减轻大鼠离体心肌细胞H/R损伤和改善线粒体功能障碍
为进一步明确KLK1是否直接作用于心肌细胞发挥效应,分离新生大鼠心肌细胞,通过腺病毒感染细胞24 h实现心肌细胞KLK1过表达,随后进行H/R造成心肌细胞损伤。结果显示,与H/R组相比,KLK1过表达组心肌细胞活力提高(P<0.05,图 4A),同时线粒体过氧化物生成量减少(P<0.05)、膜电位增加(P<0.01)、ATP生成量增加(P<0.05,图 4B~4D)。提示KLK1可直接作用于大鼠心肌细胞改善线粒体功能障碍。
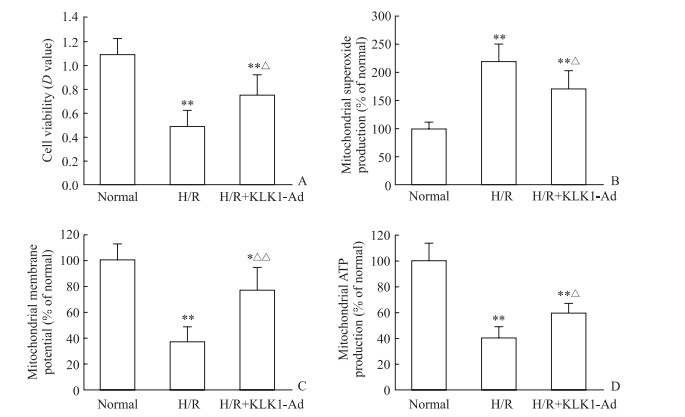
|
图 4 KLK1过表达减轻新生大鼠离体心肌细胞H/R损伤和改善线粒体功能障碍 Fig 4 KLK1 overexpression mitigating cardiomyocytes injury and mitochondrial dysfunction induced by H/R injury in vitro in neonatal rats A: Cell viability evaluated by MTT analysis; B: Mitochondrial superoxide production; C: Mitochondrial membrane potential; D: Mitochondrial ATP production. KLK1: Kallikrein 1; H/R: Hypoxia/reoxygenation; KLK1-Ad: KLK1 recombinant adenovirus infection; ATP: Adenosine triphosphate; MTT: 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide. *P < 0.05, **P < 0.01 vs normal group; △P < 0.05, △△P < 0.01 vs H/R group. n=5, x±s |
2.4 KLK1过表达改善心肌细胞H/R损伤后线粒体功能障碍的受体机制
为探讨KLK1调节心肌细胞线粒体功能的受体机制,观察了缓激肽受体阻断剂对KLK1作用的影响。结果显示,B2R阻断剂HOE140能完全抑制KLK1抗心肌细胞损伤的作用(图 5A),并完全阻断KLK1抗线粒体损伤的作用(图 5B~5D);而B1R阻断剂R715对KLK1抗心肌细胞损伤的作用没有影响(图 5A),也无法阻断其对线粒体功能的调节作用(图 5B~5D)。
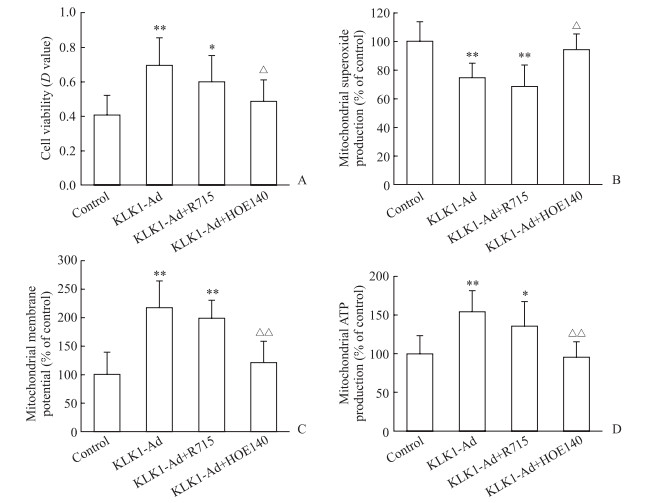
|
图 5 缓激肽受体阻断剂对KLK1改善新生大鼠离体心肌细胞H/R损伤后线粒体功能障碍的影响 Fig 5 Effect of bradykinin receptor antagonists on KLK1 in the mitigation of mitochondrial dysfunction induced by cardiomycytes H/R injury in vitro in neonatal rats A: Cell viability evaluated by MTT analysis; B: Mitochondrial superoxide production; C: Mitochondrial membrane potential; D: Mitochondrial ATP production. KLK1: Kallikrein 1; H/R: Hypoxia/reoxygenation; KLK1-Ad: KLK1 recombinant adenovirus infection; R715: Bradykinin receptor type 1 (B1R) antagonist; HOE140: Bradykinin receptor type 2 (B2R) antagonist; ATP: Adenosine triphosphate. *P < 0.05, **P < 0.01 vs control group; △P < 0.05, △△P < 0.01 vs KLK1-Ad group. n=5, x±s |
3 讨论
本研究结果显示KLK1能改善大鼠心脏I/R损伤后的线粒体功能障碍,并且其作用能被B2R受体阻断剂抑制,提示KLK1通过作用于心肌细胞的B2R进而抑制心脏I/R损伤、改善线粒体功能障碍。本研究结果进一步拓展了对KLK1心脏保护机制的认识。在心脏I/R损伤中,线粒体产生的过氧化物能导致线粒体应激,破坏线粒体结构和功能,最终促进心肌细胞功能异常和凋亡[4-6]。因此,其改善心脏I/R后的线粒体功能障碍可能是其减轻I/R后心脏损伤、抑制心肌细胞凋亡的重要机制。此外,有研究发现KLK1具有改善心脏I/R损伤后心脏重构的作用[19]。本研究发现KLK1可减轻大鼠心脏I/R损伤、增加存活的心肌组织,我们推测KLK1还可能通过其自身的抗I/R损伤作用减轻心脏损伤后期的心脏重构程度,提高心脏功能。
