2. 海军军医大学(第二军医大学)长征医院超声诊疗科, 上海 200003;
3. 解放军904医院特诊科, 苏州 215007;
4. 海军军医大学(第二军医大学)长海医院病理科, 上海 200433
2. Department of Ultrasound, Changzheng Hospital, Naval Medical University(Second Military Medical University), Shanghai 200003, China;
3. Department of Special Consultation, No. 904 Hospital of PLA, Suzhou 215007, Jiangsu, China;
4. Department of Pathology, Changhai Hospital, Naval Medical University(Second Military Medical University), Shanghai 200433, China
近年来,超声引导下甲状腺结节微波消融术凭借其超微创、无瘢痕、安全性高、疗效确切等优势,日益受到临床重视[1-6]。微波消融术后超声影像检测消融区血供缺失及体积缩小是局部疗效评估的关键,然而对消融区标本进行组织病理学评估才更严谨和准确,但是目前相关研究报道尚少,尤其缺乏连续动态的对照观察研究。有关细胞呼吸酶活性的研究表明,微波消融术后即刻消融区虽然仍保持与消融前相似的细胞结构和组织形态[7-8],但其细胞呼吸关键酶已丧失活性[9-12],然而这并未能平抑研究者们依赖组织结构对微波消融治疗效果的质疑[13]。因此,有必要对甲状腺结节热消融区组织结构的经时变化进行动态研究。
1 资料和方法 1.1 研究对象以2017年1月至2018年12月于上海国际医学中心接受消融区穿刺活组织检查评估的甲状腺结节微波消融术后患者60例为研究对象,男14例、女46例,年龄为16~57岁,平均(35.22±9.77)岁。共69个消融区,其中51例为1个消融区,9例为2个消融区。微波消融术前穿刺活组织检查病理诊断为甲状腺乳头状癌28个、结节性甲状腺肿21个、滤泡性腺瘤17个、桥本甲状腺炎结节3个。消融区行穿刺活组织检查评估的纳入标准:(1)甲状腺乳头状癌(消融术后必需对消融区进行病理评估);(2)行分期消融治疗的良性甲状腺结节(再次消融治疗时可对前次消融区进行病理评估);(3)消融区各径线均大于1 cm(方便实施切割式活组织检查并获取合格的标本)。排除标准:(1)患者出现严重出血倾向或凝血机制障碍,包括停止服用抗凝血药物未达规定时程者;(2)失去安全穿刺路径者。本研究经上海国际医学中心伦理委员会审批,所有患者均签署了知情同意书。
1.2 仪器与设备超声检查与引导穿刺活组织检查使用Hitachi Hi Vision Preirus、Esaote MyLabTM Alpha及Mindray Resona 7S彩色多普勒超声仪,探头频率为7~14 MHz。活组织检查针使用Presia 18 G HS半自动切割式活检针。
1.3 评估时间及评估手段评估时间为微波消融术后即刻和术后1、3、6、12个月。评估手段为超声引导下经皮粗针穿刺组织光镜下病理学检查。
1.4 评估过程患者取仰卧位,颈部轻度后伸,常规消毒颈部皮肤并铺巾,以2%利多卡因局部浸润麻醉,在超声引导下行经皮穿刺。应用液体隔离法分离和保护消融区周围毗邻结构,并判断消融区与毗邻结构是否粘连。以18 G HS活检针刺入甲状腺消融区内获取组织标本。每个消融区的取材部位包括:(1)消融区中央域,即消融区中线部位;(2)消融区边缘域,即消融区边界内侧3 mm内。对标本不合格者(呈碎颗粒状,最大长度<3 mm者),可再次穿刺取材至合格。所得标本均置于4%甲醛溶液中固定,以备制作石蜡组织切片。组织切片采用H-E染色和甲状腺转录因子1(thyroid transcription factor 1,TTF1)免疫组织化学染色。
1.5 病理判读将各评估节点的消融区中央域及边缘域组织切片置于光镜下全面观察,判读肿瘤细胞存活情况、细胞结构完整性、组织形态特征、坏死物及坏死范围与程度、纤维组织增生情况。综合上述判读结果确定消融区组织坏死程度:(1)完全坏死,全部标本组织内出现广泛的大片状变性坏死物,红染、无结构,或隐约可见甲状腺滤泡轮廓影,但无完整滤泡上皮细胞或肿瘤细胞;(2)部分坏死,全部标本组织内以大片状红染无结构坏死物为主,部分区域可见少量结构和形态可辨的滤泡上皮细胞或乳头状癌细胞;(3)无坏死或坏死不明显,全部标本组织内可见结构较完整的甲状腺滤泡上皮细胞或乳头状癌细胞,虽可见部分细胞的细胞核溶解但细胞整体无明显碎裂溶解,细胞仍维持滤泡形态或乳头状癌排列形态。
2 结果 2.1 标本取材结果甲状腺消融区穿刺活组织检查的时间节点及标本数分别为消融术后即刻69个消融区,术后1个月12个消融区,术后3个月19个消融区,术后6个月25个消融区,术后12个月13个消融区。各消融区的中央域及边缘域穿刺标本均成功行H-E染色和免疫组织化学染色,符合病理检查要求。
