近年来心脏磁共振(cardiac magnetic resonance,CMR)技术飞速发展,因其具有多平面、多序列、高组织分辨率及无电离辐射的“一站式”检查等优势,能够提供丰富的心肌解剖学和功能学信息及定量病灶的发展程度,是缺血及非缺血性心肌病心肌组织特征评估的有效方法之一。不同CMR技术如钆造影剂延迟增强(late gadolinium enhancement,LGE)序列、mapping序列、弥散加权成像(diffusion-weighted imaging,DWI)和磁共振波谱(magnetic resonance spectroscopy,MRS)各具优势,对不同类型心肌疾病的组织学改变均有其特异性。本文综述了这些心肌组织的特征成像方法在评估不同类型心肌疾病中的优势及临床应用。
1 LGE序列LGE序列是最早用于评估心肌组织学异常的方法,在注射钆造影剂10~15 min后应用快速自旋回波反转恢复序列可获得LGE序列图像。此时正常心肌内钆造影剂已基本廓清,虽然钆造影剂无法穿透完整的细胞膜,但可能滞留于异常扩大的细胞外间隙(如浸润性疾病、纤维化),或当细胞膜完整性破坏(如急性心肌梗死、心肌炎症)时钆造影剂可进入细胞内,这些病理状态均可缩短组织T1弛豫时间,而在LGE序列表现为高信号。LGE序列图像不仅是诊断疾病的方法,也是预测全因死亡率、心血管死亡率、室性心律失常和猝死及主要心血管不良事件的可靠依据[1]。
虽然冠状动脉粥样硬化性心脏病(以下简称“冠心病”)的病因是冠状动脉斑块及狭窄,但决定患者病情和预后的关键是心肌,因此心肌活性成为临床评估冠心病患者预后的重要指标[2]。亮血LGE序列上血池内高信号的造影剂可能影响小的心内膜下梗死灶的检出,而黑血LGE技术可消除血池和正常心肌信号,仅显示高信号的梗死灶[3]。灰血LGE技术成像的信噪比较亮血和黑血LGE技术更高,尤其是显示心内膜下心肌延迟强化病灶,并且其重复性及观察者间一致性均更佳,也能显示较亮血和黑血LGE图像上更大的瘢痕面积,甚至能较黑血LGE技术更精准地判断心肌和乳头肌瘢痕[4]。
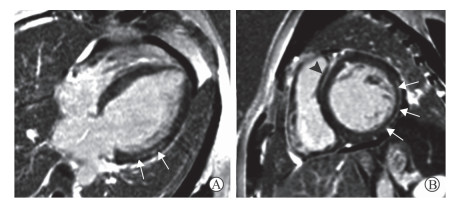
LGE序列对不同类型原发性心肌病的诊断、鉴别诊断及预后评估有重要价值。各种心肌病延迟强化病灶分布及信号具有一定特征。肥厚型心肌病(hypertrophic cardiomyopathy,HCM)以心肌增厚为主要表现,部分HCM患者可合并左心室流出道梗阻、左房室瓣前叶收缩期前向运动,以及收缩、舒张功能的改变,其心肌损伤区多位于肥厚心肌中层[5](图 1)。而扩张型心肌病(dilated cardiomyopathy,DCM)的典型表现除心腔扩张、射血分数降低外,室间隔心肌中层线样强化也具有一定特征性[6](图 2)。近期研究发现,DCM患者冠状动脉内皮功能障碍可能是心肌发生纤维化的重要因素[6]。无论是HCM还是DCM,当心肌出现大范围延迟强化时均提示患者预后不良[7]。常规的CMR检查虽不能评估冠状动脉的狭窄情况,但LGE序列可检出梗死的心肌组织,呈心内膜下心肌甚至透壁性心肌延迟强化表现,这有助于原发性心肌病合并冠心病的诊断。

|
图 1 1例HCM患者LGE表现 Fig 1 LGE features of an HCM patient A 36-year-old male patient with palpitation and dyspnea. Four-chamber heart (A) and short-axis (B) LGE images show the thickened myocardia with delayed contrast enhancement in the middle myocardia of interventricular septum and left ventricular anterior wall (arrows). HCM: Hypertrophic cardiomyopathy; LGE: Late gadolinium enhancement |
|
