2. 南部战区疾病预防控制中心, 广州 510507
2. Center for Disease Control and Prevention of Southern Theater Command of PLA, Guangzhou 510507, Guangdong, China
西尼罗病毒(West Nile virus,WNV)是有包膜的单正链RNA病毒,属于黄病毒科黄病毒属。该属成员还包括寨卡病毒、黄热病病毒、登革病毒、日本脑炎病毒和蜱传脑炎病毒等,均是高致病性病原体[1-2]。作为蚊媒叮咬传播的嗜神经性黄病毒,WNV是全球虫媒传播病毒性脑炎最重要的病原体[3]。WNV感染造成的机体炎性病理反应和中枢神经系统的严重损伤,可引起西尼罗热和西尼罗脑炎,并伴有持久的中枢神经系统后遗症或慢性肾脏疾病[4]。据报道,从我国新疆采集的蚊标本中分离出WNV,并且在当地出现了成人型西尼罗脑炎的流行[5-6]。2018年,美国、希腊、意大利和塞尔维亚各国相继发生WNV疫情,WNV感染已成为人类健康的严重威胁[7]。WNV通过在储存宿主鸟和蚊之间的循环传播而长期存在于自然界,人、马和其他脊椎动物均对WNV易感。人被携带病毒的伊蚊和库蚊叮咬感染是WNV的主要传播途径[8]。目前,尚无防治人感染WNV的特异性抗病毒药物和疫苗。
丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)家族广泛存在于哺乳动物体内,参与调控细胞的多种功能如增殖、分化、应激、凋亡和存活等。p38 MAPK是MAPK家族的重要成员,也是调控细胞应激和炎性应答的关键激酶。研究发现,抑制p38 MAPK不仅可减轻登革病毒感染导致的炎性病理反应[9],还能有效阻止寨卡病毒感染诱生的炎症反应[10]、降低WNV感染诱生的趋化因子水平[11]。这些研究均表明,虫媒病毒引发p38 MAPK途径信号转导与其致病机制密切相关。本研究拟探讨WNV感染人神经母细胞瘤细胞SH-SY5Y后对p38 MAPK途径的影响和该途径在WNV复制及调控细胞应激、炎性应答相关分子表达中的作用。
1 材料和方法 1.1 材料p38 MAPK、磷酸化p38 MAPK(phosphorylated p38 mitogen-activated protein kinase,p-p38 MAPK;Thr180/Tyr182)、β-actin兔多克隆抗体、对照siRNA、p38 MAPK siRNA(美国Cell Signaling Technology公司),辣根过氧化物酶标记的羊抗兔免疫球蛋白G(immunoglobulin G,IgG)、ECL化学发光液(美国Bio-Rad公司),DMEM培养基、Opti-MEM Ⅰ培养基、胎牛血清(fetal bovine serum,FBS)、胰蛋白酶、乙二胺四乙酸(ethylene diamine tetraacetic acid,EDTA)、Lipofectamine 2000、TRIzol试剂(美国Invitrogen公司),M-MLV反转录酶、dNTP Mix、Eastep qPCR Master Mix(美国Promega公司),随机引物、WNV非结构蛋白5、C/EBP同源蛋白(CCAAT/enhancer-binding protein homologous protein,CHOP)、干扰素刺激基因15(interferon-stimulated gene 15,ISG15)、白细胞介素(interleukin,IL)-6、活化转录因子(activating transcription factor,ATF)6α、甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GAPDH)引物(北京六合华大基因科技股份有限公司)。人神经母细胞瘤细胞SH-SY5Y、猴肾细胞Vero和仓鼠肾细胞BHK-21由海军军医大学(第二军医大学)海军医学系生物医学防护教研室保存。
