烟雾吸入性急性肺损伤(smoke inhalation-induced acute lung injury, SI-ALI)是火灾时的常见创伤,损伤发生后期肺组织可发生纤维素沉积,巨噬细胞、成纤维细胞及其分泌的多种促纤维化因子的共同作用导致后期的纤维化过程,死亡率及致残率均较高[1-3]。甲泼尼龙(methylprednisolone,MP)是一种强效糖皮质激素,可以迅速改善炎症反应,但其在SI-ALI发生后早期应用能否减轻甚至阻断肺组织纤维化的进程目前尚未见报道。本研究拟通过建立烟雾吸入性肺损伤动物模型,探索早期应用MP对SI-ALI后肺间质纤维化的保护作用。
1 材料和方法 1.1 材料成年雄性Sprague-Dawley(SD)大鼠178只,体质量220~260 g,由军事医学科学院提供,动物生产许可证号:SCXK(京)2014-0013。兔抗髓过氧化物酶(myeloperoxidase,MPO)多克隆抗体(货号ab208670,英国Abcam公司)、兔抗结缔组织生长因子(connective tissue growth factor,CTGF)多克隆抗体(货号ab5097,英国Abcam公司)、兔抗高迁移率族蛋白1(high mobility group box 1,HMGB1)多克隆抗体(货号RA2139223,美国Thermo公司)、小鼠抗白细胞介素6(interleukin 6,IL-6)单克隆抗体(货号ab9324,英国Abcam公司)、兔抗甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GAPDH)单克隆抗体(货号14C10,美国Cell Signaling公司)。注射用甲泼尼龙琥珀酸钠(生产批号:H20170197,比利时辉瑞制药公司)。
1.2 实验分组及模型建立178只SD大鼠随机分为5组:对照组(n=18)、单纯烟雾吸入组(smoke组,n=40)、烟雾吸入+MP 1 d治疗组[smoke+MP(1D)组,n=40]、烟雾吸入+MP 3 d治疗组[smoke+MP(3D)组,n=40]、烟雾吸入+MP 7 d治疗组[smoke+MP(7D)组,n=40]。产烟材料为室内火灾常见7种易燃材料,包括聚双马泡沫材料、发泡橡塑绝热制品、阻燃白胶、阻尼材料、无卤电缆、硅丙乳胶漆、装饰板。烟雾产生及实验装置为自行研制并已申请专利(专利号:ZL 2017 2 1317512.6)。将发烟材料研磨成粉等比例混合(各20 g)后,置于产烟箱的电磁炉上方的托盘中,控制电磁炉温度为300 ℃,待产烟箱中烟雾充盈后用排风机将烟雾通过管道排入试验箱中,同时用烟雾检测仪[EM-4L型,澳洲新仪器(香港)有限公司]检测箱内气体的浓度,各种气体成分控制范围为:CO(400~550)×10-6,H2S(10~15)×10-6,O2 18%~20%,上述气体成分的含量通过控制排风机进行维持,当气体浓度达到预设范围时关闭产烟箱内的排风机;若未达到预设范围,打开排风机将产烟箱内烟雾吹入试验箱。除对照组之外的各烟雾吸入组均以烟雾吸入30 min为致伤因素。烟雾吸入后第1天、第1~3天、第1~7天,smoke+MP(1D)、smoke+MP(3D)、smoke+MP(7D)组分别经腹腔注射MP(每天4 mg/kg)。每天记录各组大鼠生存率,烟雾吸入后的第7、14、28天从各组存活大鼠中随机选取6只通过腹腔注射戊巴比妥钠(40 mg/kg)麻醉后进行解剖和标本采集。
1.3 马松三色和天狼星红染色检测大鼠肺纤维化程度大鼠麻醉后放血致死,从胸正中线开胸后分离并夹闭气管,剥离大血管,完整取出全肺,结扎右主支气管,收集右肺下叶并置于10%中性甲醛溶液中固定,48 h后石蜡包埋,然后切片(厚度为6 μm),脱蜡后进行马松三色及天狼星红染色,采用单盲法由2名病理科医师于光镜下观察组织学变化。采用Ashcroft肺纤维化评分标准[4]进行评分,参数包括肺泡间隔及气管腔纤维性增宽程度、肺组织总体结构破坏程度、纤维带及纤维素沉积物的形成程度、总体肺泡闭塞程度。每位病理医师对每张切片至少选取20个随机视野(放大倍数100×)进行评分。肺组织用天狼星红染色后,用偏振显微镜进行观察,采用Image-Pro Plus 6.0软件(美国Media Cybernetics公司)对平均光密度(mean optical density,MOD)进行分析统计。
1.4 实时荧光定量PCR测定大鼠肺组织中转化生长因子β1(transforming growth factor β1,TGF-β1)和α-平滑肌肌动蛋白(α-smooth muscle actin,α-SMA)的mRNA表达水平取大鼠右上肺组织保存于液氮中。用TRIzol试剂盒(美国Life Technologies公司)提取总RNA,在260 nm处测定RNA浓度后,用PCR反转录试剂盒(美国Thermo公司)合成cDNA。用ABI Prism 7000序列探测系统(美国Applied Biosystems公司)进行实时荧光定量PCR,每个样本重复3次。反应条件:95℃ 3 min;94 ℃ 30 s、58 ℃ 30 s、72 ℃30 s,30个循环;72 ℃ 5 min。α-SMA上游引物:5′-GTC CCA GAC ATC AGG GAG TAA-3′,下游引物:5′-TCG GAT ACT TCA GCG TCA GGA-3′;TGF-β1上游引物:5′-GAC TCT CCA CCT GCA AGA CCA T-3′,下游引物:5′-GGG ACT GGC GAG CCT TAG TT-3′;GAPDH上游引物:5′-TGC GAC TTC AAC AGC AAC TC-3′,下游引物:5′-ATG TAG GCC ATG AGG TCC AC-3′。目的基因的相对表达量用2-ΔΔCt表示。