图 2 1例DCM患者LGE表现 Fig 2 LGE features of a DCM patient A 30-year-old male patient with heart failure. Four-chamber heart (A) and short-axis (B) LGE images show the dilated left ventricle, mildly thinned myocardium and linear enhancement in the middle myocardia of interventricular septum (black triangular arrow) and left ventricular free wall (white arrows). DCM: Dilated cardiomyopathy; LGE: Late gadolinium enhancement |
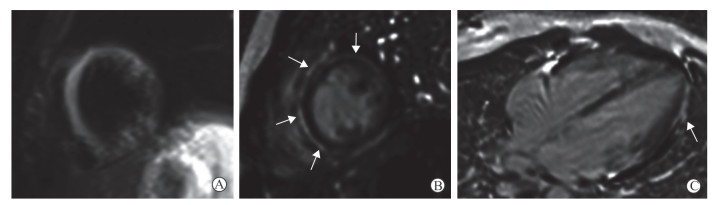
病毒性心肌炎患者LGE序列提示心肌可逆或不可逆损伤。急性期活动性炎症浸润的范围随病程发生变化,并与炎症严重程度呈正相关[8];慢性期病灶延迟强化提示存在残余局灶纤维化[9]。急性病毒性心肌炎患者心肌延迟强化范围与肌钙蛋白Ⅰ及肌酸激酶峰值显著相关,且可预示美国纽约心脏协会(New York Heart Association,NYHA)心功能分级恶化及心血管不良事件的发生[8]。延迟强化最常见于左心室外侧壁,并自心外膜区发展,病情危重者心肌可呈透壁性延迟强化表现[10](图 3)。
|
图 3 1例急性病毒性心肌炎患者LGE表现 Fig 3 LGE features of an acute viral myocarditis patient A 13-year-old female patient with acute viral myocarditis. A: Turbo inversion recovery modulus sequence shows that interventricular septum and left ventricular wall manifest diffused hyperintensity; B, C: Short-axis (B) and 4-chamber heart (C) late gadolinium enhancement images show the sub-epicardium enhancement in the corresponding location (white arrows). LGE: Late gadolinium enhancement |
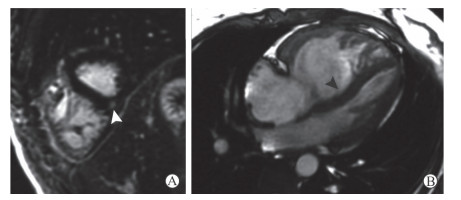
肺动脉高压(pulmonary hypertension,PH)患者右心室室间隔插入部中层心肌常见三角形或带状延迟强化信号,部分可向室间隔内延伸(图 4),这种特征性插入部中层心肌延迟强化表现与右心室功能不全显著相关[11]。有研究认为PH患者心肌延迟强化表现可能由于该区域心肌纤维环或心肌炎性改变所致[12]。随着肺动脉压的增高,右心室插入部细胞外基质沉积增多、心肌细胞排列紊乱甚至由纤维组织替代[13]。
|
图 4 1例PH患者LGE表现 Fig 4 LGE features of a PH patient A 30-year-old female PH patient with systemic lupus erythematosus and dyspnea. A: LGE image shows triangular delayed contrast enhancement in the right ventricular insertion point (white triangular arrow); B: Four-chamber heart cine image shows the obviously dilated right ventricle, thickened right ventricular free wall and interventricular septum bowing to the left ventricle (black triangular arrow). PH: Pulmonary hypertension; LGE: Late gadolinium enhancement |
2 Mapping序列