2.2 组织学观察结果 2.2.1 光镜下可见的表现(1)自然结构:视野内的滤泡结构、细胞形态、细胞核形态、细胞排列形态、间质血管等均与消融前高度相似或一致(图 1A~1C);TTF1表达阳性(图 1D)。可资明确诊断为甲状腺疾病,如结节性甲状腺肿、滤泡性腺瘤、桥本甲状腺炎结节或乳头状癌等。记为无坏死或坏死不明显。(2)部分坏死:视野内可见无滤泡结构、无上皮细胞的红染坏死物,亦可偶见尚存上皮细胞结构的完整滤泡;TTF1表达弱阳性(图 2、3)。(3)完全坏死:视野内全部为无上皮细胞、无滤泡结构的红染坏死物;TTF1表达阴性(图 4~8)。(4)纤维增生:视野内可见大量甚或全部为纤维组织,纤维细胞结构清晰,可伴有钙化、透明变性;间或可见小片状的无结构红染坏死物;TTF1表达阴性(图 3、图 5~7)。计入完全坏死。(5)淋巴细胞浸润:视野内于无结构的红染坏死物或者增生的纤维组织背景中分布丰富的淋巴细胞(图 5)。计入完全坏死。
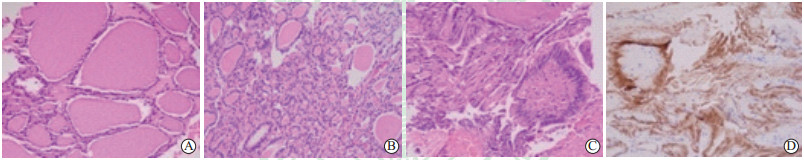
|
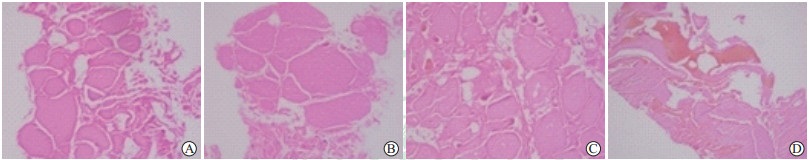
图 1 甲状腺结节微波消融术后即刻消融区边缘域腺体组织近似自然状态光镜图 Fig 1 Histology and immunohistopathology findings were similar to the natural state in marginal ablation zone of thyroid nodules immediately after microwave ablation A: Each thyroid follicle lumen was intact, and varied in size and similar in shape. The colloid in the follicular lumens was obviously dehydrated. The follicular epithelial cells were intact, cubic or flattened without atypia. The diagnosis was nodular thyroid goiter, consistent with the pre-ablation diagnosis. B: The structure of the follicle lumens was indistinct. The shapes of follicular epithelial cells were from cubic to columnar, arranged in cords or micro-follicular shape, and without atypia. Hyperplasia of fiber tissue was also seen. The diagnosis was follicular thyroid adenoma, consistent with the pre-ablation. C: The follicular epithelial cells were columnar with large oval nucleus which were overlapping and had nuclear grooves. The cells arranged in a papillary shape, and the center consisted of fibrovascular tissue. The diagnosis was papillary thyroid carcinoma, consistent with the pre-ablation. D: Histochemical staining of TTF1 was positive in papillary thyroid carcinoma immediately after ablation, consistent with pre-ablation. TTF1: Thyroid transcription factor 1. A-C: H-E staining; D: Immunohistochemical staining. Original magnification: ×200 |
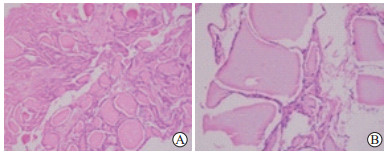
|
图 2 结节性甲状腺肿微波消融术后1个月消融区滤泡组织部分坏死光镜图 Fig 2 Partial necrosis in ablation area of nodular thyroid goiter 1 month after microwave ablation in microscopic images A: Central zone, most thyroid follicles were incomplete, and the follicular epithelial cells were indistinct; B: Marginal zone, although a few follicular epithelial cells can be seen, the follicles were incomplete. H-E staining. Original magnification: ×200 |