1.2 细胞培养与病毒制备将SH-SY5Y细胞、Vero细胞、BHK-21细胞用含10% FBS的DMEM培养基在37 ℃、5% CO2孵箱内培养。细胞生长密度达到90%以上时,用含EDTA的胰蛋白酶消化,按1︰3的比例传代培养。WNV在Vero细胞内增殖,收集病毒、分装贮存于-80 ℃冰箱[12-13]。病毒滴度在BHK-21细胞上用蚀斑法测定[14]。同时,收集无病毒感染的Vero细胞培养上清作为处理细胞的对照(mock infection)。
1.3 WNV感染细胞将SH-SY5Y、Vero细胞接种于35 mm培养皿,培养过夜。吸弃皿内培养液,加入WNV在37 ℃分别孵育5、15、30、60 min,病毒的感染复数(multiplicity of infection,MOI)为2,收集WNV短时孵育SH-SY5Y、Vero细胞的裂解物样品。将SH-SY5Y细胞接种于35 mm培养皿,培养过夜后,吸弃皿内培养液,加2 MOI WNV至皿内,37 ℃吸附1 h,吸弃病毒液,用磷酸盐缓冲液(phosphate buffer saline,PBS)洗涤1次,加入含10% FBS的DMEM培养基分别培养12、24、48、60 h。WNV吸附1 h(感染时间为0 h)后开始计时。SH-SY5Y细胞的对照组用Vero细胞培养上清吸附1 h后,加入新鲜培养基分别培养至上述各时间点。Vero细胞的WNV感染组与对照组培养48 h。
1.4 siRNA转染将SH-SY5Y细胞接种于12孔培养板内,待细胞生长密度约50%时进行siRNA转染。使用Lipofectamine 2000转染siRNA,用Opti-MEM Ⅰ培养基稀释p38 MAPK siRNA、对照siRNA至终浓度为100 nmol/L,按照产品说明书操作。转染72 h后,收集细胞裂解物样品评估siRNA干扰效率;此外,细胞用PBS洗涤2次,2 MOI WNV感染24 h后收集细胞样品,检测WNV在敲低p38 MAPK表达的细胞内复制和宿主分子表达变化。
1.5 蛋白质印迹检测将WNV短时孵育细胞、WNV感染细胞和Vero细胞培养上清处理的对照细胞用PBS洗涤2次,裂解细胞制备样品[15]。样品经十二烷基硫酸钠-聚丙烯酰胺凝胶电泳分离后,将蛋白质转移至硝酸纤维素膜上,用5%脱脂奶粉于室温封闭2 h,用1︰1 000稀释的p38 MAPK、p-p38 MAPK、β-actin兔多克隆抗体在4 ℃孵育过夜。膜用TBST洗涤3次,5 min/次,用1︰2 000稀释的辣根过氧化物酶标记的羊抗兔IgG在室温孵育2 h,再用TBST洗涤3次,5 min/次。膜上加入ECL化学发光底物,用化学发光成像仪(英国GeneGnome公司)显色成像,利用Gene Tools分析软件进行图像分析。样品蛋白质的信号经内参β-actin均一处理后计算p38 MAPK、p-p38 MAPK水平的变化。
1.6 qRT-PCR检测在确定的样品收集时间点,WNV感染细胞和Vero细胞培养上清处理的对照细胞用PBS洗涤2次,用TRIzol试剂抽提细胞RNA,用多功能酶标仪(美国BioTek公司)检测RNA纯度和浓度后,使用随机引物、M-MLV反转录酶和dNTP Mix反转录成cDNA。以cDNA为模板,使用Eastep qPCR Master Mix,在Rotor-Gene 3000 PCR仪(澳大利亚Corbett公司)上扩增目的基因,利用Rotor-Gene 6.1.81软件,采用ΔΔCt法进行相对定量分析,以GAPDH为内参,每个样品检测3次[15]。WNV非结构蛋白5引物序列:正向5'-GAG TCC AAG AAG TCA GAG GGT ACA-3',反向5'-CCA CTC TTC ATG GTG ACA ATG TTC C-3';CHOP引物序列:正向5'-AGC TGG AAC CTG AGG AGA GA-3',反向5'-TGG ATC AGT CTG GAA AAG CA-3';ISG15引物序列:正向5'-GAC AAA TGC GAC GAA CCT CT-3',反向5'-CGG CCC TTG TTA TTC CTC A-3';IL-6引物序列:正向5'-CAA TCT GGA TTC AAT GAG GAG AC-3',反向5'-CTC TGG CTT GTT CCT CAC TAC TC-3';ATF6α引物序列:正向5'-TTT TGT GAG CGG GGA AAA GC-3',反向5'-TGG TCC CCA GAG AAA ATG GT-3';GAPDH引物序列:正向5'-TGG GCT ACA CTG AGC ACC AG-3',反向5'-AAG TGG TCG TTG AGG GCA AT-3'。