尽管LGE序列可提供预后信息,但无法评判异常信号究竟是心肌急性损伤或炎症反应还是慢性损伤或纤维化。因此纵向弛豫时间定量成像(T1 mapping)、横向弛豫时间定量成像(T2 mapping)等新技术应运而生,以单纯定量参数的方法弥补了半定量或肉眼评判的局限性,并采用伪彩图标记心肌组织。Mapping序列检出病变极其灵敏,甚至能够检出LGE序列阴性的病灶,可实现心肌组织病理学改变的无创性评估[14]。
2.1 T1 mapping初始纵向弛豫时间定量成像(native T1 mapping)在未注射造影剂时采集并测量心肌组织的T1值,能够检出心肌炎症、铁过载、心肌梗死和瘢痕等导致的初始T1值升高。该技术可重复性和灵敏性高,尤其是对肾功能不全而无法使用造影剂的患者或对造影剂过敏的患者,避免了发生造影剂不良反应的风险[14]。增强后T1 mapping(post T1 mapping)技术是于注射造影剂后15 min采集T1 mapping图像并测量心肌组织增强后T1值,其可显示各种病因导致的细胞间隙扩大和纤维化,并与替代性纤维化(瘢痕)心肌鉴别,但增强后T1值受造影剂剂量、浓度、注射速率及注射后采集时间等多种因素的影响[15]。细胞外容积(extracellular volume,ECV)是根据注射细胞外顺磁性造影剂前后分别获取的T1值(初始T1值和增强后T1值)计算得到,代表心肌中细胞外基质占整个心肌组织的百分比。由于造影剂可在扩大的细胞外间隙聚集,因此可以用ECV定量相对扩大的细胞外间隙[16]。
糖尿病性心肌病患者由于心肌结构和功能异常,发生心力衰竭的风险明显增加,因此早期检出、早期干预对患者的预后至关重要。病程早期心肌收缩功能未见异常,初始T1值和ECV的增高提示心肌细胞外间隙扩大,该方法可能在病程早期患者无任何临床症状时检出心肌异常[17]。早期干预如血管紧张素转换酶抑制剂(angiotensin converting enzyme inhibitor,ACEI)治疗可能会改善心肌细胞外间隙扩大,但控制血糖和ACEI的治疗与心肌纤维环之间的关系仍需要大规模的研究证实[17-18]。
非ST段抬高型心肌梗死(non-ST-segment elevation myocardial infarction,NSTEMI)是由非闭塞罪犯血管病变引起的较小程度的心肌损伤,由于其表现多变及对急性心肌损伤区的识别引起了临床高度关注。当患者发生NSTEMI时,心肌间质水肿导致T1值延长,初始T1 mapping较T2加权成像(T2-weighted imaging,T2WI)识别心肌水肿更灵敏[19],并且能够监测坏死心肌边缘呈水肿状态的可挽救心肌[20]。初始T1 mapping图像可显示急性心肌梗死患者梗死区域内局灶性短T1值区,代表了“缺血-再灌注”导致微血管损伤(microvascular injury,MVI)而引起的心肌内出血,多预示患者预后不良[21]。另外,联合应用初始T1 mapping和T2* mapping这两种无需注射造影剂的方法,可能成为鉴别正常心肌、梗死心肌(初始T1值升高)及梗死心肌伴MVI(初始T1值及T2*值均降低)有效且准确的方法。
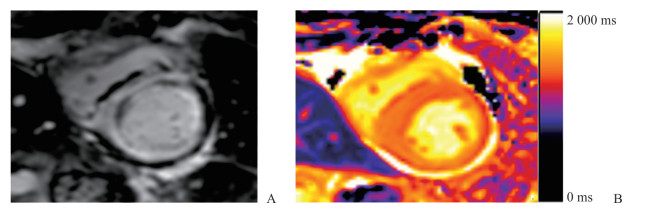
淀粉样变性是由于蛋白折叠异常导致不可溶的纤维性淀粉样蛋白沉积于器官或组织的细胞外间隙引起的一组疾病。淀粉样蛋白沉积于心肌间质导致细胞外间隙扩大,使心肌呈弥漫性受累,初始T1 mapping及ECV均可定量这种病理改变,表现为初始T1值和ECV显著升高[22](图 5)。T1 mapping技术不仅能够早期发现心肌受累,而且可用来评估淀粉样物质的负荷状态从而评价疗效[23]。当与其他表现为心肌增厚的疾病如HCM、Anderson-Fabry综合征难以鉴别时,初始T1值或ECV变化的差异具有重要诊断价值[24]。
|
图 5 1例心肌淀粉样变性患者LGE和T1 mapping表现 Fig 5 LGE and T1 mapping features of a patient with cardial amyloidosis A 65-year-old female patient with cardiac amyloidosis. LGE sequence (A) and native T1 mapping (B) show that the native T1 value (1 430.28 ms) is increased in the myocardium with diffused delayed contrast enhancement. LGE: Late gadolinium enhancement |
