随着介入治疗技术与器具的快速发展,血管内治疗已成为颅内动脉瘤的主要治疗方式。血流导向装置的问世进一步颠覆了传统血管内治疗的理念,它通过改变动脉瘤瘤腔及其载瘤动脉的血流动力学,促进瘤内血栓的形成,最终达到治愈动脉瘤的目的。在临床应用经验不断积累和机制研究不断深入的基础上,各家介入器具厂商也相继推出了自己的血流导向装置产品,目前多见Pipeline血流导向装置(美国Medtronic公司)的相关临床报道[1]。由我国自主研发的Tubridge血流导向装置[微创神通医疗科技(上海)有限公司]也已获准上市,其性能与疗效已得到多中心临床研究证实[1-2]。本研究回顾性分析我科应用上述两种血流导向装置治疗复杂颅内动脉瘤的单中心数据,进一步探讨其安全性与有效性。
1 资料和方法 1.1 病例资料回顾性连续纳入2010年8月至2017年12月我科应用血流导向装置治疗的复杂颅内动脉瘤病例,收集其临床及影像学资料进行分析。纳入标准:(1)最大径≥10.0 mm或既往治疗后复发的颅内动脉瘤;(2)应用血流导向装置进行治疗。排除标准:(1)合并其他颅内血管性疾病;(2)严重肝肾功能不全;(3)临床及影像资料不完整;(4)拒绝签署手术知情同意书。本研究通过我院伦理委员会审批。
最终纳入99例患者共101个复杂颅内动脉瘤。其中男26例(26.3%),女73例(73.7%);患者年龄为20~78岁,平均年龄为(53.9±11.9)岁。89例(89.9%)患者为首次行动脉瘤治疗,10例(10.1%)患者因既往治疗后复发而行再治疗。首次治疗的89例患者中,首发症状为头痛者20例(22.5%)、头晕者22例(24.7%)、复视或视力下降者31例(34.8%)、短暂性脑缺血或缺血性脑卒中症状者5例(5.6%)、意外发现11例(12.4%)。
1.2 动脉瘤特征101个颅内动脉瘤中未破裂动脉瘤91个(90.1%),其中囊性动脉瘤87个(86.1%)、夹层动脉瘤4个(4.0%);其余10个(9.9%)为复发动脉瘤再治疗。动脉瘤最大径范围为10.1~32.2 mm,平均最大径为(19.6±6.6)mm,其中10.0~14.9 mm者共31个(30.7%)、15.0~24.9 mm者共44个(43.6%)、≥25.0 mm者共26个(25.7%)。在动脉瘤部位分布上,94个(93.1%)位于前循环,包括颈内动脉海绵窦段50个(49.5%)、颈内动脉眼动脉段17个(16.8%)、颈内动脉床突段19个(18.8%)、颈内动脉末段8个(7.9%);其余7个(6.9%)位于后循环。
1.3 治疗策略所有手术操作均在气管插管全身静脉麻醉下经股动脉穿刺入路进行。穿刺成功后予以全身肝素化。行常规旋转数字减影血管造影(digital subtraction angiography,DSA)检查并进行三维重建,测量动脉瘤和载瘤动脉远端及近端直径,依此制定治疗策略,选择合适的血流导向装置(Tubridge血流导向装置或Pipeline血流导向装置)及弹簧圈。在血流导向装置释放过程中,以6 F或7 F导引导管为支撑,在微导丝辅助下将支架微导管[T-track微导管,微创神通医疗科技(上海)有限公司;Marksman微导管,美国Medtronic公司]超选至载瘤动脉远端,通过“Y”形阀将血流导向装置置入微导管内,将支架输送至微导管头端,在X线透视下定位。固定输送导丝,并缓慢回撤微导管释放血流导向装置,通过推送血流导向装置和回撤微导管的配合,增加瘤颈处的金属覆盖率,并促进支架的充分打开及贴壁。释放完成后使用Artis Zee Biplane VC 14医用血管造影机(德国西门子医疗系统有限公司)行动态计算机断层扫描(Dyna computed tomography,DynaCT)或使用Allura Xper FD20 Biplane造影机(荷兰Philips公司)行VasoCT,评估血流导向装置打开及贴壁情况。对于血流导向装置打开欠满意或贴壁不良者,采用微导丝成襻技术或高顺应性球囊(Hyperform或Scepter球囊,美国Medtronic公司)予以后扩张。对于拟行血流导向装置结合弹簧圈栓塞治疗者,须在释放血流导向装置前将输送弹簧圈的微导管提前置于瘤内,应用支架后释放技术进行辅助栓塞。最后进行工作角度及标准正侧位造影,评估支架打开及远端血流的情况。
1.4 抗血小板聚集方案患者于术前常规口服阿司匹林(300 mg 1次/d)+氯吡格雷(75 mg 1次/d),不少于3 d。术中对患者行全身肝素化,术后肝素自然中和。术后6周内继续口服阿司匹林(100 mg、1次/d)+氯吡格雷(75 mg 1次/d)。3个月后停用氯吡格雷,继续口服阿司匹林(100 mg、1次/d),建议终身服用。
1.5 术后评估及临床和影像学随访术后即刻,由2名高年资的神经外科医师共同评价手术效果。对于合并使用弹簧圈的动脉瘤采用Raymond分级对动脉瘤栓塞情况进行评估:RaymondⅠ级为致密栓塞,RaymondⅡ级为瘤颈残留,RaymondⅢ级为瘤体残留。将单纯行血流导向装置支架成形术的动脉瘤根据支架置入后造影剂滞留情况分为有明显滞留和无明显滞留[3]。本研究中复发动脉瘤在既往治疗中均合并使用弹簧圈,即刻结果以Raymond分级进行评价。
