肝细胞癌(hepatocellular carcinoma,HCC)是临床最常见的恶性肿瘤之一,其发病率及死亡率分别居我国恶性肿瘤的第3位和第2位,严重威胁居民的生命健康[1]。虽然近年来HCC诊疗水平不断提高,但整体疗效仍然不佳,主要原因在于大部分HCC患者就诊时就已属中晚期。中晚期HCC的一个主要特征是合并门静脉癌栓(portal vein tumor thrombus,PVTT)。在初次就诊的HCC患者中PVTT的发生率高达44%~66.2%,在中晚期患者中这一比例更高(80%~90%);患者预后极差,自然生存时间仅为2.7~4个月[2]。由于HCC合并PVTT患者例数众多,因此如能提高这部分患者的疗效,就有望提高HCC的整体疗效。对于这部分患者的诊治国际上存在争议,西方国家主要以分子靶向药物(索拉非尼)为主,有效率仅为27.7%~43.6%[3-4];东南亚及我国主要以手术、经导管肝动脉化学栓塞(transcatheter arterial chemoembolization,TACE)、放射治疗及综合治疗为主[5-6],疗效报道不一,总体生存率偏低。因此,HCC合并PVTT的诊治研究是目前国际和国内HCC领域最具挑战性的难点和热点。
然而,既往PVTT患者的诊治主要以单学科或单学科多模式为主,即主要取决于初诊医师做出的诊治决策,治疗方法的选择缺乏指导体系。如外科医师推崇手术切除,放射科、介入科及内科医师则可能推崇以放射治疗、TACE、内科保守治疗为主的综合治疗,导致临床诊治无序且疗效参差不齐。
针对目前HCC合并PVTT棘手的诊治现状,本团队建立了HCC合并PVTT多学科诊治创新体系,并展开系统化的研究和应用。本文就PVTT发生机制、PVTT的临床诊疗和PVTT多学科诊疗(multidisciplinary team,MDT)新模式展开讨论。
1 PVTT的发生机制本团队首次建立了2株来源于患者PVTT组织的能够模拟PVTT发生的细胞系(CSQT-1、CSQT-2)[7]。该细胞系开拓了HCC转移研究的新途径,为研究PVTT发生分子机制提供了珍贵的研究模型,被国内外同行广泛使用。同时本团队深入研究了PVTT的发生机制,尝试寻找潜在的治疗靶点及标志物,指导临床转化。
既往对于PVTT发生机制的研究不够深入,本团队首次利用细胞黏附分子1(intercellular adhesion molecule 1,ICAM1)分离出PVTT相关肿瘤干细胞,并发现ICAM1可促进PVTT的形成及肿瘤转移[8],且该促进作用是通过调控ICAM1相关的长链非编码RNA实现的[9]。此外,我们还发现FOXM1/miR-135a/MTSS1信号通路影响着PVTT的发生及肿瘤转移[10],14-3-3ζ/HIF-1α信号通路也参与了HCC细胞向PVTT的进展[11]。针对临床缺少PVTT标志物的难点,我们研究发现α-岩藻糖苷酶(α-L-fucosidase,AFU)、成纤维细胞生长因子诱导早期反应蛋白14(fibroblast growth factor-inducible 14,Fn14)可作为预测HCC合并PVTT预后的标志物[12]。
既往关于HCC合并PVTT研究的临床转化应用较少,制约了疗效的提升。本团队研究发现乙型肝炎病毒(hepatitis B virus,HBV)引起的炎症可以通过TGF-β/miRNA-34a/CCL22/Treg途径促进PVTT的发生[13]。我们在此基础上开展临床转化应用研究,发现术后抗病毒治疗可提升HCC合并PVTT患者2年总生存率至7.0%(对照组为0)[14]。同时我们发表多篇述评论述了抗病毒治疗对于HCC患者长期生存的重要意义[15-16]。既往以索拉非尼为代表的分子靶向药物在临床上取得了一定的疗效,为HCC的治疗开辟了新的方向和思路;但索拉非尼治疗PVTT的有效率仅为27.7%~43.6%,患者的总生存时间仅为6.5个月[3-4]。