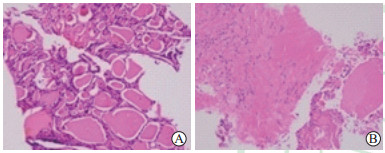
|
图 3 桥本甲状腺炎结节微波消融术后1个月消融区组织部分坏死光镜图 Fig 3 Partial necrosis in ablation area of Hashimoto's thyroiditis nodular 1 month after microwave ablation in microscopic images A: Central zone, there were follicles with different sizes in most of the area and a pink amorphous mass of necrotic material in the small part of them; B: Marginal zone, obvious fibrous tissue proliferation, diffusely distributed lymphocytes were observed, there were no follicles. H-E staining. Original magnification: ×200 |
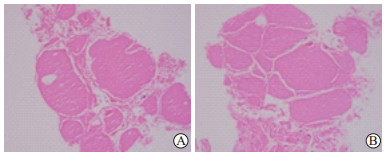
|
图 4 结节性甲状腺肿微波消融术后1个月消融区滤泡组织完全坏死光镜图 Fig 4 Complete necrosis in ablation area of nodular thyroid goiter 1 month after microwave ablation in microscopic images There were no follicular epithelial cells in both central zone (A) and marginal zone (B). H-E staining showed pink amorphous mass of necrotic material. Original magnification: ×200 |
|
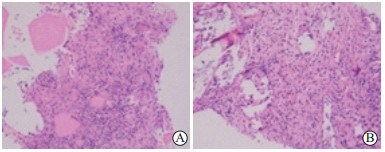
图 5 甲状腺乳头状癌微波消融术后1个月消融区肿瘤组织完全坏死光镜图 Fig 5 Complete necrosis in ablation area of papillary thyroid carcinoma 1 month after microwave ablation in microscopic images H-E staining showed obvious fibrous hyperplasia and diffusely distributed lymphocytes in the central zone (A) and marginal zone (B). There was no papillary structure. Original magnification: ×200 |
|
图 6 甲状腺乳头状癌微波消融术后3个月消融区肿瘤组织完全坏死光镜图 Fig 6 Complete necrosis in ablation area of papillary thyroid carcinoma 3 months after microwave ablation in microscopic images H-E staining showed pink amorphous mass of necrotic material in central zone (A) and marginal zone (B). A few of these follicular epithelial cell outlines were seen in the marginal zone, the interstitial fibrous tissue was proliferated with hyaline degeneration. Original magnification: ×200 |

|
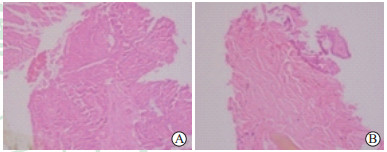
图 7 甲状腺乳头状癌微波消融术后6个月消融区肿瘤组织完全坏死伴纤维组织增生光镜图 Fig 7 Complete necrosis and fibrous tissue proliferation in ablation area of papillary thyroid carcinoma 6 months after microwave ablation in microscopic images A: Central zone, only a few flattened follicular epithelial cells were seen. The cellular structure was destroyed by ablation. Foreign body granuloma and fibrous tissue proliferation were observed. B: Marginal zone, a few incomplete follicles and a large amount of fibrous tissue proliferation were seen. C: Marginal zone, expression of TTF1 was negative. TTF1: Thyroid transcription factor 1. A, B: H-E staining; C: Immunohistochemical staining. Original magnification: ×200 |
