2. 西门子(上海)医疗系统有限公司, 上海 201318
2. Siemens Healthineers, Shanghai 201318, China
肥厚型心肌病(hypertrophic cardiomyopathy,HCM)是一种以左心室壁非对称性肥厚为主要特点的遗传性疾病[1]。心肌细胞肥大、排列紊乱及间质纤维化、心肌内小血管壁增厚为其病理特征[2-4]。通常HCM患者心外膜下大的冠状动脉多无异常,但由于心肌内小血管壁增厚、冠状动脉微循环受损等因素导致的心肌缺血却成为HCM临床恶化,甚至导致患者死亡的直接原因之一[5-6]。HCM患者心室壁肥厚及纤维化的严重程度与其心肌微血管功能障碍(myocardial microvascular dysfunction,MVD)密切相关[7]。MVD的严重程度是影响HCM患者预后及心力衰竭等心血管事件发生风险的重要指标[8],故准确评估HCM患者的MVD有重要的临床价值。目前,正电子发射计算机断层显像(positron emission tomography,PET)、心脏磁共振(cardiac magnetic resonance,CMR)、冠状动脉微循环阻力(index of microcirculatory resistance,IMR)指数等均可用于评估冠状动脉微血管功能,而CMR因无创、无辐射、分辨率高、可用多种后处理软件提供多参数定量指标等特点,成为检测微循环功能障碍有前景的方法之一[1, 8-10]。其中,双指数、多扩散敏感因子(b)体素内不相干运动(intravoxel incoherent motion,IVIM)技术可无创、定量地呈现微循环改变相关的信号变化,能更加直观地反映组织灌注的病理生理学信息[11-13],但目前IVIM在心脏中应用的研究较少。本研究初步探讨应用3.0 T CMR IVIM技术评估HCM患者MVD的价值。
1 资料和方法 1.1 病例资料回顾性分析2016年4月至2017年10月于兰州大学第一医院行3.0 T CMR检查的26例HCM患者的病历资料。纳入标准:(1)左心室舒张末期心室壁厚度(end-diastolic thickness,EDTH)≥ 15 mm;(2)有HCM家族史者左心室EDTH ≥ 13 mm,且无其他心肌肥厚相关的心脏疾病,冠状动脉造影检查未见异常。选择同期12名健康对照,纳入标准:无心血管病史及相关危险因素,心脏体格检查、心电图及超声心动图检查均未见明显异常。所有研究对象均无磁共振成像检查禁忌证,检查前均签署知情同意书。本研究通过兰州大学第一医院伦理委员会审批(LDYYLL2019-178)。
1.2 检查方法采用德国西门子医疗系统有限公司Skyra 3.0 T超导型磁共振成像系统,8通道心脏相控线圈,胸前导联心电门控、呼吸门控技术。采用平衡稳态自由进动序列进行四腔心、短轴电影序列扫描,重复时间(repetition time,TR)为2.8 ~ 3.0 ms,回波时间(echo time,TE)为1.1 ~ 1.5 ms,视野(field of view,FOV)为370 mm × 320 mm,层厚为8 mm,翻转角为60 °~ 70 °。IVIM扫描:在四腔心电影图像上,记录左心室舒张中晚期心肌运动幅度最小的时间点,作为IVIM序列的触发延迟(trigger delay,TD)时间。采集不同b值(0、50、100、200、400、600 s/mm2)的左心室短轴基底段、中间段及心尖段3层图像(图 1)。矩阵为84 × 128,TR为300 ms,TE为56.0 ms,层厚为5.0 mm,层间距为18 mm,FOV为400 mm × 400 mm,TD时间为350 ~ 600 ms;呼气末屏气扫描,扫描时间为16 ~ 25 s,心率约55 ~ 100次/min。静脉注射造影剂(钆喷酸葡胺,0.2 mmol/kg)10 ~ 15 min后行相位敏感反转恢复(phase-sensitive inversion recovery,PSIR)序列,扫描时对电影序列范围进行复制,获得钆造影剂延迟增强(late gadolinium enhancement,LGE)图像。
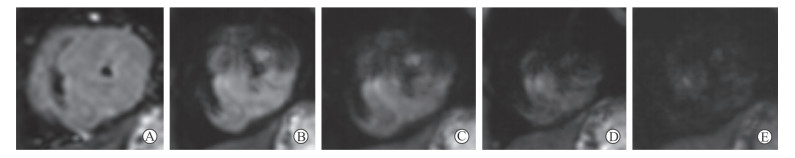
|
