2. 海军军医大学(第二军医大学)长海医院虹口院区重症医学科, 上海 200081;
3. 海军军医大学(第二军医大学)长海医院虹口院区消毒供应科, 上海 200081;
4. 解放军东海舰队上海舰艇岸勤部医院, 上海 200940
2. Intensive Care Unit, Hongkou Branch of Changhai Hospital, Naval Medical University(Second Military Medical University), Shanghai 200081, China;
3. Department of Sterilization and Supply, Hongkou Branch of Changhai Hospital, Naval Medical University(Second Military Medical University), Shanghai 200081, China;
4. Hospital of Shanghai Warship Logistics Department, East China Sea Fleet, Shanghai 200940, China
PRKAG2心脏综合征是一种罕见的常染色体显性遗传病,是由于编码单磷酸腺苷(adenosine monophosphate,AMP)活化蛋白激酶(adenosine monophosphate-activated protein kinase,AMPK)γ2亚基的PRKAG2基因遗传性缺陷导致的以心肌肥厚、心室预激、快速性心律失常以及传导系统疾病为主要表现的心脏病变[1]。截至目前,国际上已报道了不少于20个PRKAG2心脏综合征的家系,并已发现了至少14个基因位点的突变,而且PRKAG2基因突变的位置均位于AMPK γ2亚基的胱硫醚β-合成酶(cystathionine β-synthase,CBS)区域[2-6]。AMPK活性的改变被认为是它们共同的发病机制[6-10]。
PRKAG2基因突变对AMPK功能的影响有几种不同的观点。Arad等[7]通过对过表达PRKAG2(N488I)突变的转基因鼠的研究发现,PRKAG2 N488I突变引起的AMPK活性增加是导致心肌糖原过度沉积、心肌肥大、心律失常等临床症状的主要因素。Sidhu等[11]通过对过表达PRKAG2(R302Q)突变的转基因小鼠的研究却得出了截然不同的结果,该研究认为PRKAG2(R302Q)突变转基因小鼠的AMPK活性是下降的,同时也可见到心肌糖原过度沉积、心肌肥大等现象。而Banerjee等[10]在构建的PRKAG2(T400N)突变转基因鼠中却发现心肌AMPK的活性表现为双相改变,AMPK活性早期激活、随后降低,最后恢复至野生型水平,同时该研究也揭示了AMPK活性的下降伴有心肌糖原过度沉积。
2007年海军军医大学(第二军医大学)长海医院心血管内科张静等[12]首先发现了中国人群特有的PRKAG2(G100S)心脏综合征家系,但在随后的基因测序中却发现PRKAG2(G100S)突变点位于AMPK γ2亚基非CBS区域,不同于以往国外报道的在CBS区域[2-6]。此类患者同样有心肌肥厚和心律失常等PRKAG2心脏综合征的临床表型,但对于该基因突变时AMPK活性的变化研究较少,更未见对AMPK动态观察的研究。本研究通过动态观察PRKAG2(G100S)突变转基因小鼠心肌细胞中AMPK活性的变化,明确PRKAG2(G100S)突变对AMPK活性的影响,进一步认识中国人群特有的PRKAG2(G100S)心脏综合征的发病机制,为该病的诊断和治疗提供依据。
1 材料和方法 1.1 材料PRKAG质粒由广州复能基因有限公司提供;Insulator-α-MHC载体、pInsulator载体由南京大学-南京生物医药研究院提供;PrimeSTAR HS聚合酶由宝生物工程(大连)有限公司生产;ClonExpressⅡ One Step Cloning Kit连接体系由南京诺唯赞生物科技有限公司提供;T4 DNA连接酶由美国NEB公司生产;C57BL/6J、ICR小鼠由南京大学-南京生物医药研究院提供[动物生产许可证号:SCXK(苏)2015-0001;动物合格证号:201703581],小鼠均饲养在无特定病原体(specific pathogen free,SPF)级屏障设施内,光照周期为12 h/12 h。