|
图 8 结节性甲状腺肿微波消融术后6个月和12个月消融区完全坏死光镜图 Fig 8 Complete necrosis in ablation zones of nodular thyroid goiter 6 and 12 months after microwave ablation in microscopic images H-E staining showed pink amorphous mass of necrotic material in the central zone (A) and marginal zone (B) at 6 months, and in the central zone (C) and marginal zone (D) at 12 months after microwave ablation. Original magnification: ×200 |
2.2.2 消融区组织坏死程度的区域差异与动态演变
消融术后即刻随访的69个消融区中,所有消融区组织在光镜下均呈凝固变性,结节的细胞结构与排列方式仍呈自然状态,没有坏死表现,且无中央域和边缘域的区域差异。每个消融区均可作出明确的、与消融前一致的组织学诊断。
消融术后1个月随访的12个消融区中,9个(75.00%,9/12)消融区组织在光镜下均呈大片红染无结构坏死,达到完全坏死,且中央域及边缘域所见高度一致;2个消融区的中央域及边缘域除了片状坏死外仍可见滤泡结构,但滤泡上皮细胞结构模糊,达到部分坏死;1个消融区的中央域和边缘域纤维组织显著增生伴散在淋巴细胞浸润,坏死不明显,但已无滤泡结构可见。
消融术后3个月随访的19个消融区中,16个(84.21%,16/19)中央域及15个(78.95%,15/19)边缘域达到完全坏死,3个中央域及4个边缘域达到部分坏死,虽可见滤泡结构,但滤泡上皮细胞模糊。
消融术后6个月及12个月随访的共38个(100.00%,38/38)消融区的边缘域及中央域均呈现完全坏死。
自消融后1个月起至随访终点消融后12个月,消融区的中央域及边缘域的完全坏死率随时间增加,其坏死程度呈经时加重的演变趋势。见表 1。
|
|
表 1 甲状腺结节微波消融术后消融区坏死程度经时变化情况 Tab 1 Necrosis rates of tissues from central zone and marginal zone of thyroid nodules after microwave ablation |
3 讨论 3.1 消融区疗效的组织学评估理念
微波消融治疗与外科手术切除的最根本区别在于不能使甲状腺结节离开患者身体,对消融区的评估是评价甲状腺结节微波消融疗效的核心。二维超声、三维超声和弹性超声可以评价消融区的回声、形态和硬度,彩色多普勒和超声造影可以评价消融区的血供情况[14-16],而消融区的组织学状态则是影响上述超声影像评价指标的基础,因此消融后结节组织是否完全坏死便成为确定微波消融疗效的关键。光镜下组织学观察是评价消融区组织坏死程度的必备手段,组织坏死以细胞结构崩解与形态丧失为特征性标志。如若仍可见到完整的细胞结构和形态,则极可能被判断为消融治疗未达到应有的效果。笔者曾通过微波消融术后消融区的即刻穿刺取材光镜下组织学观察研究发现,细胞结构和组织形态在消融后即刻与消融术前高度相似,一度引起对热消融疗效的自我质疑[7]。马奔等[17]曾对热消融治疗后1个多月的甲状腺乳头状癌消融区行外科切除手术,手术标本病理检查发现仍有结构完整的肿瘤细胞,进而质疑微波消融治疗的彻底性和安全性。为了验证相关质疑是否正确,我们对甲状腺结节热消融区的细胞呼吸链关键酶的活性进行了动态研究,发现消融后即刻位于线粒体内调节细胞呼吸的琥珀酸脱氢酶(succinate dehydrogenase,SDH)和还原型烟酰胺腺嘌呤二核苷酸黄递酶(nicotinamide adeninedinucleotide phosphate diaphorase,NADPH-d)即已失去活性,消融后6个月时仍旧保持无活性,进而提出判断微波消融疗效更应侧重细胞呼吸酶SDH和NADPH-d活性的改变。然而受研究和检测条件的限制,多数医疗机构仅能开展光镜下组织学诊断,因此研究消融区组织结构和形态的动态变化对正确阐述热消融疗效、理解消融区经时变化规律具有普遍意义。