Anderson-Fabry综合征是一种X染色体连锁隐性遗传的溶酶体贮积病,因α-牛乳糖(α-galactosidase,α-GAL)基因缺陷使体内糖神经胺醇脂质无法代谢,不断堆积在细胞质及溶酶体内,从而引发多系统病变,形态学上同样表现为左心室心肌肥厚。与其他表现为心肌肥厚的心肌疾病相比,Anderson-Fabry综合征心肌T1值降低,为Anderson-Fabry综合征心肌受累最具特征性的表现[25]。不仅如此,初始T1值的降低甚至早于左心室心肌肥厚的发生。
2.2 T2 mapping心肌水分的增加可使T2弛豫时间延长。T2 mapping克服了传统T2WI的局限性,并且在定量评价心肌水肿方面非常灵敏,如检出急性心肌炎或应激性心肌病的灵敏度和特异度均可达90% [26]。冠心病患者缺血心肌早期即可因水肿引起T2值明显升高,这使发生NSTEMI或心肌酶升高的急性胸痛患者病变检出的灵敏度明显提高[27]。T2 mapping还可协助区分梗死心肌和可挽救心肌,虽然二者的T2值均明显高于正常心肌,但可挽救心肌表现为T2值升高而在LGE图像呈低信号,且有研究显示高T2值(≥ 62 ms)预示着患者预后不良[28]。
T2 mapping技术可检出传统T2WI短时反转恢复(short tau inversion recovery,STIR)序列无法显示的心肌炎症区,其显示的炎症心肌区域多比LGE序列显示的范围更大、更灵敏,可能提示部分受累心肌并未发生纤维化改变[29]。一项纳入经心肌活组织检查确诊的129例急性心肌炎患者的回顾性研究显示,T2 mapping对于检出表现为慢性症状的心肌炎(>14 d)更灵敏,T2值>59 ms提示心肌受累,且T2 mapping诊断心肌炎的准确度(81%)明显高于诊断路易斯湖心肌炎的准确度(56%)[30]。
结节病是一种多系统受累的肉芽肿性疾病,心脏受累是结节病患者第二大常见死因。与心肌炎不同,结节病早期应用类固醇激素或其他免疫抑制剂可改善心肌炎症、阻止心功能恶化和心肌瘢痕形成。研究显示,心电图异常的疑似心脏受累的结节病患者心肌整体T2值明显升高,其中41%患者的LGE序列未见异常强化信号,但T2 mapping可早期检出结节病累及的心肌区域,并有利于临床干预方案的实施[29]。
3 DWIDWI可通过编码水分子的随机运动提取微观的组织结构信息,无需使用造影剂即可评估心肌组织活性,这为因肾功能不全而无法使用造影剂的患者带来了福音。体素内不相干运动(intravoxel incoherent motion,IVIM)多b值双指数模型可准确提取出单纯的水分子弥散(扩散)和毛细血管网内血液微循环(灌注)信息,这为进一步探究心肌组织灌注和弥散情况提供了可能。
研究显示慢性心肌梗死区表观弥散系数(apparent diffusion coefficient,ADC)较正常心肌明显升高,并与LGE图像显示的区域一致,其灵敏度、特异度均较高[31]。对于急性心肌缺血性心肌病患者,DWI较传统T2WI序列检出心肌水肿区的灵敏性更高、面积更大[32-33]。心肌再灌注治疗后第3天,应用IVIM分析结果显示慢性表观弥散系数(slow apparent diffusion coefficient,ADCslow)和快速扩散成分所占比例(f)均显著减低,并且较其他时间点均降低,提示再灌注治疗后观察心肌水肿的最佳时间可能为再灌注治疗后的第3天[33]。
DWI不仅能早期较灵敏地发现缺血性心肌病的组织学改变,对于发现非缺血性心肌病的微循环改变也发挥重要作用。高血压患者由于长期的高左心室后负荷导致心肌损伤和重构,其ADCslow显著低于健康志愿者,提示高血压患者可能存在心肌血流灌注减低[34]。HCM患者以心肌显著增厚为特征,其微血管功能障碍的严重程度(ADCslow降低)与心肌的肥厚程度呈正相关[34],IVIM可能会检出超出延迟强化范围的低灌注区[35]。
4 MRSMRS是一种可对人体的组织代谢进行定量分析的无创、无辐射的检查方法,其中磁共振氢谱(1H-magnetic resonance spectroscopy,1H-MRS)是目前唯一可对活体细胞内脂肪酸含量进行定量检测的方法。3.0 T磁共振扫描仪获取的1H-MRS图像较1.5 T获得的图像质量更佳,定量心肌脂肪酸含量的准确度更高[36]。目前的研究结果显示,健康人群中心肌三酰甘油的含量无明显的性别差异[37],但会随着年龄增长而增高[38]。