治疗前、治疗后及出院时均采用改良Rankin量表(modified Rankin scale,mRS)对患者临床症状进行评分,并记录新发的神经系统症状,之后对患者进行门诊或电话随访。建议所有患者术后3个月行头部磁共振血管造影(magnetic resonance angiography,MRA),6、12个月行DSA检查,以后每年定期行头部MRA检查。通过与前次影像学结果比较,将动脉瘤影像学结果判定为完全闭塞、进一步血栓形成、稳定、复发4类情况[2]。
1.6 统计学处理采用SPSS 18.0软件进行统计学分析。计量资料以x±s表示,两组间比较采用非参数检验;计数资料以例数和百分数表示,组间比较采用χ2检验或其校正公式。检验水准(α)为0.05。
2 结果 2.1 手术情况对99例患者101个动脉瘤共置入116枚血流导向装置(Tubridge 74枚、Pipeline 42枚),均成功输送并释放。其中,61个(60.4%)动脉瘤应用Tubridge血流导向装置治疗(Tubridge组),40个(39.6%)应用Pipeline血流导向装置治疗(Pipeline组)。Tubridge组共61例患者61个动脉瘤,平均年龄为(52.3±12.8)岁,男18例、女43例;Pipeline组共38例患者40个动脉瘤,男8例、女30例,平均年龄为(56.8±10.1)岁。两组性别和年龄差异均无统计学意义(P均>0.05)。
在治疗策略上,71个(70.3%)动脉瘤行血流导向装置合并使用弹簧圈治疗(图 1),20个(19.8%)行单纯血流导向装置置入治疗(图 2),10个(9.9%)复发动脉瘤再次行血流导向装置合并使用弹簧圈治疗。4个颈内动脉海绵窦段动脉瘤行Pipeline血流导向装置治疗时为进一步改善支架在血管迂曲处的贴壁情况行高顺应性球囊扩张,扩张后支架贴壁情况良好,载瘤动脉通畅。81个行血流导向装置合并使用弹簧圈治疗的动脉瘤(含复发动脉瘤),术后即刻评估2个(2.5%)达到RaymondⅠ级,4个(4.9%)达到RaymondⅡ级,75个(92.6%)为RaymondⅢ级;20个行单纯血流导向装置置入治疗的动脉瘤中,17个(85.0%)在血流导向装置置入后动脉瘤腔内造影剂明显滞留,3个(15.0%)未见造影剂明显滞留。详见表 1。
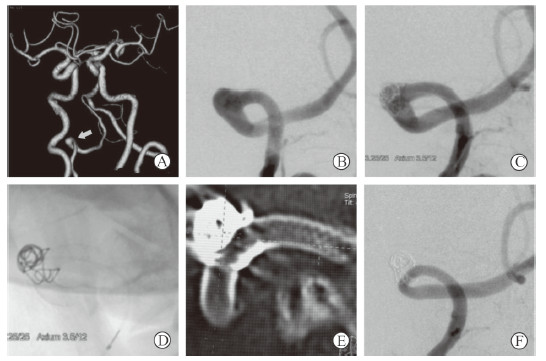
|
图 1 1例行血流导向装置合并使用弹簧圈治疗的颅内动脉瘤患者影像学资料 Fig 1 Imaging data of a patient with intracranial aneurysm treated by embolization device with adjunctive coil Male, 69 years old, dizziness and headache for 3 months and hypertension for 10 years. Computed tomography angiography showed an aneurysm (arrow) in right vertebral artery (A), and intraoperative angiography confirmed the right vertebral artery dissecting aneurysm (B), with proximal vascular diameter of 3.3 mm and distal vascular diameter of 3.2 mm. After a Pipeline embolization device was successfully deployed with 1 adjunctive coil, aneurysm body remained and the parent artery was unobstructed immediately (C), and the shapes of stent and coils were satisfactory (D, E). At 8 months after operation, the aneurysm was completely undetected and the parent artery was unobstructed (F). There was no new neurological dysfunction after operation |
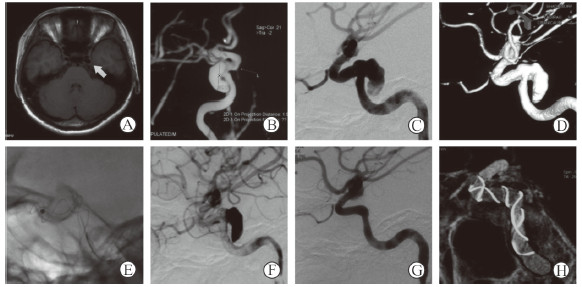
|