图 1 HCM患者左心室短轴中段层面不同b值IVIM图像 Fig 1 IVIM images with different b values in middle view of left ventricular short-axis of HCM patients A 45-year-old female HCM patient. Left ventricular myocardial signal is of uniformity, hypertrophic myocardial segment signal is higher than non-hypertrophic segment, and myocardial signal decreases with increase of b values. A: b=0 s/mm2; B: b=50 s/mm2; C: b=100 s/mm2; D: b=200 s/mm2; E: b=400 s/mm2. HCM: Hypertrophic cardiomyopathy; b: Diffusion sensitive factor; IVIM: Intravoxel incoherent motion |
1.3 图像分析
将IVIM原始图像导入后处理软件(MITK-Diffusion)获得双指数模型拟合图,按照美国心脏协会的17节段划分法,测量不同节段心肌感兴趣区(region of interest,ROI)的快速表观扩散系数(fast apparent diffusion coefficient,ADCfast)、慢速表观扩散系数(slow apparent diffusion coefficient,ADCslow)和快速扩散成分所占比例(fraction of ADCfast,f),测量时避免将心腔内血池和心外膜外脂肪成分画入ROI。每个节段相同位置、相同大小的ROI测量3次,取平均值。
图像分级标准:Ⅰ级,心脏、呼吸运动和变形伪影较严重,>2个心肌节段信号丢失,无法测量;Ⅱ级,心脏、呼吸运动和变形伪影较严重,≤2个心肌节段信号丢失,无法测量;Ⅲ级,各节段心肌轮廓锐利、信号清晰,无伪影干扰[14]。在短轴电影图像上测量各心肌节段EDTH,因心尖17段显示较差不对其进行测量。依据各心肌节段EDTH将HCM患者分为无肥厚节段组(EDTH<15 mm)和肥厚节段组(EDTH≥15 mm);并将肥厚节段组进一步细分为轻度(EDTH为15~20 mm)、中度(EDTH为21~25 mm)、重度(EDTH为26~30 mm)和极重度(EDTH>30 mm)肥厚节段亚组[8]。所有数据由2位放射科副高级以上职称CMR诊断医师进行图像分析和数据测量。
1.4 统计学处理采用SPSS 22.0软件进行数据处理。呈正态分布的计量资料以x±s表示;呈偏态分布的计量资料以中位数(下四分位数,上四分位数)表示,多组间比较采用Kruskal-Wallis H检验,组间两两比较采用Mann-Whitney U检验。各节段IVIM参数与EDTH的相关性分析采用Spearman相关分析。检验水准(α)为0.05。
2 结果 2.1 基本资料12名健康对照,其中男6例、女6例,年龄为30 ~ 63岁,平均年龄为(48.8 ± 11.2)岁,共192个心肌节段,图像质量均为Ⅲ级。26例HCM患者,其中男19例、女7例,年龄为12 ~ 71岁,平均年龄为(54.7 ± 13.6)岁,共416个心肌节段。去除HCM患者34个Ⅰ级和Ⅱ级心肌节段(第4、5、6、11、12、13、14、15、16节段分别为2、7、3、9、3、1、1、3、5个),最终纳入382个心肌节段,其中无肥厚节段组心肌节段284个(74.3%),肥厚节段组心肌节段98个(25.7%)。肥厚节段组中,轻度、中度、重度、极重度心肌节段分别为66、24、4、4个(分别占67.3%、24.5%、4.1%、4.1%)。HCM患者中22例(84.6%)出现LGE,4例(15.4%)无LGE;无LGE节段294个(77.0%),LGE节段88个(23.0%)。
2.2 健康对照与HCM患者f值、ADCslow、ADCfast的比较HCM患者ADCslow和ADCfast均低于健康对照,差异均有统计学意义(P<0.05,P<0.01);而f值在健康对照和HCM患者之间差异无统计学意义(P>0.05)。见表 1。
|
|
表 1 健康对照与HCM患者f值、ADCslow、ADCfast的比较 Tab 1 Comparison of f value, ADCslow and ADCfast between healthy controls and HCM patients |