1.2 心脏过表达人源PRKAG2(G100S)突变转基因小鼠模型的建立以PRKAG2基因为模板,利用PCR技术进行定点突变,制作PRKAG2(G100S)片段。以PRKAG2(G100S)突变片段的质粒为模板,用PrimeSTAR HS聚合酶进行高保真PCR扩增,获得目的片段。使用ClonExpressⅡ One Step Cloning Kit连接体系对pInsulator-α-MHC载体及PRKAG2(G100S)突变片段进行连接,酶切鉴定正确后,经抽提质粒、纯化等程序成功制成pInsulator-Myh6-PRKAG2-G100S转基因片段,通过原核注射将纯化后的转基因片段注射到事先收集的受精卵中并移植至假孕ICR雌鼠输卵管内。受体雌鼠妊娠后成功分娩,子代小鼠经PCR鉴定基因型,获得转基因阳性的小鼠(首建鼠)[13]。将首建鼠与野生型C57BL/6J小鼠回交建系,得到的后代为N1代,将N1代转基因阳性鼠与野生型C57BL/6J小鼠交配,得到的后代为N2代,继续向下传代扩大繁育依次为N3代、N4代、……,并保持种群。
1.3 蛋白质印迹法检测小鼠心脏组织中PRKAG2(G100S)蛋白水平随机选取剪尾鉴定阳性及阴性的N4代转基因小鼠各3只,采用颈椎脱臼法处死小鼠,取出0.2 g心脏组织,经裂解、离心、变性等程序制成蛋白样本,经十二烷基硫酸钠-聚丙烯酰胺凝胶电泳分离后转移至硝酸纤维素膜;以10%脱脂奶粉溶液封闭,依次加入兔抗人PRKAG2一抗(英国Abcam公司)及辣根过氧化物酶标记的山羊抗兔二抗(美国Santa Cruz公司)孵育,以电发学发光法显色发光。用Quality One软件行灰度扫描,以甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GAPDH)为内参照计算PRKAG2(G100S)蛋白的相对表达量。
1.4 小鼠心肌细胞AMPK活性检测随机选取N4代4周龄和12周龄的PRKAG2(G100S)转基因小鼠与同窝野生型小鼠各6只,用颈椎脱臼法处死小鼠,纵行切开心脏并剪取心脏尖端部组织,反复剪碎,予以胰酶(江苏恩莫阿赛生物技术有限公司)消化后,筛网过滤,收集细胞,将细胞悬液种于多聚赖氨酸包被的96孔板中,放入37 ℃、5% CO2培养箱中孵育。待细胞完全贴壁后将细胞取出,用37%的甲醛溶液固定,弃去甲醛溶液,连续清洗3次,以3%的H2O2阻断内源性过氧化物酶对实验的影响。弃去阻断剂,连续清洗3次,弃去洗液后加封闭剂。弃去封闭剂,加入一抗(英国Abcam公司)孵育,清洗后加入二抗(美国Santa Cruz公司)孵育,清洗弃去二抗,最后弃去清洗液,依次加入辣根过氧化物酶底物、染色剂,室温避光孵育5 min后用磷酸化AMPK(phospho-AMPK,pAMPK)检测试剂盒(上海浩然生物技术有限公司)检测AMPK活性。在发射波长为530 nm、激发波长为585 nm时检测pAMPK蛋白的含量,在发射波长为360 nm、激发波长为450 nm时检测总AMPK蛋白的含量。计算小鼠心肌细胞AMPK的活性,计算公式:AMPK活性=pAMPK含量/总AMPK含量。
1.5 统计学处理采用SPSS 18.0软件进行统计学分析。计数资料表示为频数(%),组间差异的比较采用χ2 检验;计量资料以x±s表示,如符合正态分布和方差齐性则采用两独立样本t检验进行组间比较,否则采用非参数检验进行组间比较。检验水准(α)为0.05。
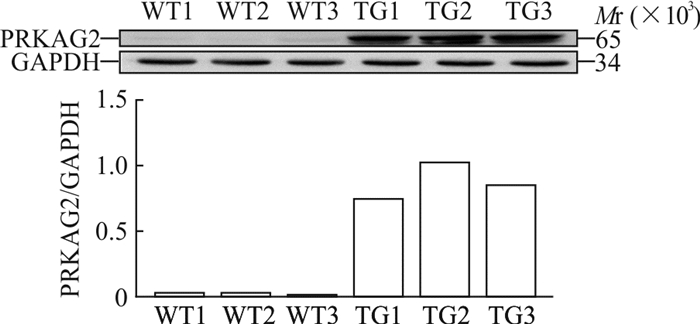
2 结果 2.1 转基因小鼠心脏组织中PRKAG2(G100S)蛋白的表达用蛋白质印迹法检测PCR鉴定阳性及阴性的N4代转基因小鼠心脏组织中PRKAG2(G100S)蛋白水平,结果(图 1)显示,转基因小鼠心脏组织PRKAG2(G100S)相对表达量高于野生型小鼠(0.874±0.139 vs 0.027±0.008,P=0.007)。证实PRKAG2(G100S)基因在转基因小鼠心脏上成功表达。
|
图 1 蛋白质印迹法检测N4代转基因小鼠与同窝野生型小鼠心脏组织中PRKAG2(G100S)的表达量 Fig 1 Expression of PRKAG2 (G100S) in heart tissues of N4 generation transgenic mice and wildtype littermate by Western blotting WT1-WT3: Wildtype littermate; TG1-TG3: Transgenic mouse; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase |
2.2 转基因小鼠心肌细胞中AMPK活性的变化
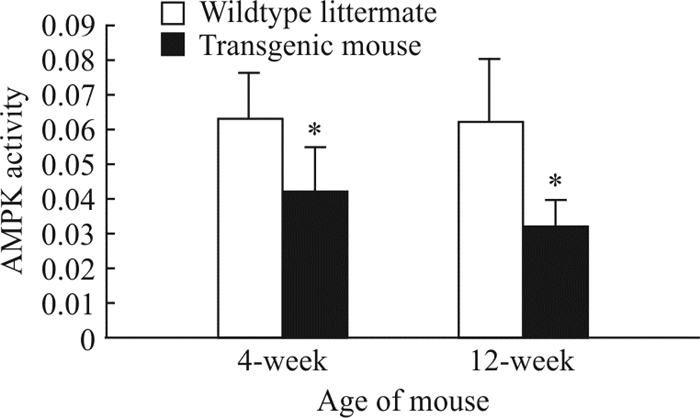