图 2 1例行单纯血流导向装置治疗的颅内动脉瘤患者影像学资料 Fig 2 Imaging data of a patient with intracranial aneurysm treated with embolization device alone Female, 24 years old, blurred vision for 2 months. Magnetic resonance angiography showed a large aneurysm (arrow) of internal carotid artery cavernous segment (A, B). Left internal carotid artery angiography confirmed the cavernous sinus aneurysm of the left internal carotid artery (C). The maximum diameter of the aneurysm was 15.4 mm, with a wide neck of 6.7 mm. The proximal and distal diameters of the parent artery were 4.5 mm and 4.8 mm, respectively (D). A Tubridge embolization device was deployed successfully (E). Immediately after operation, the contrast agent was obviously retained and the parent artery was unobstructed (F). At 12 months after operation, the aneurysm was completely occluded (G), and the shape of stent was satisfactory (H) |
|
|
表 1 颅内动脉瘤一般资料及患者血流导向装置治疗情况 Tab 1 Baseline characteristics of intracranial aneurysms and treatment outcomes of the patients treated by embolization devices |
2.2 临床随访结果
围手术期内Pipeline血流导向装置组1例患者于置入术后2 d发生致死性动脉瘤破裂后死亡。2例发生术后缺血性并发症,其中1例左侧海绵窦段动脉瘤在Pipeline血流导向装置置入术后2 h突发言语含糊,磁共振成像(magnetic resonance imaging,MRI)检查示左侧基底节区新鲜梗死灶,5 d后发生肢体无力,头颅计算机断层扫描(computed tomography,CT)示梗死区域出血转化,出院时mRS评分为3分;另1例为颈内动脉眼动脉段动脉瘤患者行Tubridge血流导向装置治疗,脉络膜前动脉发生闭塞,出院时mRS评分为2分,随访过程中功能缺损进一步恢复。98例患者治疗前、出院时及末次随访mRS评分见表 2,Tubridge组和Pipeline组各时间点mRS评分差异均无统计学意义(P均>0.05)。
|
|
表 2 颅内动脉瘤治疗前、出院时及随访时改良Rankin量表评分 Tab 2 Clinical outcomes of patients treated by embolization devices |
共91例患者获得临床随访,随访时间为6~74个月,平均(28.7±12.2)个月。7例患者失访。随访过程中无新发出血及缺血事件。以复视或视力下降为首发症状的31例患者中,23例(74.2%)症状缓解,8例(25.8%)症状稳定,无一例患者症状进一步加重。
2.3 影像学随访结果在101个颅内动脉瘤中,共88个(87.1%)获得了影像学随访,失访(含死亡病例)13个(12.9%)。63个动脉瘤获得了短期随访(0~6个月),其中42个(66.7%)动脉瘤完全闭塞,15个(23.8%)进一步血栓形成,5个(7.9%)稳定,1个(1.6%)复发;49个动脉瘤获得了中长期随访(7~18个月),其中36个(73.5%)动脉瘤完全闭塞,11个(22.4%)进一步血栓形成,1个(2.0%)稳定,1个(2.0%)复发;28个动脉瘤获得了长期随访(>18个月),其中25个(89.3%)动脉瘤完全闭塞,2个(7.1%)进一步血栓形成,1个(3.6%)稳定,无动脉瘤复发。全部动脉瘤末次影像学随访动脉瘤完全闭塞率为72.7%(64/88),Tubridge组和Pipeline组末次随访动脉瘤完全闭塞率差异无统计学意义(χ2=0.049,P=0.824;表 3)。载瘤动脉及覆盖分支均保持通畅,远端血流未受影响,无相关临床症状。
|
|
表 3 血流导向装置治疗颅内动脉瘤影像学随访动脉瘤完全闭塞率 Tab 3 Complete occlusion rate of intracranial aneurysms treated by embolization devices during follow-up |