既往研究发现,KLK1抗心脏I/R损伤作用主要通过B2R实现,B2R阻断剂HOE140能完全阻断KLK1的心肌保护作用[9-11],本研究结果与此一致。KLK1可能通过2种机制激活B2R:(1)KLK1可通过分解激肽原生成激肽,进而激活B2R[8, 11, 20];(2)KLK1自身能通过其酶活性作用直接激活B2R[9]。由于本课题组无激肽原缺失动物模型,本研究不能明确KLK1调节线粒体功能的作用是直接激活B2R,还是通过促进激肽生成间接激活B2R。KLK1激活B2R后,主要通过激活蛋白激酶B(protein kinase B, Akt)-糖原合成激酶3β(glycogen synthase kinase 3β, GSK3β)信号通路实现抗心肌细胞凋亡[11]、促进心脏血管生成[21]、抑制压力负荷导致的心肌细胞肥大[12]等作用,提示Akt-GSK3β可能是KLK1激活B2R后在心肌细胞内发挥生物学效应的主要信号通路。KLK1改善心肌细胞线粒体损伤是否也通过Akt-GSK3β通路实现有待进一步研究。
此外,I/R后线粒体损伤的调节可能还存在其他机制。过氧化物酶体增殖物激活受体γ共激活因子1α(peroxisome proliferator-activated receptor γ coactivator 1α,PGC1α)是调节线粒体生成、能量代谢的重要调控因子[22-23]。组蛋白去乙酰化酶sirtuin 1(SIRT1)能与PGC1α上多个赖氨酸位点发生作用使之去乙酰化,进而增强PGC1α活性[24],两者共同抑制I/R导致的线粒体损伤、维持线粒体功能的稳定[18, 25-26]。KLK1对心肌细胞线粒体功能的调节是否最终通过影响SIRT1和PGC1α实现,是我们后续的研究方向。
| [1] |
KIRK J A, CINGOLANI O H. Thrombospondins in the transition from myocardial infarction to heart failure[J]. J Mol Cell Cardiol, 2016, 90: 102-110. DOI:10.1016/j.yjmcc.2015.12.009 |
| [2] |
RAEDSCHELDERS K, ANSLEY D M, CHEN D D. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion[J]. Pharmacol Ther, 2012, 133: 230-255. DOI:10.1016/j.pharmthera.2011.11.004 |
| [3] |
CHEN Y R, ZWEIER J L. Cardiac mitochondria and reactive oxygen species generation[J]. Circ Res, 2014, 114: 524-537. DOI:10.1161/CIRCRESAHA.114.300559 |
| [4] |
SHINTANI-ISHIDA K, INUI M, YOSHIDA K. Ischemia-reperfusion induces myocardial infarction through mitochondrial Ca2+ overload[J]. J Mol Cell Cardiol, 2012, 53: 233-239. DOI:10.1016/j.yjmcc.2012.05.012 |
| [5] |
FAZAL L, LAUDETTE M, PAULA-GOMES S, PONS S, CONTE C, TORTOSA F, et al. Multifunctional mitochondrial Epac1 controls myocardial cell death[J]. Circ Res, 2017, 120: 645-657. DOI:10.1161/CIRCRESAHA.116.309859 |
| [6] |
LESNEFSKY E J, CHEN Q, TANDLER B, HOPPEL C L. Mitochondrial dysfunction and myocardial ischemiareperfusion:implications for novel therapies[J]. Annu Rev Pharmacol Toxicol, 2017, 57: 535-565. DOI:10.1146/annurev-pharmtox-010715-103335 |