2型糖尿病(type 2 diabetes mellitus,T2DM)患者易发生心室舒张功能障碍,甚至发生心力衰竭。研究发现T2DM患者心肌舒张功能障碍与心肌三酰甘油含量有关,与心肌灌注储备能力无明显相关性,提示心肌脂肪变性可能是患者发生糖尿病性心肌病早期的预测因素,并预示存在亚临床性心功能障碍的可能[39-40]。
人体代谢异常可导致脂肪酸在心肌内异常贮积,尤其是在室间隔内。近年来3.0 T高场强磁共振扫描设备的应用提高了心脏1H-MRS成像的化学位移分辨率和图像信噪比,并能够分辨心肌内脂肪酸和不饱和脂肪酸的沉积。Liao等[36]应用1H-MRS发现急性心力衰竭患者出院后心肌不饱和脂肪酸含量显著升高,且与CMR获取的左心室心功能参数呈负相关,不饱和脂肪酸含量可能为患者预后提供证据。
综上所述,CMR组织学成像能够实现从定性到定量评估缺血及非缺血性疾病引起的心肌组织学异常。各种CMR成像方法相互补充,可有效发挥各方法在疾病诊疗和机制探究方面的优势。但目前我们需要在熟悉、掌握现有技术的基础上,挖掘各种成像方法的潜力并将其转化到心肌疾病的临床应用中。
| [1] |
GANESAN A N, GUNTON J, NUCIFORA G, MCGAVIGAN A D, SELVANAYAGAM J B. Impact of late gadolinium enhancement on mortality, sudden death and major adverse cardiovascular events in ischemic and nonischemic cardiomyopathy:a systematic review and meta-analysis[J]. Int J Cardiol, 2018, 254: 230-237. DOI:10.1016/j.ijcard.2017.10.094 |
| [2] |
BULLUCK H, DHARMAKUMAR R, ARAI A E, BERRY C, HAUSENLOY D J. Cardiovascular magnetic resonance in acute ST-segment-elevation myocardial infarction:recent advances, controversies, and future directions[J]. Circulation, 2018, 137: 1949-1964. DOI:10.1161/CIRCULATIONAHA.117.030693 |
| [3] |
FRANCIS R, KELLMAN P, KOTECHA T, BAGGIANO A, NORRINGTON K, MARTINEZ-NAHARRO A, et al. Prospective comparison of novel dark blood late gadolinium enhancement with conventional bright blood imaging for the detection of scar[J/OL]. J Cardiovasc Magn Reson, 2017, 19: 91. doi: 10.1186/s12968-017-0407-x.
|
| [4] |
FAHMY A S, NEISIUS U, TSAO C W, BERG S, GODDU E, PIERCE P, et al. Gray blood late gadolinium enhancement cardiovascular magnetic resonance for improved detection of myocardial scar[J/OL]. J Cardiovasc Magn Reson, 2018, 20: 22. doi: 10.1186/s12968-018-0442-2.
|
| [5] |
MARON M S. Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy[J/OL]. J Cardiovasc Magn Reson, 2012, 14: 13. doi: 10.1186/1532-429X-14-13.