3 讨论
血流导向装置的临床应用改变了颅内动脉瘤介入治疗的传统理念,随着临床经验的不断积累,已成为复杂颅内动脉瘤的重要治疗手段[5-6]。相较于常规颅内支架,血流导向装置通过重塑颅内动脉瘤及其载瘤动脉的血流动力学环境,促进瘤内血栓的形成,并为新生血管内膜提供附着,从而最终实现颅内动脉瘤的治愈性闭塞[7]。既往研究显示,应用血流导向装置治疗大型、巨大型等复杂颅内动脉瘤,其完全闭塞率显著高于常规弹簧圈辅助栓塞治疗[8]。Kallmes等[1]报道应用Pipeline血流导向装置治疗1 221例颅内动脉瘤,随访显示6个月完全闭塞率达75%,1年完全闭塞率达85.5%;而Liu等[2]应用Tubridge血流导向装置治疗复杂动脉瘤的前瞻性多中心随机对照研究结果显示,Tubridge治疗组术后6个月的完全闭塞率达75.3%,显著高于支架辅助弹簧圈栓塞组的24.5%。
除了具有较高的治愈率外,血流导向装置相较于载瘤动脉闭塞、弹簧圈辅助栓塞等常规介入治疗方法能够有效缓解大型、巨大型动脉瘤产生的占位效应。Zanaty等[9]比较了应用血流导向装置、支架辅助栓塞、单纯栓塞及载瘤动脉闭塞4种方法治疗颈内动脉海绵窦段动脉瘤的临床疗效,结果显示接受血流导向装置治疗的患者症状缓解率(92.16%)显著高于其他治疗方案组。Puffer等[10]报道使用Pipeline血流导向装置治疗44例颈内动脉海绵窦段动脉瘤患者,随访时90%的患者症状改善。而在本组病例中以复视或视力下降为首发症状的31例患者,在临床随访过程中23例(74.2%)症状缓解,8例(25.8%)症状稳定,均未出现症状加重。
虽然血流导向装置对于治疗复杂颅内动脉瘤较常规介入治疗方法更具优势,但仍有部分病例在接受血流导向装置治疗后未能实现动脉瘤的有效闭塞。Brasiliense等[11]研究显示,动脉瘤的大小与部位会影响血流导向装置治疗闭塞率,体积较小、位于颈内动脉近端的动脉瘤其闭塞率较高。除了动脉瘤本身的特征,Rouchaud等[12]应用兔动脉瘤模型对影响血流导向装置闭塞率的因素进行了研究,结果显示血流导向装置支架的贴壁情况是影响动脉瘤闭塞的关键因素;Aquarius等[13]应用大鼠动脉瘤模型也得到了相似结论,认为提高血流导向装置支架贴壁性比提高金属覆盖率更重要。在本组病例中,有2例患者在血流导向装置释放过程中发生支架贴壁不良,因此应用球囊对支架进行了后扩张,后期也取得了良好的治疗效果。合并应用弹簧圈被认为能够加快瘤内血栓的形成,提高血流导向装置的远期疗效[14];但一项基于Pipeline血流导向装置的前瞻性研究显示,合并应用弹簧圈治疗较单独血流导向装置治疗的平均手术时间增加,同时其残死率也较高,仍需进一步研究[15]。目前仍多主张在硬膜环内,特别是对大型、巨大型动脉瘤的治疗应合并应用弹簧圈[6]。
血流导向装置治疗颅内动脉瘤整体上是安全的,但仍存在一定的并发症风险。一项meta分析显示血流导向装置治疗颅内动脉瘤的总体并发症率为17%,其中永久性致残率为3.7%,致死率为2.8%[16]。动脉瘤迟发性出血是血流导向装置置入后最严重的并发症之一[17],在本组病例中,亦有1例患者在Pipeline血流导向装置置入后2 d发生致死性的动脉瘤破裂出血。Cebral等[18]应用流体力学模拟技术对血流导向装置置入后的血流动力学变化进行分析,结果显示血流导向装置置入后会引起瘤内压力增高,这可能是导致动脉瘤发生破裂的原因之一。同时,Ikeda等[19]通过对血流导向装置术后动脉瘤破裂的患者进行尸体解剖发现,虽然动脉瘤的流出道已形成血栓,但在流入道仍有入射血流的存在,也提示血流导向装置置入后动脉瘤血流动力学环境的变化与动脉瘤破裂有关。相较于血流导向装置术后动脉瘤破裂,迟发性的脑实质出血发生率可能更高,文献报道其发生率为4.0%~8.5%,但其发生原因尚不明确[20-21]。本组病例中也有1例患者发生实质性脑出血,但其术后早期MRI检查有明确的缺血性脑卒中证据,提示缺血性脑卒中后出血转化可能是血流导向装置术后脑实质出血的机制之一。由于血流导向装置的金属覆盖率较常规颅内支架更高,其发生缺血性事件的风险不容忽视,缺血性事件主要由支架内血栓、载瘤动脉或分支血管闭塞所致[16]。本组病例中有1例患者血流导向装置覆盖的脉络膜前动脉发生闭塞导致缺血性事件。文献报道血流导向装置覆盖分支在随访中发生血流减少或闭塞的比例为15.8%~20%,其是否会导致相关缺血性并发症仍需进一步的长期随访数据[22-23]。