分别对4周龄、12周龄的6只转基因小鼠和6只同窝野生型小鼠心肌细胞进行AMPK活性检测,结果(图 2)显示,4周龄和12周龄的转基因小鼠心肌细胞中AMPK活性均低于同窝野生型小鼠(0.042±0.013 vs 0.063±0.013,0.032±0.008 vs 0.062±0.018),差异均有统计学意义(P=0.019,P=0.004)。12周龄和4周龄的转基因小鼠心肌细胞中AMPK活性(0.032±0.008 vs 0.042±0.013)差异无统计学意义(P=0.135)。
|
图 2 不同周龄转基因小鼠与同窝野生型小鼠心肌细胞AMPK活性变化 Fig 2 Changes of AMPK activity in cardiomyocytes of transgenic mice and wildtype littermate at different weeks of age AMPK: Adenosine monophosphate-activated protein kinase. *P < 0.05 vs wildtype litttermate. n=6, x±s |
3 讨论
AMPK作为一种重要的蛋白激酶,对体内多种代谢过程都起到十分重要的作用。AMPK通过哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)抑制剂、真核细胞延伸因子2(eukaryotic translation elongation factor 2,eEF2)、p70S6激酶等下游信号途径调节蛋白合成,可以抑制心肌细胞的增殖和心肌肥大。AMPK可以诱导葡萄糖转运蛋白(glucose transporter,GLUT)向细胞膜转位,磷酸化转录因子开启GLUT基因的表达,从而促进周围组织摄取葡萄糖、抑制糖原合成、促进葡萄糖向糖酵解方向转化,调节糖代谢。研究证实AMPK对心肌细胞电离子通道的活性也起着十分重要的作用。Light等[14]发现AMPK α1亚基的组织性激活突变基因可导致心肌细胞动作电位时程明显延长,显著延迟了钠通道开放、失活,使钠通道更加趋向于超极化从而导致心律失常的发生。Yoshida等[15]研究表明AMPK直接与三磷酸腺苷(adenosine triphosphate,ATP)敏感性钾通道(ATP-sensitive potassium channel,KATP)结合,并且在代谢抑制时AMPK的活化增加了KATP通道开放概率。其他研究也证实在心肌缺血性预处理过程中,AMPK通过激活KATP通道,缩短动作电位持续时间,起到心肌保护作用[16-17]。Alesutan等[18]研究发现,在非洲爪蟾卵母细胞中表达的Kir2.1同样也受AMPK的调节。AMPK活性的改变是PRKAG2心脏综合征心肌肥厚、心肌细胞糖原过度沉积以及心律失常等心脏综合征症状的主要机制[19]。
AMPK是一种丝氨酸/苏氨酸激酶,由α催化亚基和β、γ调节亚基组成的异质三联体,γ亚基目前已知有3种亚型,分别为γ1、γ2和γ3。与γ1、γ3相比,γ2亚型的氨基末端延伸出约300个氨基酸,这是γ1、γ3所没有的[20]。在心脏的生长发育中,AMPKγ2是唯一发挥作用的AMPKγ复合体。γ亚单氨基端含有4个串联重复序列和由60个氨基酸组成的CBS区域,它们串联形成Bateman结构域,Bateman结构域是AMP和ATP的结合位点,该结构域的突变会引起AMPK结合AMP的能力变化,并导致AMPK活性改变。AMPK活性的改变会带来严重的病理生理学改变[21]。目前为止,国外发现的PRKAG2基因位点的突变均位于AMPKγ2亚基的CBS区域。
PRKAG2基因不同突变位点导致的AMPK活性的改变不一定相同。Sidhu等[11]研究发现PRKAG2(R302Q)突变导致AMPK活性下降;Arad等[7]发现PRKAG2(N488I)突变转基因小鼠AMPK活性较野生型小鼠增高,心肌细胞中的糖原较正常的心肌细胞增加30倍;Banerjee等[10]构建的PRKAG2(T400N)突变转基因鼠心肌AMPK的活性表现为双相改变,在转基因小鼠出生的第2天AMPK活性较对照野生型小鼠增高1.1倍,1~2周时AMPK活性进一步升高至野生型小鼠的1.4~1.5倍,而在4周、8周、12周时下降至野生型小鼠的0.54倍、0.52倍、0.41倍,20周时又恢复到野生型小鼠水平。不同类型的基因突变对AMPK活性的影响不同,与突变位点处于CBS不同的区域有关[21]。AMPK通过促进周围组织对葡萄糖的摄取、抑制糖原的合成、促进葡萄糖向糖酵解方向转化等途径调节糖代谢,AMPK活性下降可导致心肌细胞糖原过度沉积;而PRKAG2(N488I)突变时AMPK活性增高,但同样会导致心肌细胞糖原过度沉积,可能与AMPK活性增高时机体对糖的吸收增加有关[7]。