1.7 统计学处理数据以 x± s表示。采用Student’s t检验分析病毒RNA、宿主分子mRNA和p38 MAPK水平的差异。检验水准(α)为0.05。
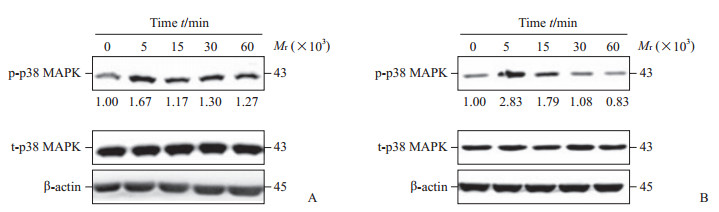
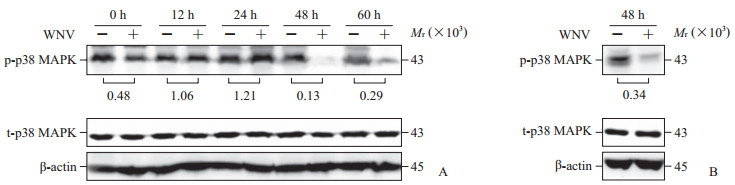
2 结果 2.1 WNV感染早期促进p38 MAPK磷酸化而晚期抑制其磷酸化蛋白质印迹分析结果显示,WNV孵育SH-SY5Y细胞5、15、30、60 min激活了p38 MAPK途径,表现为p38 MAPK磷酸化水平升高,其中病毒孵育5 min时能明显促进p38 MAPK的磷酸化(图 1A)。在Vero细胞,与对照组(0 min)相比,WNV孵育5、15、30 min也导致p38 MAPK磷酸化水平升高,并且病毒孵育5 min时也明显促进p38 MAPK磷酸化(图 1B)。如图 2A所示,WNV感染SH-SY5Y细胞12 h、24 h时激活了p38 MAPK途径,而感染48 h、60 h时则明显抑制了p38 MAPK途径。此外,WNV感染Vero细胞48 h,p38 MAPK磷酸化水平也明显降低(图 2B)。上述结果表明WNV感染早期(24 h以内)激活了SH-SY5Y细胞内p38 MAPK途径。
|
图 1 WNV短时孵育细胞内p38 MAPK的磷酸化水平 Fig 1 Levels of p38 MAPK phosphorylation in cells incubated with WNV for short durations A: SH-SY5Y cells; B: Vero cells. WNV: West Nile virus; MAPK: Mitogen-activated protein kinase; p-p38 MAPK: Phosphorylated p38 mitogen-activated protein kinase; t-p38 MAPK: Total p38 mitogen-activated protein kinase. Fold change of p-p38 MAPK levels in SH-SY5Y cells and Vero cells incubated with WNV over the levels in cells without WNV incubation (set to 1.00) was shown below each blot after being normalized to β-actin levels. Representative results out of three experiments were shown |
|
图 2 WNV感染细胞内p38 MAPK磷酸化的变化 Fig 2 Phosphorylation of p38 MAPK in cells during WNV infection A: SH-SY5Y cells; B: Vero cells. WNV: West Nile virus; MAPK: Mitogen-activated protein kinase; p-p38 MAPK: Phosphorylated p38 mitogen-activated protein kinase; t-p38 MAPK: Total p38 mitogen-activated protein kinase. Fold change of p-p38 MAPK levels in SH-SY5Y cells and Vero cells with WNV infection (+) over the levels in cells with mock infection (-) was shown below each blot after being normalized to β-actin levels. Results were representative of at least three experiments |