|
| [6] |
EHARA S, MATSUMOTO K, KITADA R, NISHIMURA S, SHIMADA K, YOSHIYAMA M. Clinical significance of discrepant mid-wall late gadolinium enhancement in patients with nonischemic dilated cardiomyopathy[J]. Heart Vessels, 2018, 33: 1482-1489. DOI:10.1007/s00380-018-1196-3 |
| [7] |
MACHⅡ M, SATOH H, SHIRAKI K, SAOTOME M, URUSHIDA T, KATOH H, et al. Distribution of late gadolinium enhancement in end-stage hypertrophic cardiomyopathy and dilated cardiomyopathy:differential diagnosis and prediction of cardiac outcome[J]. Magn Reson Imaging, 2014, 32: 118-124. DOI:10.1016/j.mri.2013.10.011 |
| [8] |
MEWTON N, DERNIS A, BRESSON D, ZOUAGHI O, CROISILLE P, FLOCARD E, et al. Myocardial biomarkers and delayed enhanced cardiac magnetic resonance relationship in clinically suspected myocarditis and insight on clinical outcome[J]. J Cardiovasc Med (Hagerstown), 2015, 16: 696-703. DOI:10.2459/JCM.0000000000000024 |
| [9] |
MAVROGENI S, BRATIS K, GEORGAKOPOULOS D, KARANASIOS E, KOLOVOU G, PAVLIDES G, et al. Evaluation of myocarditis in a pediatric population using cardiovascular magnetic resonance and endomyocardial biopsy[J]. Int J Cardiol, 2012, 160: 192-195. DOI:10.1016/j.ijcard.2011.04.019 |
| [10] |
BANKA P, ROBINSON J D, UPPU S C, HARRIS M A, HASBANI K, LAI W W, et al. Cardiovascular magnetic resonance techniques and findings in children with myocarditis: a multicenter retrospective study[J/OL]. J Cardiovasc Magn Reson, 2015, 17: 96. doi: 10.1186/s12968-015-0201-6.
|
| [11] |
MCCANN G P, GAN C T, BEEK A M, NIESSEN H W, VONK NOORDEGRAAF A, VAN ROSSUM A C. Extent of MRI delayed enhancement of myocardial mass is related to right ventricular dysfunction in pulmonary artery hypertension[J]. AJR Am J Roentgenol, 2007, 188: 349-355. DOI:10.2214/AJR.05.1259 |
| [12] |
BRADLOW W M, ASSOMULL R, KILNER P J, GIBBS J S, SHEPPARD M N, MOHIADDIN R H. Understanding late gadolinium enhancement in pulmonary hypertension[J]. Circ Cardiovasc Imaging, 2010, 3: 501-503. DOI:10.1161/CIRCIMAGING.109.919779 |
| [13] |
EGEMNAZAROV B, CRNKOVIC S, NAGY B M, OLSCHEWSKI H, KWAPISZEWSKA G. Right ventricular fibrosis and dysfunction:actual concepts and common misconceptions[J]. Matrix Biol, 2018, 68/69: 507-521. DOI:10.1016/j.matbio.2018.01.010 |
| [14] |
FERREIRA V M, PIECHNIK S K, DALL'ARMELLINA E, KARAMITSOS T D, FRANCIS J M, NTUSI N, et al. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents[J]. J Cardiovasc Magn Reson, 2014, 16: 36. DOI:10.1186/1532-429X-16-36 |
| [15] |
TAYLOR A J, SALERNO M, DHARMAKUMAR R, JEROSCH-HEROLD M. T1 mapping:basic techniques and clinical applications[J]. JACC Cardiovasc Imaging, 2016, 9: 67-81. DOI:10.1016/j.jcmg.2015.11.005 |
| [16] |
JEROSCH-HEROLD M, SHERIDAN D C, KUSHNER J D, NAUMAN D, BURGESS D, DUTTON D, et al. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy[J]. Am J Physiol Heart Circ Physiol, 2008, 295. |
| [17] |
CAO Y, ZENG W, CUI Y, KONG X, WANG M, YU J, et al. Increased myocardial extracellular volume assessed by cardiovascular magnetic resonance T1 mapping and its determinants in type 2 diabetes mellitus patients with normal myocardial systolic strain[J/OL]. Cardiovasc Diabetol, 2018, 17: 7. doi: 10.1186/s12933-017-0651-2.