我们前期在中国人群特有的PRKAG2心脏综合征家系中发现了新的错义突变PRKAG2(G100S)突变,经基因测序发现PRKAG2(G100S)位于AMPKγ2亚基非CBS区,通过构建过表达PRKAG2(G100S)突变动物模型,对该疾病的临床特征、分子机制进行了大量的研究。Zhang等[22]成功构建了过表达PRKAG2(G100S)突变斑马鱼模型,并发现基因突变组心肌细胞AMPK活性明显低于野生型组,但该实验未对PRKAG2(G100S)突变时AMPK活性的动态变化进行研究。在本研究中,我们分别检测了4周龄、12周龄的PRKAG2(G100S)突变转基因小鼠和同窝野生型小鼠心肌细胞AMPK活性,结果显示不同周龄的转基因小鼠心肌细胞中AMPK活性均低于同窝野生型小鼠,12周龄与4周龄转基因小鼠相比心肌细胞中AMPK活性差异无统计学意义。以上研究表明,PRKAG2(G100S)突变导致心肌细胞的AMPK活性变化特点是:(1)导致AMPK活性下降;(2)AMPK活性较稳定,不随年龄增长升高或降低。本研究结果与国外报道的PRKAG2(R302Q)突变、PRKAG2(R531G)突变等转基因小鼠模型的结果[9, 11]较一致。至于为何位于AMPKγ2亚基非CBS区的PRKAG2(G100S)突变也可导致AMPK活性下降,可能也与AMPK结合AMP的能力下降有关,具体机制仍有待于进一步研究。
| [1] |
GOLLOB M H, GREEN M S, TANG A S, ROBERTS R. PRKAG2 cardiac syndrome:familial ventricular preexcitation, conduction system disease, and cardiac hypertrophy[J]. Curr Opin Cardiol, 2002, 17: 229-234. DOI:10.1097/00001573-200205000-00004 |
| [2] |
THEVENON J, LAURENT G, ADER F, LAFORÊT P, KLUG D, DUVA PENTIAH A, et al. High prevalence of arrhythmic and myocardial complications in patients with cardiac glycogenosis due to PRKAG2 mutations[J]. Europace, 2017, 19: 651-659. |
| [3] |
GOLLOB M H, GREEN M S, TANG A S, GOLLOB T, KARIBE A, ALI HASSAN A S, et al. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome[J]. N Engl J Med, 2001, 344: 1823-1831. DOI:10.1056/NEJM200106143442403 |
| [4] |
GOLLOB M H, SEGER J J, GOLLOB T N, TAPSCOTT T, GONZALES O, BACHINSKI L, et al. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy[J]. Circulation, 2001, 104: 3030-3033. DOI:10.1161/hc5001.102111 |
| [5] |
BAYRAK F, KOMURCU-BAYRAK E, MUTLU B, KAHVECI G, BASARAN Y, ERGINEL-UNALTUNA N. Ventricular pre-excitation and cardiac hypertrophy mimicking hypertrophic cardiomyopathy in a Turkish family with a novel PRKAG2 mutation[J]. Eur J Heart Fail, 2006, 8: 712-715. DOI:10.1016/j.ejheart.2006.03.006 |
| [6] |