Feng等[17]研究发现,术后早期应用索拉非尼可通过阻断肝再生ERK信号通路有效抑制PVTT生长和HCC转移,在此基础上开展的临床研究发现,术后早期使用索拉非尼治疗的患者中位生存时间可达15个月,明显长于对照组(10个月),该结论为合理应用索拉非尼提供了理论支持。针对HCC合并PVTT诊治过程中出现索拉非尼或静脉FOLFOX4方案(氟尿嘧啶、亚叶酸钙及奥沙利铂灌注)化学治疗耐药的难题,本团队研究发现全反式维甲酸可通过降低EpCAM阳性肿瘤干细胞的比例降低肿瘤干细胞的干性及耐药性,从而提高疗效[18-19]。该结果为解决索拉非尼或化学治疗药物耐药开辟了一种潜在有效的临床治疗手段,为临床转化奠定了基础。
2 PVTT的临床诊疗HCC合并PVTT患者的病情复杂,PVTT的侵犯程度及类型是指导治疗及评估预后的关键因素。既往尚无公认的PVTT分型标准,从而导致治疗方法笼统、诊治不规范、不当治疗或过度治疗。针对这一难点,本团队建立了具有中国特色的PVTT分型标准(又称程氏分型)[20]:Ⅰ型,癌栓侵犯肝叶或肝段的门静脉分支;Ⅱ型,癌栓侵犯至门静脉左支或右支;Ⅲ型,癌栓侵犯至门静脉主干;Ⅳ型,癌栓侵犯至肠系膜上静脉;术后病理学诊断微血管癌栓为Ⅰ0型。该分型标准与目前国际上主流的HCC分期如TNM分期[21]、意大利的CLIP评分系统[22]、日本的JIS评分系统[23]相比更科学、更简单、更实用,也更有利于患者病情评估、治疗选择、预后监测。香港中文大学刘允怡院士评价该分型标准优于日本的VP分型系统,目前该分型标准已被全国肝癌合并癌栓诊治研究协作组推荐为中国的PVTT分型标准。我们据此分型也明确了PVTT的治疗指征,即Ⅰ型与Ⅱ型HCC合并PVTT患者适合手术治疗,Ⅲ型慎重手术,Ⅳ型患者禁忌手术治疗,同时也推荐了PVTT的介入治疗指征[24-25]。
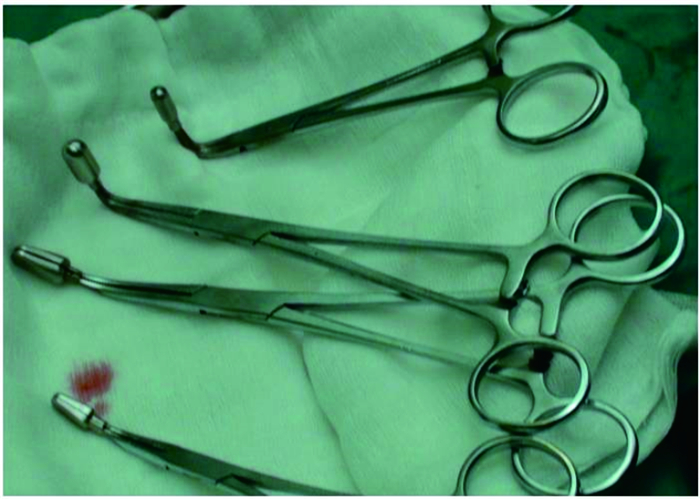
针对不同分型HCC合并PVTT治疗的难点,我们进行了一系列的技术革新:(1)针对Ⅲ型PVTT患者手术切除有争议、手术切除率低、术后复发率高等难题,发现PVTT对放射治疗的反应比HCC原发灶更敏感,并以此为理论依据首创了三维适形调强放射治疗联合病理降期手术切除[26]。通过PVTT直线加速器三维调强适形放射治疗技术,采用计算机断层扫描(computed tomography,CT)或磁共振成像(magnetic resonance imaging,MRI)精确定位PVTT的范围,设定靶区,以30 Gy精确分割剂量连续照射6 d,待癌栓缩小降期后再行手术切除,使Ⅲ型PVTT患者1、2年总生存率分别提升至69.0%、20.4%,远高于对照组(分别为35.6%、0)[26]。(2)针对HCC合并PVTT范围不清、切缘难以把握、术后残肝体积小等难点,将三维数字成像技术引入PVTT的治疗领域,精确判断手术范围,改良术式[27]。相比传统的CT或MRI,基于多层螺旋CT数据的三维数字成像技术能更清楚地显示瘤体及PVTT的范围,更有利于判断手术指征、指导手术操作。创用该技术可将PVTT患者术后2年的总生存率提升至40.0%,明显高于对照组(18.0%)[28]。(3)针对无法行手术治疗的PVTT患者既往采用的治疗方案疗效不佳这一难点,本团队使用三维调强适形放射治疗联合TACE治疗可提高疗效。首先采用PVTT直线加速器三维调强适形放射治疗技术精确照射癌栓组织,待癌栓回缩、门静脉部分或全部再通后行TACE治疗,可将患者中位生存时间延长至11个月,远长于TACE(3.98个月)、TACE联合索拉非尼(6.96个月)、索拉非尼(6.5个月)等治疗方案[29]。(4)针对PVTT断端取栓术缺乏有效工具、无法彻底清除PVTT组织、疗效差等难点,本团队发明了专门针对PVTT组织的癌栓取栓钳(专利号:CN 204797942 U,图 1)。相比传统取栓器械,该取栓钳能够防止取栓过程中PVTT播散,满足术中无瘤操作的需求。我们还开创了内镜辅助下取栓术(图 2),这一术式可彻底清除肉眼癌栓,减少术后复发,提高患者的总生存率。腹腔镜手术用PVTT取栓钳、医用癌栓吸引器、HCC癌栓蛐纹弹簧吸引管、可旋转肝脏外科手术用下腔静脉根部血管解剖分离钳、PVTT激光光纤消融针调节固定装置、新型双腔药物缓释输注装备、医用放射性双膜镍钛合金血管内支架等技术也均可能促进PVTT治疗手段的发展,目前正处于临床转化研发阶段。