2.2 WNV在p38 MAPK敲低的细胞内复制增强
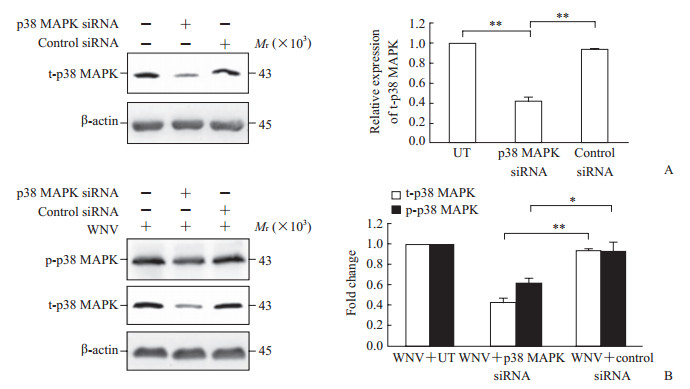
如图 3A所示,与未转染的对照细胞与转染对照siRNA的细胞相比,p38 MAPK siRNA转染72 h后SH-SY5Y细胞内p38 MAPK表达降低(P<0.01),表明p38 MAPK siRNA转染可敲低p38 MAPK。因WNV感染24 h激活SH-SY5Y细胞内p38 MAPK途径(图 2A),故p38 MAPK siRNA转染72 h后再以WNV感染转染细胞24 h。qRT-PCR检测显示,与对照siRNA转染的细胞相比,p38 MAPK siRNA转染的细胞内WNV RNA水平升高(168% vs 100%,P<0.05)。同时,WNV对p38 MAPK磷酸化的上调作用也因p38 MAPK siRNA转染而削弱(P<0.05,图 3B)。结果表明,在敲低p38 MAPK表达的SH-SY5Y细胞内WNV复制增强。
|
图 3 WNV在敲低p38 MAPK表达的SH-SY5Y细胞内复制 Fig 3 WNV replication in p38 MAPK knockdown SH-SY5Y cells A: t-p38 MAPK expression; B: Influence of WNV on p38 MAPK signaling. WNV: West Nile virus; MAPK: Mitogen-activated protein kinase; t-p38 MAPK: Total p38 mitogen-activated protein kinase; p-p38 MAPK: Phosphorylated p38 mitogen-activated protein kinase; UT: Untransfected cells. *P < 0.05, **P < 0.01. n=3, s |
2.3 WNV经p38 MAPK途径调控CHOP、ATF6α与IL-6的表达
如图 4所示,WNV感染促进CHOP、ISG15和IL-6 mRNA表达而抑制ATF6α mRNA表达,并且CHOP、ISG15、IL-6 mRNA表达水平的升高趋势一致,呈WNV感染时间依赖性升高。与感染12 h相比,WNV感染24、48、60 h可促进ISG15和IL-6 mRNA表达(P<0.05,P<0.01),感染48 h、60 h可促进CHOP mRNA表达(P<0.01,P<0.05)。与感染12 h相比,WNV感染24、48、60 h抑制了ATF6α mRNA表达(P<0.05,P<0.01)。

|
图 4 WNV感染诱生SH-SY5Y细胞内相关分子mRNA表达的动态变化 Fig 4 Kinetics of related molecule mRNA expression in SH-SY5Y cells during WNV infection A: CHOP; B: ISG15; C: IL-6; D: ATF6α. CHOP: CCAAT/enhancer-binding protein homologous protein; ISG15: Interferon-stimulated gene 15; IL-6: Interleukin 6; ATF6α: Activating transcription factor 6α. Data were shown as fold induction of the mRNA levels in WNV-infected cells over the corresponding mRNA levels in mock-infected cells at the same time point. *P < 0.05, **P < 0.01. n=3, x±s |
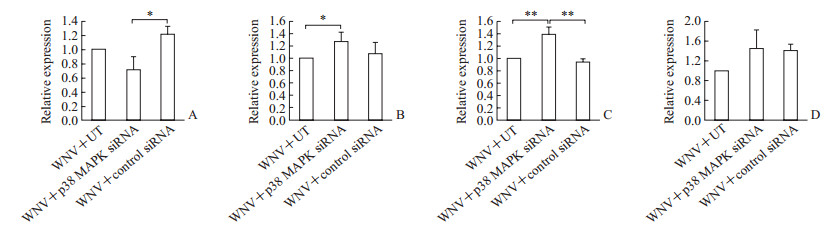