| [7] |
PATHAK M, WONG S S, DREVENY I, EMSLEY J. Structure of plasma and tissue kallikreins[J]. Thromb Haemost, 2013, 110: 423-433. DOI:10.1160/TH12-11-0840 |
| [8] |
SOTIROPOULOU G, PAMPALAKIS G. Targeting the kallikrein-related peptidases for drug development[J]. Trends Pharmacol Sci, 2012, 33: 623-634. DOI:10.1016/j.tips.2012.09.005 |
| [9] |
CHAO J, YIN H, GAO L, HAGIWARA M, SHEN B, YANG Z R, et al. Tissue kallikrein elicits cardioprotection by direct kinin β2 receptor activation independent of kinin formation[J]. Hypertension, 2008, 52: 715-720. DOI:10.1161/HYPERTENSIONAHA.108.114587 |
| [10] |
YOSHIDA H, ZHANG J J, CHAO L, CHAO J. Kallikrein gene delivery attenuates myocardial infarction and apoptosis after myocardial ischemia and reperfusion[J]. Hypertension, 2000, 35. |
| [11] |
YIN H, CHAO L, CHAO J. Kallikrein/kinin protects against myocardial apoptosis after ischemia/reperfusion via Aktglycogen synthase kinase-3 and Akt-Bad.14-3-3 signaling pathways[J]. J Biol Chem, 2005, 280: 8022-8030.
|
| [12] |
LI H J, YIN H, YAO Y Y, SHEN B, BADER M, CHAO L, et al. Tissue kallikrein protects against pressure overload-induced cardiac hypertrophy through kinin β2 receptor and glycogen synthase kinase-3β activation[J]. Cardiovasc Res, 2007, 73: 130-142. DOI:10.1016/j.cardiores.2006.10.014 |
| [13] |
YAO Y, SHENG Z, LI Y, YAN F, FU C, LI Y, et al. Tissue kallikrein promotes cardiac neovascularization by enhancing endothelial progenitor cell functional capacity[J]. Hum Gene Ther, 2012, 23: 859-870. DOI:10.1089/hum.2011.123 |
| [14] |
GAO L, BLEDSOE G, YIN H, SHEN B, CHAO L, CHAO J. Tissue kallikrein-modified mesenchymal stem cells provide enhanced protection against ischemic cardiac injury after myocardial infarction[J]. Circ J, 2013, 77: 2134-2144. DOI:10.1253/circj.CJ-12-1585 |
| [15] |
CAO B, YU Q, ZHAO W, TANG Z, CONG B, DU J, et al. Kallikrein-related peptidase 8 is expressed in myocardium and induces cardiac hypertrophy[J/OL]. Sci Rep, 2016, 7: 20024. doi: 10.1038/srep20024.