3.2 热消融后细胞结构和形态具有时效性本研究再次证实,微波消融后即刻光镜下细胞保持与消融前几乎相同的自然结构和形态,没有明显坏死的表现,这是因为细胞受到不可逆损伤后至光镜下出现其结构和形态学改变需要经过一定的时间。消融后1个月观察,大部分消融区呈现完全坏死,部分消融区呈现部分坏死,个别消融区仍可见到形态可辨、结构完整的细胞;消融后3个月时观察,形态、结构完整可辨的细胞数量减少,而无结构的坏死物质增多;而至消融后6个月及12个月时,消融区组织则均呈现红染无结构的凝固性坏死物质。这样的动态演变结果提示,热消融后细胞结构和形态的光镜下所见具有时间依赖性。众所周知,用于解剖学教学的尸体标本早已丧失活性,但仍旧保持完好的细胞和组织结构。穿刺活组织检查取得的新鲜活性细胞或组织置入甲醛溶液后,细胞很快凝固变性,封存若干年后其结构、形态仍完好,但活性早已丧失[18]。微波消融致甲状腺结节细胞迅速丧失活性,但坏死并非消融后即刻发生,而是随着时间推移呈渐进式发展与加重。电镜下可以观察到微波消融治疗后即刻肿瘤细胞的细胞膜中断,线粒体和内质网等超微细胞器失去正常结构[19],而光镜下观察所见却与消融前差别不大。本研究发现,甲状腺乳头状癌和桥本甲状腺炎结节消融后边缘域出现显著的纤维组织增生和(或)淋巴细胞浸润,而甲状腺腺瘤和结节性甲状腺肿则少见此现象。这可能与乳头状癌、桥本甲状腺炎结节具有诱导胶原纤维增生的潜能有关。增生的纤维组织几乎无或仅有极少的甲状腺滤泡可见,但是TTF1表达呈阴性。TTF1是存在于甲状腺滤泡上皮细胞核的重要遗传物质,是证明所见细胞是否为甲状腺滤泡上皮的直接佐证,消融后即刻消融区组织的TTF1呈阳性表达。当TTF1表达转为阴性,提示消融区内甲状腺组织已然达到完全坏死,吸收殆尽。
3.3 消融区穿刺时间节点和部位的选择穿刺活组织检查因其有创不宜反复多次实施,因而寻找合适的穿刺活组织检查评估时间点显得尤为必要。本研究发现消融后1个月、3个月光镜下均可观察到较多细胞结构,与术后6个月差别较大;而消融术后6个月起消融区内呈现大片坏死结构,与消融后12个月的镜下所见形态差别不大,均很难见到细胞结构。因此,建议将消融术后6个月作为消融后病理评估的时间节点。粗针穿刺取材部位包括消融区的中央域和边缘域,中央域系整个消融热场内温度最高、细胞灭活速度最快和程度最强之处,而边缘域接近原病灶边界,为正常甲状腺腺体到消融坏死区的过渡区域,其组织坏死程度较中央域更具有评估的必要性。尤其是对于邻近气管或食管的乳头状癌消融区,边缘域组织应予优先穿刺取材,以判断其是否尚有存活的肿瘤组织,因为残存肿瘤有向气管或食管浸润生长的潜能,需要格外重视和及时处置。
本研究在多个时间节点评估了甲状腺结节微波消融区组织的动态演变,如能对每例患者在各时间节点均穿刺取材并进行自身对照研究是最为理想的。穿刺活组织检查虽然微创,但多次穿刺方案仍无法获得研究对象的依从与配合,因此导致各时间节点的病例数量和病例构成均不相同,有待后续研究中增加病例数,进一步提高结果的验证力度和质量。
| [1] |
章建全.经皮热消融治疗在甲状腺乳头状癌及其区域淋巴结转移中的应用前景[J/CD].中华医学超声杂志(电子版), 2014, 11: 606-609. http://www.cnki.com.cn/Article/CJFDTotal-ZHCD201408001.htm
|
| [2] |
马芳花, 黄品同, 戚瑞祥, 王平, 游向东. 超声引导下微波消融治疗甲状腺微小乳头状癌与外科手术切除的研究[J]. 中国超声医学杂志, 2017, 33: 399-402. |
| [3] |
JUNG S L, BAEK J H, LEE J H, SHONG Y K, SUNG J Y, KIM K S, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules:a prospective multicenter study[J]. Korean J Radiol, 2018, 19: 167-174. DOI:10.3348/kjr.2018.19.1.167 |
| [4] |
陈杭军, 张超, 黄品同.甲状腺微小乳头状癌经微波消融与手术切除的疗效、安全性及经济性比较[J/CD].中华医学超声杂志(电子版), 2018, 15: 275-280. http://cdmd.cnki.com.cn/Article/CDMD-10335-1019010262.htm
|
| [5] |
LIU Y J, QIAN L X, LIU D, ZHAO J F. Ultrasound-guided microwave ablation in the treatment of benign thyroid nodules in 435 patients[J]. Exp Biol Med (Maywood), 2017, 242: 1515-1523. DOI:10.1177/1535370217727477 |
| [6] |
ZHENG B W, WANG J F, JU J X, WU T, TONG G, REN J. Efficacy and safety of cooled and uncooled microwave ablation for the treatment of benign thyroid nodules:a systematic review and meta-analysis[J]. Endocrine, 2018, 62: 307-317. DOI:10.1007/s12020-018-1693-2 |