qRT-PCR检测结果(图 5)显示,与未转染的细胞相比,p38 MAPK siRNA转染细胞内IL-6(P<0.05)、ATF6α(P<0.01)mRNA水平升高;与对照siRNA转染的细胞相比,p38 MAPK siRNA转染细胞内ATF6α mRNA水平升高(P<0.01)、CHOP mRNA水平降低(P<0.05);ISG15 mRNA水平在各组差异无统计学意义。结果表明,WNV感染差异调控SH-SY5Y细胞内CHOP、IL-6、ATF6α和ISG15的表达;WNV可经p38 MAPK途径调控与应激应答相关的CHOP、ATF6α表达和与炎性应答相关的IL-6表达。
|
图 5 敲低p38 MAPK表达对WNV调控SH-SY5Y细胞内相关分子mRNA表达的影响 Fig 5 Influence of p38 MAPK knockdown on related molecule mRNA expression in SH-SY5Y cells induced by WNV infection A: CHOP; B: IL-6; C: ATF6α; D: ISG15. CHOP: CCAAT/enhancer-binding protein homologous protein; IL-6: Interleukin 6; ATF6α: Activating transcription factor 6α; ISG15: Interferon-stimulated gene 15; UT: Untransfected cells. Data were shown as fold induction of the mRNA levels in the siRNA-transfected cells over the corresponding mRNA levels in UT (set to 1.0). *P < 0.05, **P < 0.01. n=3, x±s |
3 讨论
许多黄病毒通过调控宿主细胞的多条信号转导途径(如固有免疫途径、应激应答途径和凋亡途径等)维持自身复制增殖,因此揭示黄病毒如何调控宿主细胞信号转导从而建立感染对于阐明病毒致病机制、研发抗病毒治疗的新策略具有重要意义[16]。细胞和小动物模型的研究均表明,宿主免疫应答对于控制WNV向中枢神经系统播散、炎性病理反应及病毒的清除至关重要[17]。在WNV感染过程中,与免疫应答、细胞应激相关的干扰素途径、ATF6途径和炎性因子受到关注。
人视网膜Müller细胞能表达高水平的寨卡病毒入侵因子anexelekto(AXL),故对病毒高度易感。寨卡病毒感染Müller细胞可激活p38 MAPK途径,该途径的激活与细胞产生大量促炎细胞因子密切相关,研究者们认为阻断病毒感染引发p38 MAPK途径信号转导可能是控制寨卡病毒感染导致眼部炎症的新靶点[10]。肝脏是登革病毒感染的重要器官,病毒在肝脏复制并产生较高的病毒载量。利用登革病毒感染的小鼠模型,研究表明病毒感染诱生p38 MAPK和其下游信号分子(如MAPKAPK2、HSP27和ATF2)发生磷酸化;以p38 MAPK抑制剂SB203580处理后可降低p38 MAPK下游信号分子的磷酸化水平,从而减轻登革病毒造成的肝损伤[18]。WNV感染人小神经胶质细胞后通过激活MAPK途径促进促炎细胞因子和趋化因子的产生,抑制p38 MAPK可降低趋化因子的表达,这可能与WNV的神经致病机制相关[11]。本研究发现,WNV短时孵育(1 h以内)和早期感染(24 h以内)激活了SH-SY5Y细胞p38 MAPK途径,表现为p38 MAPK磷酸化水平升高。该结果与寨卡病毒、登革病毒和WNV对p38 MAPK途径调控的报道[9-11, 18]一致。p38 MAPK磷酸化的动态变化显示WNV感染48 h、60 h时抑制了p38 MAPK途径,提示病毒感染引发细胞信号转导的时空性和复杂性。同时,WNV对Vero细胞p38 MAPK途径具有相似的调控作用。qRT-PCR检测结果显示,在敲低p38 MAPK表达的SH-SY5Y细胞内WNV复制增强。上述结果表明,在病毒感染早期,WNV激活了人神经母细胞瘤SH-SY5Y细胞p38 MAPK途径,而该途径下调可促进病毒复制。