ARAD M, BENSON D W, PEREZ-ATAYDE A R, MCKENNA W J, SPARKS E A, KANTER R J, et al. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy[J]. J Clin Invest, 2002, 109: 357-362. DOI:10.1172/JCI0214571 |
| [7] |
ARAD M, MOSKOWITZ I P, PATEL V V, AHMAD F, PEREZ-ATAYDE A R, SAWYER D B, et al. Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy[J]. Circulation, 2003, 107: 2850-2856. DOI:10.1161/01.CIR.0000075270.13497.2B |
| [8] |
SCOTT J W, HAWLEY S A, GREEN K A, ANIS M, STEWART G, SCULLION G A, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations[J]. J Clin Invest, 2004, 113: 274-284. DOI:10.1172/JCI19874 |
| [9] |
DAVIES J K, WELLS D J, LIU K, WHITROW H R, DANIEL T D, GRIGNANI R, et al. Characterization of the role of γ2 R531G mutation in AMP-activated protein kinase in cardiac hypertrophy and Wolff-Parkinson-White syndrome[J]. Am J Physiol Heart Circ Physiol, 2006, 290: H1942-H1951. DOI:10.1152/ajpheart.01020.2005 |
| [10] |
BANERJEE S K, RAMANI R, SABA S, RAGER J, TIAN R, MATHIER M A, et al. A PRKAG2 mutation causes biphasic changes in myocardial AMPK activity and does not protect against ischemia[J]. Biochem Biophys Res Commun, 2007, 360: 381-387. DOI:10.1016/j.bbrc.2007.06.067 |
| [11] |
SIDHU J S, RAJAWAT Y S, RAMI T G, GOLLOB M H, WANG Z, YUAN R, et al. Transgenic mouse model of ventricular preexcitation and atrioventricular reentrant tachycardia induced by an AMP-activated protein kinase loss-of-function mutation responsible for Wolff-ParkinsonWhite syndrome[J]. Circulation, 2005, 111: 21-29. DOI:10.1161/01.CIR.0000151291.32974.D5 |
| [12] |
张静, 郑兴, 秦永文, 周炳炎, 吴弘, 王洪如. 家族性传导系统异常伴心室预激及心肌肥厚一家系调查分析[J]. 中华心血管病杂志, 2007, 35: 258-259. DOI:10.3760/j.issn:0253-3758.2007.03.016 |
| [13] |
陈挺, 刘杰, 肖良, 鲍礼智, 余云华, 郑兴. 心脏过表达人源PRKAG2(G100S)转基因小鼠模型的建立[J]. 第二军医大学学报, 2016, 37: 273-278. CHEN T, LIU J, XIAO L, BAO L Z, YU Y H, ZHENG X. Establishment of transgenic mouse model with cardiac overexpression of PRKAG2-G100S[J]. Acad J Sec Mil Med Univ, 2016, 37: 273-278. |
| [14] |