2.3 HCM患者非肥厚节段组、肥厚节段组与健康对照组间f值、ADCslow、ADCfast的比较
见表 2,HCM患者肥厚节段组f值和ADCfast均低于非肥厚节段组,差异均有统计学意义(P均<0.01),而ADCslow在两组间差异无统计学意义(P>0.05)。HCM患者非肥厚节段组及肥厚节段组的ADCfast均低于健康对照,差异均有统计学意义(Z=- 5.505、- 8.144,P均<0.01);HCM患者非肥厚节段组的ADCslow及肥厚节段组的f值均低于健康对照,差异均有统计学意义(Z=- 2.411、- 3.000,P= 0.016、0.003);而HCM患者非肥厚节段组的f值及肥厚节段组的ADCslow与健康对照相比差异均无统计学意义(Z=- 0.844、- 1.548,P= 0.399、0.122)。
|
|
表 2 HCM患者非肥厚节段组及肥厚节段组f值、ADCslow、ADCfast的比较 Tab 2 Comparison of f value, ADCslow and ADCfast between non-hypertrophic and hypertrophic segment groups in HCM patients |
2.4 HCM患者肥厚节段组不同肥厚程度亚组之间f值、ADCslow、ADCfast的比较
见表 3,HCM患者肥厚节段组轻度、中度、重度、极重度肥厚程度亚组之间ADCfast差异有统计学意义(H= 9.769,P= 0.021),但两两比较结果示各组之间ADCfast差异均无统计学意义(P均>0.05);f值及ADCslow在4个不同肥厚程度亚组之间差异均无统计学意义(P均>0.05)。
|
|
表 3 HCM患者肥厚节段组不同肥厚程度亚组f值、ADCslow、ADCfast的比较 Tab 3 Comparison of f value, ADCslow and ADCfast among different hypertrophy subgroups in HCM patients |
2.5 HCM患者无LGE及有LGE节段心肌f值、ADCslow、ADCfast的比较
HCM患者有LGE心肌节段的f值及ADCfast均低于无LGE心肌节段,差异均有统计学意义(Z=- 2.704、- 2.143,P均<0.05),而两者之间ADCslow差异无统计学意义(P= 0.130)。见表 4。
|
|
表 4 HCM患者无LGE及有LGE心肌节段f值、ADCslow、ADCfast的比较 Tab 4 Comparison of f value, ADCslow and ADCfast between myocardial segments with and without LGE in HCM patients |
2.6 EDTH与IVIM各参数相关性分析
对HCM患者各心肌节段IVIM参数f值、ADCfast、ADCslow与EDTH进行Spearman相关分析,结果显示EDTH与f值及ADCfast均呈负相关(r=- 0.195、- 0.282,P均<0.01),与ADCslow无明显相关性(r=- 0.041,P= 0.422)。
3 讨论MVD是HCM的一个重要病理特征[15],其微循环损伤程度可作为评价HCM预后及危险分层的因素之一,严重MVD是HCM患者不良结局的重要预测因素[16-17]。CMR可在一次检查中同时评价形态、功能及心肌组织特性[18],是冠状动脉微血管功能障碍综合诊断效能最高的无创性检查方法[19]。
既往对于HCM微循环障碍的研究主要采用CMR心肌首过灌注成像[8, 20],但是该方法需要注射造影剂,造影剂在脑、肾脏的沉积会引起造影剂毒性反应,尤其不适用于肾功能受损的患者。多b值、双指数模型IVIM技术可同时实现组织内的水分子扩散和微循环灌注的无造影剂定量评价[21-22]。通过后处理软件勾画ROI可获取反映组织微循环灌注的参数f值、ADCfast及反映水分子扩散的参数ADCslow。该技术已经广泛用于神经、骨骼肌肉系统、腹盆部及乳腺等组织器官[23-26],但应用于心脏方面的研究较少。Callot等[27]首次在犬体内进行了心脏的IVIM成像及参数测量,证明IVIM在技术层面具有可行性,使IVIM评价心肌微循环功能成为可能。此后也有外学者采用不同的技术方法证实了IVIM成像应用于健康人及部分心脏疾病患者活体心脏的可行性[1, 8, 12, 28-29]。