在WNV感染的中枢神经系统,神经元是产生促炎细胞因子的潜在来源,而促炎细胞因子是WNV神经毒性作用的主要因素。研究报道,WNV诱生IL-1β、IL-6、IL-8和肿瘤坏死因子α(tumor necrosis factor α,TNFα)的表达与病毒导致死亡神经元数量的升高具一致性;用IL-1β或TNFα抗体处理神经元可降低WNV对神经元的毒性作用[19]。黄病毒感染诱导内质网应激可激活细胞非折叠蛋白应答,病毒通过调控此应答完成自身复制[20]。WNV诱生非折叠蛋白应答可抑制抗病毒信号转导途径以利于病毒复制和免疫逃逸[21]。本研究考察了WNV感染后非折叠蛋白应答途径(CHOP和ATF6α)、干扰素途径(ISG15)的相关分子和促炎细胞因子(IL-6)表达的动态变化,结果表明WNV感染促进了CHOP、IL-6和ISG15 mRNA的表达,并且表达水平呈WNV感染时间依赖性升高;WNV感染24、48、60 h抑制了ATF6α mRNA表达。有研究者提出,WNV感染促进CHOP表达代表宿主防御反应,因CHOP依赖的细胞凋亡能限制病毒的增殖和中枢神经系统的侵袭[22]。在敲除ATF6的鼠胚成纤维细胞,WNV蛋白表达和病毒产量均下降,表明ATF6信号转导可促进细胞存活、抑制固有免疫应答,从而维持病毒复制[23]。本研究提示,WNV对宿主细胞相关分子的差异调控与其致病机制有关。考察敲低p38 MAPK表达对WNV调控上述分子mRNA表达的影响,结果证实,WNV经p38 MAPK途径调控与应激应答相关的CHOP、ATF6α表达,并调控与炎性应答相关的IL-6表达。
综上所述,WNV激活p38 MAPK途径是病毒感染早期的重要信号转导事件,p38 MAPK途径的激活可能负反馈调控WNV复制,WNV经该途径调控细胞应激和炎性应答反应。对WNV感染引发宿主细胞信号转导的深入解析有助于阐明病毒致病的分子机制和提供抗病毒治疗的新靶点。
| [1] |
OUHOUMMANE N, TCHOUAKET E, LOWE A M, FORTIN A, KAIRY D, VIBIEN A, et al. Economic burden of West Nile virus disease, Quebec, Canada, 2012-2013[J]. Emerg Infect Dis, 2019, 25: 1943-1950. DOI:10.3201/eid2510.181608 |
| [2] |
PASTORINO B, NOUGAIRÈDE A, WURTZ N, GOULD E, DE LAMBALLERIE X. Role of host cell factors in flavivirus infection:implications for pathogenesis and development of antiviral drugs[J]. Antiviral Res, 2010, 87: 281-294. DOI:10.1016/j.antiviral.2010.04.014 |
| [3] |
SUTHAR M S, DIAMOND M S, GALE M Jr. West Nile virus infection and immunity[J]. Nat Rev Microbiol, 2013, 11: 115-128. DOI:10.1038/nrmicro2950 |
| [4] |
SAXENA V, BOLLING B G, WANG T. West Nile virus[J]. Clin Lab Med, 2017, 37: 243-252. DOI:10.1016/j.cll.2017.01.001 |
| [5] |
梁国栋. 我国西尼罗病毒和Tahyna病毒的发现与流行[J]. 微生物与感染, 2016, 11: 66-71. |
| [6] |
梁国栋. 虫媒病毒——重要的被忽略的热带传染病病原体[J]. 中国热带医学, 2018, 18: 1-5. |
| [7] |
韩硕.欧洲多国遭西尼罗河病毒侵袭[EB/OL]. (2018-08-28)[2019-06-02]. http://m.people.cn/n4/2018/0828/c3535-11515528.html.