|
图 1 门静脉癌栓取栓钳(专利号CN 204797942 U) Fig 1 Thrombectomy forceps for portal vein tumor thrombus (Patent No. CN 204797942 U) |

|
图 2 门静脉癌栓内镜辅助下取栓术 Fig 2 Endoscopic assisted thrombectomy for portal vein tumor thrombus A: Portal vein tumor thrombus before thrombectomy; B: Portal venous lumen after completely removing tumor thrombus with thrombectomy |
3 PVTT MDT新模式
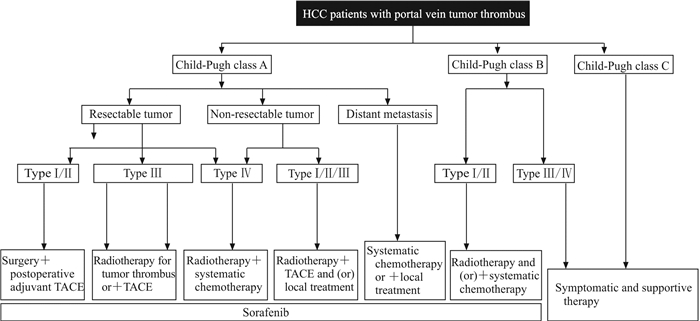
既往HCC合并PVTT的诊治主要以单学科或单学科多模式诊治为主,即治疗策略的制定主要依赖初诊医师或初诊科室。由于各学科医师专业背景的差异导致临床工作中HCC合并PVTT的诊治策略五花八门、疗效参差不齐。针对这一难题,本团队在国内较早地提出了MDT新理念,即根据患者基本情况、癌栓类型及肿瘤是否可切除等情况,经多学科医师讨论后决定治疗方案,并在国内首次制定了HCC合并PVTT患者MDT流程图(图 3)[20]。同时联合我院介入科、医学影像科、放射治疗科、肝内科等科室共同组建了国内首家PVTT多学科专病诊治中心——海军军医大学(第二军医大学)门静脉癌栓诊治中心及全国HCC合并PVTT研究协作组。
|
图 3 HCC合并门静脉癌栓患者多学科联合诊治流程图 Fig 3 Diagnosis and treatment of HCC patients with portal vein tumor thrombus HCC: Hepatocellular carcinoma; TACE: Transcatheter arterial chemoembolization |
针对HCC合并PVTT临床诊治工作不规范、缺乏有效合理的诊治体系的难题,本团队在MDT理念的引领下率先组织东方肝胆外科医院多名专家制定了《肝细胞癌合并门静脉癌栓多学科诊治——东方肝胆外科医院专家共识》(2015)[30];并联合全国肝胆外科、放射治疗科、医学影像科、介入科、肝内科80多名专家共同起草编订了《肝细胞癌合并门静脉癌栓多学科诊治——中国专家共识(2016)》[20],该共识为HCC合并PVTT MDT诊治领域国内外首部专家共识,首次对HCC合并PVTT的诊治给出了规范性意见。
4 小结HCC合并PVTT患者治疗难度大,病死率高,预后较差[31]。根据特定的PVTT分型制定MDT创新体系的个体化治疗方案能使患者获得较好预后。MDT创新体系的综合治疗方案可减少微小癌栓灶的肝内转移,降低术后复发率,提高患者的远期存活率。MDT创新体系的治疗策略是以患者个体化的治疗为导向,根据患者的具体情况提供最佳的治疗方案,在肯定手术治疗PVTT疗效的同时,突出了TACE治疗、放射治疗、化学治疗的效果[32]。然而,目前关于PVTT MDT创新体系的几种联合治疗方式多为回顾性研究,尚缺乏多中心、大样本、前瞻性的随机对照研究。同时,需要进一步细化不同分型PVTT患者的治疗方式,为患者提供更加个体化的治疗方案。
| [1] |
TORRE L A, BRAY F, SIEGEL R L, FERLAY J, LORTET-TIEULENT J, JEMAL A. Global cancer statistics, 2012[J]. CA Cancer J Clin, 2015, 65: 87-108. DOI:10.3322/caac.21262 |