由于血流导向装置为编织支架,其释放技术有别于以往的激光雕刻支架,在操作过程中应注重推拉技术的配合,以使血流导向装置在精确定位的同时能够充分打开和贴壁。血流导向装置释放后建议常规进行DynaCT或VasoCT重建,以准确判断其打开及贴壁情况。如果血流导向装置打开不满意,可以使用“J”形导丝技术进行支架内按摩或应用球囊进行扩张。本研究中应用的两种血流导向装置在安全性与有效性上相似,但在支架特性和实际操作中又略有不用,其根本在于两种血流导向装置采用的金属材质不同。Pipeline血流导向装置为钴铬合金材料,具有较好的径向支撑力,打开后远端可提供较好的锚定,在长直血管中贴壁性良好,也正是因为较大的径向支撑力,Pipeline血流导向装置第1代的Classic和第2代的Flex均设计了头端保护装置,避免在输送过程中对血管造成损伤。而Tubridge为镍钛合金材料,能提供良好的柔顺性,通过推拉技术的配合在弯曲血管甚至是“S”形血管均能获得良好的贴壁,整体操作简单。以上支架性能及操作技巧上的不同在病例选择过程中应予以考虑。
综上所述,本研究结果显示应用Tubrdige和Pipeline两种血流导向装置治疗复杂颅内动脉瘤均能取得良好的疗效,但也存在一定的并发症风险。其有效性和安全性的进一步提升有赖于更深入的临床研究和机制研究。
| [1] |
KALLMES D F, BRINJIKJI W, CEKIRGE S, FIORELLA D, HANEL R A, JABBOUR P, et al. Safety and efficacy of the Pipeline embolization device for treatment of intracranial aneurysms:a pooled analysis of 3 large studies[J]. J Neurosurg, 2017, 127: 775-780. DOI:10.3171/2016.8.JNS16467 |
| [2] |
LIU J M, ZHOU Y, LI Y, LI T, LENG B, ZHANG P, et al. Parent artery reconstruction for large or giant cerebral aneurysms using the Tubridge flow diverter:a multicenter, randomized, controlled clinical trial (PARAT)[J]. AJNR Am J Neuroradiol, 2018, 39: 807-816. DOI:10.3174/ajnr.A5619 |
| [3] |
RAYMOND J, GUILBERT F, WEILL A, GEORGANOS S A, JURAVSKY L, LAMBERT A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils[J]. Stroke, 2003, 34: 1398-1403. DOI:10.1161/01.STR.0000073841.88563.E9 |
| [4] |
QUINN T J, DAWSON J, WALTERS M R, LEES K R. Reliability of the modified Rankin scale:a systematic review[J]. Stroke, 2009, 40: 3393-3395. DOI:10.1161/STROKEAHA.109.557256 |
| [5] |
WALCOTT B P, STAPLETON C J, CHOUDHRI O, PATEL A B. Flow diversion for the treatment of intracranial aneurysms[J]. JAMA Neurol, 2016, 73: 1002-1008. DOI:10.1001/jamaneurol.2016.0609 |
| [6] |
BRINJIKJI W, MURAD M H, LANZINO G, CLOFT H J, KALLMES D F. Endovascular treatment of intracranial aneurysms with flow diverters:a meta-analysis[J]. Stroke, 2013, 44: 442-447. DOI:10.1161/STROKEAHA.112.678151 |
| [7] |
LI Z F, FANG X G, YANG P F, HUANG Q H, ZHAO W Y, LIANG C, et al. Endothelial progenitor cells contribute to neointima formation in rabbit elastase-induced aneurysm after flow diverter treatment[J]. CNS Neurosci Ther, 2013, 19: 352-357. DOI:10.1111/cns.12086 |
| [8] |