本研究应用IVIM技术评估HCM患者心肌微循环功能,结果显示HCM患者ADCfast低于健康对照,差异有统计学意义,说明HCM患者较健康对照血流灌注减低,存在MVD。进一步分析发现,HCM患者肥厚节段组f值及ADCfast均低于非肥厚节段组,差异均有统计学意义,但肥厚节段组中不同肥厚程度亚组之间仅ADCfast的差异有统计学意义,因此可以推测在反映微循环灌注的这2个参数中,ADCfast较f值更灵敏,这与Callot等[27]及陈玉涛和刘文华[30]的研究结果一致。同时本研究结果显示与健康对照相比,无论是HCM患者非肥厚节段还是肥厚节段心肌均存在MVD,且与非肥厚节段组比较肥厚节段MVD更严重,这与既往研究结果[28, 31]一致,也符合HCM病理生理学机制:(1)自身心肌细胞过度肥大,心肌纤维紊乱而无再生毛细血管,使单位面积心肌血管量相对减少,血流灌注减少;(2)肥厚心肌中的微小血管内膜和(或)中膜异常增生会导致微血管的管壁增厚、管腔狭窄,于扩张时血管阻力显著增加;(3)肥厚心肌节段的僵硬程度相应增加,使左心室充盈受阻,舒张功能下降,增加了血流阻力;(4)伴随着舒张功能下降,后负荷增加,继而心肌细胞耗氧量也随之增加[1]。上述原因导致MVD引起心肌缺血。
HCM组不同肥厚程度亚组分析结果显示,中度、重度与极重度亚组ADCfast均低于轻度肥厚亚组,但不同肥厚程度亚组间两两比较ADCfast差异均无统计学意义,可能是因为重度与极重度肥厚节段亚组样本量小(均仅4例)。IVIM各参数与EDTH相关性分析结果显示,EDTH与f值及ADCfast均呈负相关,说明心肌肥厚程度越严重MVD越严重,由此引起的心肌缺血、心力衰竭等一系列复杂终点事件的风险可能越大。
心肌纤维化是HCM患者发生心源性猝死的危险因素[32],CMR晚期LGE序列能够准确、直观地反映心肌纤维化的形态及范围。本研究结果显示,有LGE心肌节段灌注低于无LGE心肌节段,说明出现纤维化的MVD更严重。有学者认为纤维化由心肌缺血所致[33],但两者的关系尚不明确。本研究中无LGE心肌节段f值及ADCfast也均低于健康对照,说明IVIM可在心肌纤维化出现之前更早地检出MVD。目前,临床对于HCM的评价主要集中在形态、功能及心肌纤维化等方面,但越来越多的研究表明,MVD也是影响HCM患者预后的主要因素之一。因此,通过IVIM技术早期发现MVD有助于对存在MVD的患者进行早期干预和治疗,从而改善患者的临床症状及预后。
本研究样本量小,可能是导致不同肥厚程度亚组之间ADCfast差异无统计学意义的原因。另外,未按照有无梗死及LGE严重程度进行分层分析,也未对随访数据进行分析。
综上所述,IVIM技术无需注射造影剂,可实现HCM MVD的无创、早期、定量评估,为HCM患者心肌缺血的早期诊断及干预提供了可靠的参考依据。
| [1] |
YIN L, XU H Y, ZHENG S S, ZHU Y, XIAO J X, ZHOU W, et al. 3.0 T magnetic resonance myocardial perfusion imaging for semi-quantitative evaluation of coronary microvascular dysfunction in hypertrophic cardiomyopathy[J]. Int J Cardiovasc Imaging, 2017, 33: 1949-1959. DOI:10.1007/s10554-017-1189-9 |
| [2] |
朱黎. CMRI多技术对肥厚型心肌病诊断和风险评估及研究进展[J]. 临床放射学杂志, 2017, 36: 151-153. |
| [3] |
喻思思, 俞瑶涵, 唐雪培, 邹倩, 李淑豪, 郑甜, 等. MR特征追踪技术定量评估肥厚型心肌病心肌应变[J]. 中国医学影像技术, 2017, 33: 1129-1133. |
| [4] |
HINDIEH W, CHAN R, RAKOWSKI H. Complementary role of echocardiography and cardiac magnetic resonance in hypertrophic cardiomyopathy[J/OL]. Curr Cardiol Rep, 2017, 19: 81. doi: 10.1007/s11886-017-0897-z.