| [2] |
ZHANG Z M, LAI E C, ZHANG C, YU H W, LIU Z, WAN B J, et al. The strategies for treating primary hepatocellular carcinoma with portal vein tumor thrombus[J]. Int J Surg, 2015, 20: 8-16. DOI:10.1016/j.ijsu.2015.05.009 |
| [3] |
CHENG A L, KANG Y K, CHEN Z, TSAO C J, QIN S, KIM J S, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma:a phase Ⅲ randomised, double-blind, placebocontrolled trial[J]. Lancet Oncol, 2009, 10: 25-34. DOI:10.1016/S1470-2045(08)70285-7 |
| [4] |
SCHWARTZ J D. Sorafenib in advanced hepatocellular carcinoma[J]. N Engl J Med, 2008, 359: 378-390. DOI:10.1056/NEJMoa0708857 |
| [5] |
KOKUDO T, HASEGAWA K, MATSUYAMA Y, TAKAYAMA T, IZUMI N, KADOYA M, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion[J]. J Hepatol, 2016, 65: 938-943. DOI:10.1016/j.jhep.2016.05.044 |
| [6] |
YANG M, FANG Z, YAN Z, LUO J, LIU L, ZHANG W, et al. Transarterial chemoembolisation (TACE) combined with endovascular implantation of an iodine-125 seed strand for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis versus TACE alone:a two-arm, randomised clinical trial[J]. J Cancer Res Clin Oncol, 2014, 140: 211-219. DOI:10.1007/s00432-013-1568-0 |
| [7] |
WANG T, HU H S, FENG Y X, SHI J, LI N, GUO W X, et al. Characterisation of a novel cell line (CSQT-2) with high metastatic activity derived from portal vein tumour thrombus of hepatocellular carcinoma[J]. Br J Cancer, 2010, 102: 1618-1626. DOI:10.1038/sj.bjc.6605689 |
| [8] |
LIU S, LI N, YU X, XIAO X, CHENG K, HU J, et al. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells[J/OL]. Gastroenterology, 2013, 144: 1031-1041.e10. doi: 10.1053/j.gastro.2013.01.046.