|
| [16] |
CONG B, ZHU X, CAO B, XIAO J, WANG Z, NI X. Estrogens protect myocardium against ischemia/reperfusion insult by up-regulation of CRH receptor type 2 in female rats[J]. Int J Cardiol, 2013, 168: 4755-4760. DOI:10.1016/j.ijcard.2013.07.231 |
| [17] |
DU J K, CONG B H, YU Q, WANG H, WANG L, WANG C N, et al. Upregulation of microRNA-22 contributes to myocardial ischemia-reperfusion injury by interfering with the mitochondrial function[J]. Free Radic Biol Med, 2016, 96: 406-417. DOI:10.1016/j.freeradbiomed.2016.05.006 |
| [18] |
CONG B, WANG L, ZHU X, LI X, LIU B, NI X. SGK1 is involved in cardioprotection of urocortin-1 against hypoxia/reoxygenation in cardiomyocytes[J]. Can J Cardiol, 2014, 30: 687-695. DOI:10.1016/j.cjca.2014.03.024 |
| [19] |
余惠珍, 朱鹏立, 黄舒洁, 向红, 潘玮, 张枫, 等. 组织激肽释放酶改善大鼠心肌重塑的实验研究[J]. 中国医科大学学报, 2014, 43: 830-836. |
| [20] |
WESTERMANN D, SCHULTHEISS H P, TSCHÖPE C. New perspective on the tissue kallikrein-kinin system in myocardial infarction:role of angiogenesis and cardiac regeneration[J]. Int Immunopharmacol, 2008, 8: 148-154. DOI:10.1016/j.intimp.2007.07.022 |
| [21] |
YAO Y Y, YIN H, SHEN B, SMITH R S Jr, LIU Y, GAO L, et al. Tissue kallikrein promotes neovascularization and improves cardiac function by the Akt-glycogen synthase kinase-3β pathway[J]. Cardiovasc Res, 2008, 80: 354-364. DOI:10.1093/cvr/cvn223 |
| [22] |
LIU D, MA Z, DI S, YANG Y, YANG J, XU L, et al. AMPK/PGC1α activation by melatonin attenuates acute doxorubicin cardiotoxicity via alleviating mitochondrial oxidative damage and apoptosis[J]. Free Radic Biol Med, 2018, 129: 59-72. DOI:10.1016/j.freeradbiomed.2018.08.032 |
| [23] |
VAN DER PLUIJM I, BURGER J, VAN HEIJNINGEN P M, IJPMA A, VAN VLIET N, MILANESE C, et al. Decreased mitochondrial respiration in aneurysmal aortas of Fibulin-4 mutant mice is linked to PGC1A regulation[J]. Cardiovasc Res, 2018, 114: 1776-1793. DOI:10.1093/cvr/cvy150 |
| [24] |
WINNIK S, AUWERX J, SINCLAIR D A, MATTER C M. Protective effects of sirtuins in cardiovascular diseases:from bench to bedside[J]. Eur Heart J, 2015, 36: 3404-3412. DOI:10.1093/eurheartj/ehv290 |
| [25] |
GERHART-HINES Z, RODGERS J T, BARE O, LERIN C, KIM S H, MOSTOSLAVSKY R, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α[J]. EMBO J, 2007, 26: 1913-1923. DOI:10.1038/sj.emboj.7601633 |
| [26] |
WU B, FENG J Y, YU L M, WANG Y C, CHEN Y Q, WEI Y, et al. Icariin protects cardiomyocytes against ischaemia/reperfusion injury by attenuating sirtuin 1-dependent mitochondrial oxidative damage[J]. Br J Pharmacol, 2018, 175: 4137-4153. DOI:10.1111/bph.v175.21 |
 2019, Vol. 40
2019, Vol. 40