| [7] |
闫磊, 章建全, 曹昆昆, 吴震中, 盛建国, 张航. 微波消融改善甲状腺结节粗针穿刺活检的过程与结果[J]. 第二军医大学学报, 2017, 38: 1250-1255. YAN L, ZHANG J Q, CAO K K, WU Z Z, SHENG J G, ZHANG H. Microwave ablation improves the process and outcome of core needle biopsy in thyroid nodules[J]. Acad J Sec Mil Med Univ, 2017, 38: 1250-1255. |
| [8] |
刘大全, 吕明德, 谭进富, 王竹, 周忠信. 不同温度微波消融肝癌效果的酶组织化学检测[J]. 南方医科大学学报, 2006, 26: 1149-1151. |
| [9] |
章建全, 闫磊, 陈红琼, 吴震中. 微波消融致甲状腺结节细胞活性的快速变化分析[J]. 中华医学杂志, 2018, 98: 3524-3527. |
| [10] |
闫磊, 章建全, 陈红琼, 吴震中, 程杰. 甲状腺结节微波消融治疗后6个月消融区细胞活性的酶组织化学检测[J]. 第二军医大学学报, 2019, 40: 20-24. YAN L, ZHANG J Q, CHEN H Q, WU Z Z, CHENG J. Enzyme histochemical detection of cell viability in ablation area of thyroid nodules at 6 months after microwave ablation[J]. Acad J Sec Mil Med Univ, 2019, 40: 20-24. |
| [11] |
冯炼强, 刘大全, 朱兆玲, 吕明德, 谭进富, 王竹, 等. 微波凝固肝癌细胞活性的酶组织化学检测[J]. 热带医学杂志, 2006, 6: 679-681. |
| [12] |
OZAKI T, TABUSE K, TSUJI T, NAKAMURA Y, KAKUDO K, MORI I. Microwave cell death:Enzyme histochemical evaluation for metastatic carcinoma of the liver[J]. Pathol Int, 2003, 53: 837-845. DOI:10.1046/j.1440-1827.2003.01571.x |
| [13] |
董文武, 张浩, 张平, 邵亮, 张挺, 吕承洲, 等. 甲状腺乳头状癌射频消融治疗后再手术5例临床分析[J]. 中国实用外科杂志, 2015, 35: 653-655. |
| [14] |
刁宗平, 章建全, 盛建国, 赵璐璐, 张航. 超声弹性成像在甲状腺良性结节射频消融中的应用价值及相关动态分析[J]. 第二军医大学学报, 2017, 38: 947-950. DIAO Z P, ZHANG J Q, SHENG J G, ZHAO L L, ZHANG H. Application value of ultrasound elastography in treatment of benign thyroid nodules with radio-frequency ablation and correlative dynamic analysis[J]. Acad J Sec Mil Med Univ, 2017, 38: 947-950. |
| [15] |
徐斌, 张梅香, 章建全. 超声引导下甲状腺良性结节经皮热消融的临床研究[J]. 第二军医大学学报, 2011, 32: 1189-1192. XU B, ZHANG M X, ZHANG J Q. Ultrasound-guided percutaneous thermal ablation of benign thyroid nodules:a clinical study[J]. Acad J Sec Mil Med Univ, 2011, 32: 1189-1192. |
| [16] |
陈吉东, 岳林先, 尹立雪, 陈琴, 熊晏群, 冯超, 等. 经皮微波消融治疗自主功能性甲状腺结节的临床研究[J]. 中华超声影像学杂志, 2018, 27: 958-962. |
| [17] |
马奔, 王宇, 嵇庆海, 赵志泓, 陈彤箴, 常彬, 等. 原发性甲状腺癌热消融治疗后再手术2例分析[J]. 中国实用外科杂志, 2016, 36: 875-879. |
| [18] |
范卫君, 叶欣. 肿瘤微波消融治疗学[M]. 北京: 人民卫生出版社, 2012: 25-27.
|
| [19] |
卫刚, 戚晓东, 马静, 宋瑾, 王静, 任扩军, 等. 乳腺癌高强度聚焦超声治疗后病理形态学观察[J]. 诊断病理学杂志, 2014, 21: 290-292. |
 2019, Vol. 40
2019, Vol. 40