|
| [9] |
GUO W, LIU S, CHENG Y, LU L, SHI J, XU G, et al. ICAM-1-related noncoding RNA in cancer stem cells maintains ICAM-1 expression in hepatocellular carcinoma[J]. Clin Cancer Res, 2016, 22: 2041-2050. DOI:10.1158/1078-0432.CCR-14-3106 |
| [10] |
LIU S, GUO W, SHI J, LI N, YU X, XUE J, et al. MicroRNA-135a contributes to the development of portal vein tumor thrombus by promoting metastasis in hepatocellular carcinoma[J]. J Hepatol, 2012, 56: 389-396. |
| [11] |
TANG Y, LIU S, LI N, GUO W, SHI J, YU H, et al. 14-3-3ζ promotes hepatocellular carcinoma venous metastasis by modulating hypoxia-inducible factor-1α[J]. Oncotarget, 2016, 7: 15854-15867. |
| [12] |
WANG K, GUO W, LI N, SHI J, ZHANG C, LAU W Y, et al. Alpha-L-fucosidase as a prognostic indicator for hepatocellular carcinoma following hepatectomy:a large-scale, long-term study[J]. Br J Cancer, 2014, 110: 1811-1819. DOI:10.1038/bjc.2014.102 |
| [13] |
YANG P, LI Q J, FENG Y, ZHANG Y, MARKOWITZ G J, NING S, et al. TGF-β-miR-34a-CCL22 signalinginduced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma[J]. Cancer Cell, 2012, 22: 291-303. DOI:10.1016/j.ccr.2012.07.023 |
| [14] |
LI N, LAI E C, SHI J, GUO W X, XUE J, HUANG B, et al. A comparative study of antiviral therapy after resection of hepatocellular carcinoma in the immune-active phase of hepatitis B virus infection[J]. Ann Surg Oncol, 2010, 17: 179-185. DOI:10.1245/s10434-009-0694-z |
| [15] |
YU L H, LI N, SHI J, GUO W X, WU M C, CHENG S Q. Does anti-HBV therapy benefit the prognosis of HBV-related hepatocellular carcinoma following hepatectomy?[J]. Ann Surg Oncol, 2014, 21: 1010-1015. DOI:10.1245/s10434-013-3320-z |
| [16] |
YU L H, LI N, CHENG S Q. The role of antiviral therapy for HBV-related hepatocellular carcinoma[J/OL]. Int J Hepatol, 2011, 2011: 416459. doi: 10.4061/2011/416459.
|
| [17] |
FENG Y X, WANG T, DENG Y Z, YANG P, LI J J, GUAN D X, et al. Sorafenib suppresses postsurgical recurrence and metastasis of hepatocellular carcinoma in an orthotopic mouse model[J]. Hepatology, 2011, 53: 483-492. DOI:10.1002/hep.24075 |
| [18] |
ZHANG Y, GUAN D X, SHI J, GAO H, LI J J, ZHAO J S, et al. All-trans retinoic acid potentiates the chemotherapeutic effect of cisplatin by inducing differentiation of tumor initiating cells in liver cancer[J]. J Hepatol, 2013, 59: 1255-1263. DOI:10.1016/j.jhep.2013.07.009 |
| [19] |
GUAN D X, SHI J, ZHANG Y, ZHAO J S, LONG L Y, CHEN T W, et al. Sorafenib enriches epithelial cell adhesion molecule-positive tumor initiating cells and exacerbates a subtype of hepatocellular carcinoma through TSC2-AKT cascade[J]. Hepatology, 2015, 62: 1791-1803. DOI:10.1002/hep.28117 |
| [20] |
CHENG S, CHEN M, CAI J; National Research Cooperative Group for Diagnosis and Treatment of Hepatocellular Carcinoma with Tumor Thrombus. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus:2016 edition[J]. Oncotarget, 2017, 8: 8867-8876. |
| [21] |
AMIN M B, GREENE F L, EDGE S B, COMPTON C C, GERSHENWALD J E, BROOKLAND R K, et al. The Eighth Edition AJCC Cancer Staging Manual:continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging[J]. CA Cancer J Clin, 2017, 67: 93-99. DOI:10.3322/caac.21388 |
| [22] |
A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators[J]. Hepatology, 1998, 28: 751-755.