ZHANG Y, ZHOU Y, YANG P, LIU J, XU Y, HONG B, et al. Comparison of the flow diverter and stent-assisted coiling in large and giant aneurysms:safety and efficacy based on a propensity score-matched analysis[J]. Eur Radiol, 2016, 26: 2369-2377. DOI:10.1007/s00330-015-4052-1 |
| [9] |
ZANATY M, CHALOUHI N, STARKE R M, BARROS G, SAIGH M P, SCHWARTZ E W, et al. Flow diversion versus conventional treatment for carotid cavernous aneurysms[J]. Stroke, 2014, 45: 2656-2661. DOI:10.1161/STROKEAHA.114.006247 |
| [10] |
PUFFER R C, PIANO M, LANZINO G, VALVASSORI L, KALLMES D F, QUILICI L, et al. Treatment of cavernous sinus aneurysms with flow diversion:results in 44 patients[J]. AJNR Am J Neuroradiol, 2014, 35: 948-951. DOI:10.3174/ajnr.A3826 |
| [11] |
BRASILIENSE L B C, AGUILAR-SALINAS P, MILLER D A, TAWK R G, SAUVAGEAU E A, HANEL R A. Analysis of predictors and probability of aneurysm occlusion in the internal carotid artery after treatment with Pipeline embolization device[J]. World Neurosurg, 2017, 107: 641-648. DOI:10.1016/j.wneu.2017.08.099 |
| [12] |
ROUCHAUD A, RAMANA C, BRINJIKJI W, DING Y H, DAI D, GUNDERSON T, et al. Wall apposition is a key factor for aneurysm occlusion after flow diversion:a histologic evaluation in 41 rabbits[J]. AJNR Am J Neuroradiol, 2016, 37: 2087-2091. DOI:10.3174/ajnr.A4848 |
| [13] |
AQUARIUS R, DE KORTE A, SMITS D, GOUNIS M, VERRIJP K, DRIESSEN L, et al. The importance of wall apposition in flow diverters[J]. Neurosurgery, 2019, 84: 804-810. DOI:10.1093/neuros/nyy092 |
| [14] |
BENDER M T, JIANG B, CAMPOS J K, LIN L M, BEATY N, VO C D, et al. Single-stage flow diversion with adjunctive coiling for cerebral aneurysm:outcomes and technical considerations in 72 cases[J]. J Neurointerv Surg, 2018, 10: 843-850. |
| [15] |
PARK M S, KILBURG C, TAUSSKY P, ALBUQUERQUE F C, KALLMES D F, LEVY E I, et al. Pipeline embolization device with or without adjunctive coil embolization:analysis of complications from the IntrePED Registry[J]. AJNR Am J Neuroradiol, 2016, 37: 1127-1131. DOI:10.3174/ajnr.A4678 |
| [16] |
ZHOU G, SU M, YIN Y L, LI M H. Complications associated with the use of flow-diverting devices for cerebral aneurysms: a systematic review and meta-analysis[J/OL]. Neurosurg Focus, 2017, 42: E17. doi: 10.3171/2017.3.FOCUS16450. https://www.ncbi.nlm.nih.gov/pubmed/28565981