|
| [18] |
SWOBODA P P, MCDIARMID A K, ERHAYIEM B, RIPLEY D P, DOBSON L E, GARG P, et al. Diabetes mellitus, microalbuminuria, and subclinical cardiac disease: identification and monitoring of individuals at risk of heart failure[J/OL]. J Am Heart Assoc, 2017, 6. pii: e005539. doi: 10.1161/JAHA.117.005539.
|
| [19] |
LAYLAND J, RAUHALAMMI S, LEE M M, AHMED N, CARBERRY J, TENG YUE MAY V, et al. Diagnostic accuracy of 3.0-T magnetic resonance T1 and T2 mapping and T2-weighted dark-blood imaging for the infarct-related coronary artery in non-ST-segment elevation myocardial infarction[J/OL]. J Am Heart Assoc, 2017, 6. pii: e004759. doi: 10.1161/JAHA.116.004759.
|
| [20] |
DALL'ARMELLINA E, PIECHNIK S K, FERREIRA V M, SI Q L, ROBSON M D, FRANCIS J M, et al. Cardiovascular magnetic resonance by non contrast T1-mapping allows assessment of severity of injury in acute myocardial infarction[J/OL]. J Cardiovasc Magn Reson, 2012, 14: 15. doi: 10.1186/1532-429X-14-15.
|
| [21] |
ROBBERS L F H J, NIJVELDT R, BEEK A M, TEUNISSEN P F A, HOLLANDER M R, BIESBROEK P S, et al. The influence of microvascular injury on native T1 and T2* relaxation values after acute myocardial infarction:implications for non-contrast-enhanced infarct assessment[J]. Eur Radiol, 2018, 28: 824-832. DOI:10.1007/s00330-017-5010-x |
| [22] |
NAM B D, KIM S M, JUNG H N, KIM Y, CHOE Y H. Comparison of quantitative imaging parameters using cardiovascular magnetic resonance between cardiac amyloidosis and hypertrophic cardiomyopathy:inversion time scout versus T1 mapping[J]. Int J Cardiovasc Imaging, 2018, 34: 1769-1777. DOI:10.1007/s10554-018-1385-2 |
| [23] |
BANYPERSAD S M, FONTANA M, MAESTRINI V, SADO D M, CAPTUR G, PETRIE A, et al. T1 mapping and survival in systemic light-chain amyloidosis[J]. Eur Heart J, 2015, 36: 244-251. DOI:10.1093/eurheartj/ehu444 |
| [24] |
PATEL A R, KRAMER C M. Role of cardiac magnetic resonance in the diagnosis and prognosis of nonischemic cardiomyopathy[J]. JACC Cardiovasc Imaging, 2017, 10(10 Pt A): 1180-1193. |
| [25] |
THOMPSON R B, CHOW K, KHAN A, CHAN A, SHANKS M, PATERSON I, et al. T1 mapping with cardiovascular MRI is highly sensitive for Fabry disease independent of hypertrophy and sex[J]. Circ Cardiovasc Imaging, 2013, 6: 637-645. DOI:10.1161/CIRCIMAGING.113.000482 |
| [26] |
THAVENDIRANATHAN P, WALLS M, GIRI S, VERHAERT D, RAJAGOPALAN S, MOORE S, et al. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping[J]. Circ Cardiovasc Imaging, 2012, 5: 102-110. DOI:10.1161/CIRCIMAGING.111.967836 |
| [27] |
MONTANT P, SIGOVAN M, REVEL D, DOUEK P. MR imaging assessment of myocardial edema with T2 mapping[J]. Diagn Interv Imaging, 2015, 96: 885-890. DOI:10.1016/j.diii.2014.07.008 |
| [28] |
ZIA M I, ROIFMAN I, GHUGRE N R, IGNATIUS A J, STRAUSS B H, DICK A, et al. Prognostic value of myocardial T2 mapping post reperfused acute myocardial infarction[J/OL]. J Cardiovasc Magn Reson, 2015, 17(Suppl 1): P150. doi: 10.1186/1532-429X-17-S1-P150.