|
| [5] |
GUTIÉRREZ-BARRIOS A, CAMACHO-JURADO F, DÍAZ-RETAMINO E, GAMAZA-CHULIÁN S, AGARRADO-LUNA A, ONETO-OTERO J, et al. Invasive assessment of coronary microvascular dysfunction in hypertrophic cardiomyopathy:the index of microvascular resistance[J]. Cardiovasc Revasc Med, 2015, 16: 426-428. DOI:10.1016/j.carrev.2015.06.008 |
| [6] |
曾晓聪, 文宏, 李醒三. 肥厚型心肌病微循环障碍的研究进展[J]. 内科理论与实践, 2017, 12: 66-69. |
| [7] |
ISMAIL T F, HSU L Y, GREVE A M, GONÇALVES C, JABBOUR A, GULATI A, et al. Coronary microvascular ischemia in hypertrophic cardiomyopathy-a pixel-wise quantitative cardiovascular magnetic resonance perfusion study[J/OL]. J Cardiovasc Magn Reson, 2014, 16: 49. doi: 10.1186/s12968-014-0049-1.
|
| [8] |
黄璐, 夏黎明, 韩瑞, 孙子燕, 白杨. 磁共振心肌首过灌注成像评价肥厚型心肌病微循环功能障碍[J]. 中国医学影像技术, 2013, 29: 394-397. |
| [9] |
ZHANG Y D, LI M, QI L, WU C J, WANG X. Hypertrophic cardiomyopathy:cardiac structural and microvascular abnormalities as evaluated with multi-parametric MRI[J]. Eur J Radiol, 2015, 84: 1480-1486. DOI:10.1016/j.ejrad.2015.04.028 |
| [10] |
TROALEN T, CAPRON T, BERNARD M, KOBER F. In vivo characterization of rodent cyclic myocardial perfusion variation at rest and during adenosine-induced stress using cine-ASL cardiovascular magnetic resonance[J/OL]. J Cardiovasc Magn Reson, 2014, 16: 18. doi: 10.1186/1532-429X-16-18.
|
| [11] |
MOULIN K, CROISILLE P, FEIWEIER T, DELATTRE B M, WEI H, ROBERT B, et al. In vivo free-breathing DTI and IVIM of the whole human heart using a real-time slice-followed SE-EPI navigator-based sequence:a reproducibility study in healthy volunteers[J]. Magn Reson Med, 2016, 76: 70-82. DOI:10.1002/mrm.v76.1 |
| [12] |
SPINNER G R, VON DEUSTER C, TEZCAN K C, STOECK C T, KOZERKE S. Bayesian intravoxel incoherent motion parameter mapping in the human heart[J/OL]. J Cardiovasc Magn Reson, 2017, 19: 85. doi: 10.1186/s12968-017-0391-1.
|
| [13] |
罗马, 张卫东. 体素内不相干运动双指数模型的技术现况[J]. 磁共振成像, 2017, 8: 265-269. |
| [14] |
刘明熙, 张挽时, 张子衡, 孟利民, 龚万沣, 刘洁, 等. 多个低b值DWI技术在心脏成像中的初步研究[J]. 中国医疗设备, 2016, 31: 42-47. |
| [15] |
HERNANDEZ L E. Myocardial stress perfusion magnetic resonance in children with hypertrophic cardiomyopathy[J]. Cardiol Young, 2018, 28: 702-708. DOI:10.1017/S1047951118000094 |
| [16] |
BRAVO P E, DI CARLI M F, DORBALA S. Role of PET to evaluate coronary microvascular dysfunction in non-ischemic cardiomyopathies[J]. Heart Fail Rev, 2017, 22: 455-464. DOI:10.1007/s10741-017-9628-1 |
| [17] |
OLIVOTTO I, GIROLAMI F, SCIAGRÀ R, ACKERMAN M J, SOTGIA B, BOS J M, et al. Microvascular function is selectively impaired in patients with hypertrophic cardiomyopathy and sarcomere myofilament gene mutations[J]. J Am Coll Cardiol, 2011, 58: 839-848. DOI:10.1016/j.jacc.2011.05.018 |
| [18] |
WENG Z, YAO J, CHAN R H, HE J, YANG X, ZHOU Y, et al. Prognostic value of LGE-CMR in HCM:a meta-analysis[J]. JACC Cardiovasc Imaging, 2016, 9: 1392-1402. DOI:10.1016/j.jcmg.2016.02.031 |
| [19] |
中华医学会心血管病学分会基础研究学组, 中华医学会心血管病学分会介入心脏病学组, 中华医学会心血管病学分会女性心脏健康学组, 中华医学会心血管病学分会动脉粥样硬化和冠心病学组. 冠状动脉微血管疾病诊断和治疗的中国专家共识[J]. 中国循环杂志, 2017, 32: 421-430. DOI:10.3969/j.issn.1000-3614.2017.05.003 |