LIGHT P E, WALLACE C H, DYCK J R. Constitutively active adenosine monophosphate-activated protein kinase regulates voltage-gated sodium channels in ventricular myocytes[J]. Circulation, 2003, 107: 1962-1965. DOI:10.1161/01.CIR.0000069269.60167.02 |
| [15] |
YOSHIDA H, BAO L, KEFALOYIANNI E, TASKIN E, OKORIE U, HONG M, et al. AMP-activated protein kinase connects cellular energy metabolism to KATP channel function[J]. J Mol Cell Cardiol, 2012, 52: 410-418. DOI:10.1016/j.yjmcc.2011.08.013 |
| [16] |
SUKHODUB A, JOVANOVIĆ S, DU Q, BUDAS G, CLELLAND A K, SHEN M, et al. A M P-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K+ channels[J]. J Cell Physiol, 2007, 210: 224-236. DOI:10.1002/(ISSN)1097-4652 |
| [17] |
TURRELL H E, RODRIGO G C, NORMAN R I, DICKENS M, STANDEN N B. Phenylephrine preconditioning involves modulation of cardiac sarcolemmal KATP current by PKC delta, AMPK and p38 MAPK[J]. J Mol Cell Cardiol, 2011, 51: 370-380. DOI:10.1016/j.yjmcc.2011.06.015 |
| [18] |
ALESUTAN I, MUNOZ C, SOPJANI M, DËRMAKUSOPJANI M, MICHAEL D, FRASER S, et al. Inhibition of Kir2.1(KCNJ2) by the AMP-activated protein kinase[J]. Biochem Biophys Res Commun, 2011, 408: 505-510. DOI:10.1016/j.bbrc.2011.04.015 |
| [19] |
DYCK J R, LOPASCHUK G D. AMPK alterations in cardiac physiology and pathology:enemy or ally?[J]. J Physiol, 2006, 574(Pt 1): 95-112. |
| [20] |
CHEUNG P C, SALT I P, DAVIES S P, HARDIE D G, CARLING D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding[J]. Biochem J, 2000, 346(Pt 3): 659-669. |
| [21] |
DANIEL T, CARLING D. Functional analysis of mutations in the γ2 subunit of AMP-activated protein kinase associated with cardiac hypertrophy and WolffParkinson-White syndrome[J]. J Biol Chem, 2002, 277: 51017-51024. DOI:10.1074/jbc.M207093200 |
| [22] |
ZHANG B L, YE Z, XU R L, YOU X H, QIN Y W, WU H, et al. Overexpression of G100S mutation in PRKAG2 causes Wolff-Parkinson-White syndrome in zebrafish[J]. Clin Genet, 2014, 86: 287-291. DOI:10.1111/cge.12267 |
 2019, Vol. 40
2019, Vol. 40