|
| [17] |
ROUCHAUD A, BRINJIKJI W, LANZINO G, CLOFT H J, KADIRVEL R, KALLMES D F. Delayed hemorrhagic complications after flow diversion for intracranial aneurysms:a literature overview[J]. Neuroradiology, 2016, 58: 171-177. DOI:10.1007/s00234-015-1615-4 |
| [18] |
CEBRAL J R, MUT F, RASCHI M, SCRIVANO E, CERATTO R, LYLYK P, et al. Aneurysm rupture following treatment with flow-diverting stents:computational hemodynamics analysis of treatment[J]. AJNR Am J Neuroradiol, 2011, 32: 27-33. DOI:10.3174/ajnr.A2398 |
| [19] |
IKEDA H, ISHⅡ A, KIKUCHI T, ANDO M, CHIHARA H, ARAI D, et al. Delayed aneurysm rupture due to residual blood flow at the inflow zone of the intracranial paraclinoid internal carotid aneurysm treated with the Pipeline embolization device:histopathological investigation[J]. Interv Neuroradiol, 2015, 21: 674-683. DOI:10.1177/1591019915609121 |
| [20] |
WHITE A C, KUMPE D A, ROARK C D, CASE D E, SEINFELD J. Patterns, predictors, and outcomes of postprocedure delayed hemorrhage following flow diversion for intracranial aneurysm treatment[J/OL]. World Neurosurg, 2018, 115: e97-e104. doi: 10.1016/j.wneu.2018.03.190. https://www.sciencedirect.com/science/article/pii/S1878875018306727
|
| [21] |
CRUZ J P, CHOW M, O'KELLY C, MAROTTA B, SPEARS J, MONTANERA W, et al. Delayed ipsilateral parenchymal hemorrhage following flow diversion for the treatment of anterior circulation aneurysms[J]. AJNR Am J Neuroradiol, 2012, 33: 603-608. DOI:10.3174/ajnr.A3065 |
| [22] |
RANGEL-CASTILLA L, MUNICH S A, JALEEL N, CRESS M C, KRISHNA C, SONIG A, et al. Patency of anterior circulation branch vessels after Pipeline embolization:longer-term results from 82 aneurysm cases[J]. J Neurosurg, 2017, 126: 1064-1069. DOI:10.3171/2016.4.JNS16147 |
| [23] |
BHOGAL P, GANSLANDT O, BÄZNER H, HENKES H, PÉREZ M A. The fate of side branches covered by flow diverters-results from 140 patients[J]. World Neurosurg, 2017, 103: 789-798. DOI:10.1016/j.wneu.2017.04.092 |
 2019, Vol. 40
2019, Vol. 40