| [20] |
KNAAPEN P, GERMANS T, CAMICI P G, RIMOLDI O E, TEN CATE F J, TEN BERG J M, et al. Determinants of coronary microvascular dysfunction in symptomatic hypertrophic cardiomyopathy[J]. Am J Physiol Heart Circ Physiol, 2008, 294: H986-H993. DOI:10.1152/ajpheart.00233.2007 |
| [21] |
GUO T, CHEN J, WU B, ZHENG D, JIAO S, SONG Y, et al. Use of intravoxel incoherent motion diffusion-weighted imaging in identifying the vascular and avascular zones of human meniscus[J]. J Magn Reson Imaging, 2017, 45: 1090-1096. DOI:10.1002/jmri.25476 |
| [22] |
NGUYEN A, LEDOUX J B, OMOUMI P, BECCE F, FORGET J, FEDERAU C. Application of intravoxel incoherent motion perfusion imaging to shoulder muscles after a lift-off test of varying duration[J]. NMR Biomed, 2016, 29: 66-73. DOI:10.1002/nbm.3449 |
| [23] |
PAVILLA A, ARRIGO A, MEJDOUBI M, DUVAUFERRIER R, GAMBAROTA G, SAINT-JALMES H. Measuring cerebral hypoperfusion induced by hyperventilation challenge with intravoxel incoherent motion magnetic resonance imaging in healthy volunteers[J]. J Comput Assist Tomogr, 2018, 42: 85-91. |
| [24] |
LIM H K, JEE W H, JUNG J Y, PAEK M Y, KIM I, JUNG C K, et al. Intravoxel incoherent motion diffusion-weighted MR imaging for differentiation of benign and malignant musculoskeletal tumours at 3 T[J/OL]. Br J Radiol, 2018, 91: 20170636. doi: 10.1259/bjr.20170636.
|
| [25] |
孟闫凯, 张翀达, 张红梅, 叶枫, 欧阳汉, 赵心明, 等. 直肠癌体素内不相干运动参数测量的可重复性研究[J]. 中华肿瘤杂志, 2017, 39: 910-915. DOI:10.3760/cma.j.issn.0253-3766.2017.12.006 |
| [26] |
JIANG L, LU X, HUA B, GAO J, ZHENG D, ZHOU Y. Intravoxel incoherent motion diffusion-weighted imaging versus dynamic contrast-enhanced magnetic resonance imaging:comparison of the diagnostic performance of perfusion-related parameters in breast[J]. J Comput Assist Tomogr, 2018, 42: 6-11. |
| [27] |
CALLOT V, BENNETT E, DECKING U K, BALABAN R S, WEN H. In vivo study of microcirculation in canine myocardium using the IVIM method[J]. Magn Reson Med, 2003, 50: 531-540. DOI:10.1002/(ISSN)1522-2594 |
| [28] |
MOU A, ZHANG C, LI M, JIN F, SONG Q, LIU A, et al. Evaluation of myocardial microcirculation using intravoxel incoherent motion imaging[J]. J Magn Reson Imaging, 2017, 46: 1818-1828. DOI:10.1002/jmri.v46.6 |
| [29] |
SAEED M, VAN T A, KRUG R, HETTS S W, WILSON M W. Cardiac MR imaging:current status and future direction[J]. Cardiovasc Diagn Ther, 2015, 5: 290-310. |
| [30] |
陈玉涛, 刘文华. 体素内非相干运动(IVIM)扩散加权成像对骨骼肌运动血流灌注变化的相关性研究[J]. 泰山医学院学报, 2015, 36: 978-981. DOI:10.3969/j.issn.1004-7115.2015.09.005 |
| [31] |
TO A C, DHILLON A, DESAI M Y. Cardiac magnetic resonance in hypertrophic cardiomyopathy[J]. JACC Cardiovasc Imaging, 2011, 4: 1123-1137. DOI:10.1016/j.jcmg.2011.06.022 |
| [32] |
O'HANLON R, GRASSO A, ROUGHTON M, MOON J C, CLARK S, WAGE R, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy[J]. J Am Coll Cardiol, 2010, 56: 867-874. DOI:10.1016/j.jacc.2010.05.010 |
| [33] |
MARON B J. Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy[J]. Circulation, 2010, 121: 445-456. DOI:10.1161/CIRCULATIONAHA.109.878579 |
 2019, Vol. 40
2019, Vol. 40