|
| [23] |
KUDO M, CHUNG H, HAJI S, OSAKI Y, OKA H, SEKI T, et al. Validation of a new prognostic staging system for hepatocellular carcinoma:the JIS score compared with the CLIP score[J]. Hepatology, 2004, 40: 1396-1405. DOI:10.1002/(ISSN)1527-3350 |
| [24] |
ZHANG X P, WANG K, CHEN Z H, CHENG S Q. Hepatocellular carcinoma with hepatic vein invasion should not be considered a contraindication for liver resection[J/OL]. Hepatology, 2017. doi: 10.1002/hep.29665.
|
| [25] |
ZHANG X P, WANG K, GUO W X, CHEN Z H, CHENG S Q. Is sorafenib an optimal treatment for hepatocellular carcinoma with macrovascular invasion or metastatic disease?[J/OL]. Hepatology, 2018, 68: 786. doi: 10.1002/hep.29862.
|
| [26] |
LI N, FENG S, XUE J, WEI X B, SHI J, GUO W X, et al. Hepatocellular carcinoma with main portal vein tumor thrombus:a comparative study comparing hepatectomy with or without neoadjuvant radiotherapy[J]. HPB (Oxford), 2016, 18: 549-556. DOI:10.1016/j.hpb.2016.04.003 |
| [27] |
SU F, CHEN K H, LIANG Z G, WU C H, LI L, QU S, et al. Comparison of three-dimensional conformal radiotherapy and hepatic resection in hepatocellular carcinoma with portal vein tumor thrombus[J]. Cancer Med, 2018, 7: 4387-4395. DOI:10.1002/cam4.2018.7.issue-9 |
| [28] |
WEI X B, XU J, LI N, YU Y, SHI J, GUO W X, et al. The role of three-dimensional imaging in optimizing diagnosis, classification and surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus[J]. HPB (Oxford), 2016, 18: 287-295. DOI:10.1016/j.hpb.2015.10.007 |
| [29] |
LI X L, GUO W X, HONG X D, YANG L, WANG K, SHI J, et al. Efficacy of the treatment of transarterial chemoembolization combined with radiotherapy for hepatocellular carcinoma with portal vein tumor thrombus:a propensity score analysis[J]. Hepatol Res, 2016, 46: 1088-1098. DOI:10.1111/hepr.v46.11 |
| [30] |
CHENG S, YANG J, SHEN F, ZHOU W, WANG Y, CONG W, et al. Multidisciplinary management of hepatocellular carcinoma with portal vein tumor thrombus-Eastern Hepatobiliary Surgical Hospital consensus statement[J]. Oncotarget, 2016, 7: 40816-40829. |
| [31] |
MARRERO J A, KULIK L M, SIRLIN C B, ZHU A X, FINN R S, ABECASSIS M M, et al. Diagnosis, staging and management of hepatocellular carcinoma:2018 practice guidance by the American Association for the Study of Liver Diseases[J]. Hepatology, 2018, 68: 723-750. DOI:10.1002/hep.v68.2 |
| [32] |
GIANNINI E G, BUCCI L, GARUTI F, BRUNACCI M, LENZI B, VALENTE M, et al. Italian Liver Cancer (ITA.LI.CA) group. Patients with advanced hepatocellular carcinoma need a personalized management:a lesson from clinical practice[J]. Hepatology, 2018, 67: 1784-1796. DOI:10.1002/hep.v67.5 |
 2019, Vol. 40
2019, Vol. 40