|
| [8] |
LIM S M, KORAKA P, OSTERHAUS A D, MARTINA B E. West Nile virus:immunity and pathogenesis[J]. Viruses, 2011, 3: 811-828. DOI:10.3390/v3060811 |
| [9] |
FU Y, YIP A, SEAH P G, BLASCO F, SHI P Y, HERVÉ M. Modulation of inflammation and pathology during dengue virus infection by p38 MAPK inhibitor SB203580[J]. Antiviral Res, 2014, 110: 151-157. DOI:10.1016/j.antiviral.2014.08.004 |
| [10] |
ZHU S, LUO H, LIY H, HA Y, MAYS E R, LAWRENCE R E, et al. p38 MAPK plays a critical role in induction of a pro-inflammatory phenotype of retinal Müller cells following Zika virus infection[J]. Antiviral Res, 2017, 145: 70-81. DOI:10.1016/j.antiviral.2017.07.012 |
| [11] |
CHEERAN M C, HU S, SHENG W S, RASHID A, PETERSON P K, LOKENSGARD J R. Differential responses of human brain cells to West Nile virus infection[J]. J Neurovirol, 2005, 11: 512-524. DOI:10.1080/13550280500384982 |
| [12] |
LI S H, LI X F, ZHAO H, JIANG T, DENG Y Q, YU X D, et al. Cross protection against lethal West Nile virus challenge in mice immunized with recombinant E protein domain Ⅲ of Japanese encephalitis virus[J]. Immunol Lett, 2011, 138: 156-160. DOI:10.1016/j.imlet.2011.04.003 |
| [13] |
张培, 梁克峰, 李春缘, 陈迪嘉, 陈颖龙, 管文升, 等. 基孔肯雅热IgG抗体胶体金快速检测试纸条初步评价[J]. 检验医学, 2019, 34: 82-83. |
| [14] |
张浩旸, 郭佳慧, 赵兰娟, 姚茜茜, 文荣, 徐娅佳, 等. DC-SIGN与mCEACAM1a分子相互作用调控鼠冠状病毒复制[J]. 微生物与感染, 2018, 13: 136-145. DOI:10.3969/j.issn.1673-6184.2018.03.002 |
| [15] |
ZHAO L J, HE S F, LIU Y, ZHAO P, BIAN Z Q, QI Z T. Inhibition of STAT pathway impairs anti-hepatitis C virus effect of interferon alpha[J]. Cell Physiol Biochem, 2016, 40(1/2): 77-90. |
| [16] |
ZHANG H, SUN J, YE J, ASHRAF U, CHEN Z, ZHU B, et al. Quantitative label-free phosphorproteomics reveals differentially regulated protein phosphorylation involved in West Nile virus-induced host inflammatory response[J]. J Proteome Res, 2015, 14: 5157-5168. DOI:10.1021/acs.jproteome.5b00424 |
| [17] |
LUO H, WANG T. Recent advances in understanding West Nile virus host immunity and viral pathogenesis[J]. F1000Res, 2018, 7: 338. DOI:10.12688/f1000research.13362.1 |
| [18] |
SREEKANTH G P, CHUNCHARUNEE A, SIRIMONTAPORN A, PANAAMPON J, NOISAKRAN S, YENCHITSOMANUS P T, et al. SB203580 modulates p38 MAPK signaling and dengue virus-induced liver injury by reducing MAPKAPK2, HSP27, and ATF2 phosphorylation[J/OL]. PLoS One, 2016, 11: e0149486. doi: 10.1371/journal.pone.0149486.
|
| [19] |
KUMAR M, VERMA S, NERURKAR V R. Pro-inflammatory cytokines derived from West Nile virus (WNV)-infected SK-N-SH cells mediate neuroinflammatory markers and neuronal death[J/OL]. J Neuroinflammation, 2010, 7: 73. doi: 10.1186/1742-2094-7-73.
|
| [20] |
BLÁZQUEZ A B, ESCRIBANO-ROMERO E, MERINO-RAMOS T, SAIZ J C, MARTIN-ACEBES M A. Stress responses in flavivirus-infected cells:activation of unfolded protein response and autophagy[J]. Front Microbiol, 2014, 5: 266. DOI:10.3389/fmicb.2014.00266 |
| [21] |
AMBROSE R L, MACKENZIE J M. West Nile virus differentially modulates the unfolded protein response to facilitate replication and immune evasion[J]. J Virol, 2011, 85: 2723-2732. DOI:10.1128/JVI.02050-10 |
| [22] |
MEDIGESHI G R, LANCASTER A M, HIRSCH A J, BRIESE T, LIPKIN W I, DEFILIPPIS V, et al. West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis[J]. J Virol, 2007, 81: 10849-10860. DOI:10.1128/JVI.01151-07 |
| [23] |
AMBROSE R L, MACKENZIE J M. ATF6 signaling is required for efficient West Nile virus replication by promoting cell survival and inhibition of innate immune responses[J]. J Virol, 2013, 87: 2206-2214. DOI:10.1128/JVI.02097-12 |
 2019, Vol. 40
2019, Vol. 40