|
| [29] |
THAVENDIRANATHAN P, WALLS M, GIRI S, VERHAERT D, RAJAGOPALAN S, MOORE S, et al. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping[J]. Circ Cardiovasc Imaging, 2012, 5: 102-110. DOI:10.1161/CIRCIMAGING.111.967836 |
| [30] |
LURZ P, LÜECKE C, EITEL I, FÖHRENBACH F, FRANK C, GROTHOFF M, et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis the MyoRacer-trial[J]. J Am Coll Cardiol, 2016, 67: 1800-1811. |
| [31] |
NGUYEN C, FAN Z, XIE Y, DAWKINS J, TSELIOU E, BI X, et al. In vivo contrast free chronic myocardial infarction characterization using diffusion-weighted cardiovascular magnetic resonance[J/OL]. J Cardiovasc Magn Reson, 2014, 16: 68. doi: 10.1186/s12968-014-0068-y.
|
| [32] |
KOCIEMBA A, PYDA M, KATULSKA K, ŁANOCHA M, SINIAWSKI A, JANUS M, et al. Comparison of diffusion-weighted with T2-weighted imaging for detection of edema in acute myocardial infarction[J/OL]. J Cardiovasc Magn Reson, 2013, 15: 90. doi: 10.1186/1532-429X-15-90.
|
| [33] |
DEUX J F, MAATOUK M, VIGNAUD A, LUCIANI A, LENCZNER G, MAYER J, et al. Diffusion-weighted echo planar imaging in patients with recent myocardial infarction[J]. Eur Radiol, 2011, 21: 46-53. DOI:10.1007/s00330-010-1912-6 |
| [34] |
MOU A, ZHANG C, LI M, JIN F, SONG Q, LIU A, et al. Evaluation of myocardial microcirculation using intravoxel incoherent motion imaging[J]. J Magn Reson Imaging, 2017, 46: 1818-1828. DOI:10.1002/jmri.v46.6 |
| [35] |
JABLONOWSKI R, FERNLUND E, ALETRAS A H, ENGBLOM H, HEIBERG E, LIUBA P, et al. Regional stress-induced ischemia in non-fibrotic hypertrophied myocardium in young HCM patients[J]. Pediatr Cardiol, 2015, 36: 1662-1669. DOI:10.1007/s00246-015-1214-5 |
| [36] |
LIAO P A, LIN G, TSAI S Y, WANG C H, JUAN Y H, LIN Y C, et al. Myocardial triglyceride content at 3 T cardiovascular magnetic resonance and left ventricular systolic function: a cross-sectional study in patients hospitalized with acute heart failure[J/OL]. J Cardiovasc Magn Reson, 2016, 18: 9. doi: 10.1186/s12968-016-0228-3.
|
| [37] |
PETRITSCH B, KÖSTLER H, GASSENMAIER T, KUNZ A S, BLEY T A, HORN M. An investigation into potential gender-specific differences in myocardial triglyceride content assessed by 1H-magnetic resonance spectroscopy at 3Tesla[J]. J Int Med Res, 2016, 44: 585-591. DOI:10.1177/0300060515603884 |
| [38] |
PETRITSCH B, GASSENMAIER T, KUNZ A S, DONHAUSER J, GOLTZ J P, BLEY T A, et al. Age dependency of myocardial triglyceride content:a 3T high-field 1H-MR spectroscopy study[J]. Rofo, 2015, 187: 1016-1021. DOI:10.1055/s-00000066 |
| [39] |
KOROSOGLOU G, HUMPERT P M, AHRENS J, OIKONOMOU D, OSMAN N F, GITSIOUDIS G, et al. Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve[J]. J Magn Reson Imaging, 2012, 35: 804-811. DOI:10.1002/jmri.v35.4 |
| [40] |
RIJZEWIJK L J, VAN DER MEER R W, SMIT J W, DIAMANT M, BAX J J, HAMMER S, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus[J]. J Am Coll Cardiol, 2008, 52: 1793-1799. DOI:10.1016/j.jacc.2008.07.062 |
 2019, Vol. 40
2019, Vol. 40