1.5 蛋白质印迹法检测大鼠肺组织中MPO、CTGF、HMGB1、IL-6的表达水平取20 mg液氮中保存的标本进行组织匀浆并用包含蛋白酶抑制剂的裂解液在冰上裂解30 min,然后22 000×g离心15 min获得上清液,用BCA蛋白检测试剂盒(美国Thermo公司)测定蛋白浓度。煮沸变性后,取相应体积进行十二烷基硫酸钠-聚丙烯酰胺凝胶电泳,转膜(湿转),5%脱脂牛奶封闭1 h,TBST洗膜3次(10 min/次),加入抗MPO、抗CTGF、抗HMGB1、抗IL-6抗体4 ℃孵育过夜,TBST洗膜;加入二抗(1:2 000)温室孵育,洗膜3次;化学发光法检测。采用Image-Pro Plus 6.0软件分析MOD,GAPDH为内参蛋白。
1.6 统计学处理应用SPSS 24.0软件进行统计分析,所有数据均以x±s表示,2组间的比较采用Mann-Whitney U检验,多组间比较采用单因素方差分析。统计图采用GraphPad Prism 6.0软件绘制,大鼠Kaplan-Meier生存曲线采用log-rank检验。检验水准(α)为0.05。
2 结果 2.1 不同MP治疗方案对大鼠烟雾吸入后28 d生存率的影响图 1显示,烟雾吸入后,smoke组、smoke+MP(1D)组、smoke+MP(3D)、smoke+MP(7D)组大鼠28 d生存率分别为47.50%、82.50%、85.05%、83.33%。3个治疗组大鼠28 d的生存率与smoke组比较,差异均有统计学意义(P均<0.01),但3个治疗组之间差异无统计学意义(P均>0.05)。

|
图 1 烟雾吸入后各组大鼠28 d生存曲线 Fig 1 Survival rates of rats in each group at 28 d after smoke inhalation Smoke+MP (1D) group: Smoke+4 mg/kg methylprednisolone (MP) for 1 d; Smoke+MP (3D) group: Smoke+4 mg/kg MP for 3 d; Smoke+MP (7D) group: Smoke+4 mg/kg MP for 7 d |
2.2 烟雾吸入后大鼠肺组织胶原沉积分析
马松三色染色结果(图 2A)显示,烟雾吸入后7 d,smoke组大鼠的肺泡间隔可见明显增宽,伴有间质内少量胶原蓝染,而smoke+MP(3D)组大鼠虽然伴有轻度的肺泡间隔增宽,但是没有显著的胶原沉积;14 d时smoke组和smoke+MP(1D)组大鼠肺泡间隔内胶原沉积呈增高趋势,smoke+MP(3D)组和smoke+MP(7D)组仍无显著改变;28 d时smoke组大鼠肺泡间隔增宽明显,伴有间隔内胶原沉积显著增多,smoke+MP(1D)组有轻度增高,smoke+MP(3D)和smoke+MP(7D)组无显著改变。Ashcroft评分结果(图 2C)显示,烟雾吸入后14 d,smoke组评分高于3个治疗组(P<0.05或P<0.01);28 d时smoke组评分达最高值,高于3个治疗组(P均<0.01),且smoke+MP(1D)组高于smoke+MP(3D)和smoke+MP(7D)组(P均<0.01);smoke +MP(3D)组与smoke+MP(7D)组之间差异无统计学意义((P>0.05)。
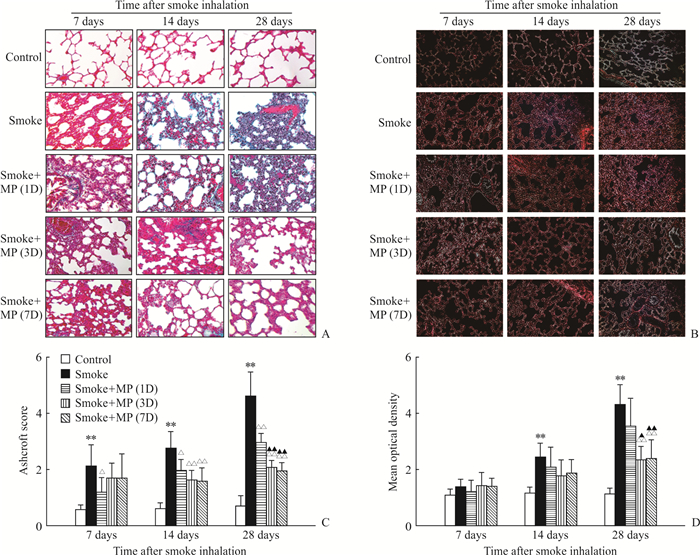
|
图 2 烟雾吸入后各组大鼠的肺组织病理学评价 Fig 2 Histopathology of lung tissues of rats in each group after smoke inhalation A: Masson's trichrome staining shows the severity of fibrosis in each group; B: Sirius Red staining shows deposition of collagenⅠin each group; C: Semi-quantitative evaluation of lung fibrosis in each group; D: Semi-quantitative evaluation of collagenⅠdeposition in each group by mean optical density. Smoke+MP (1D) group: Smoke+4 mg/kg methylprednisolone (MP) for 1 d; Smoke+MP (3D) group: Smoke+4 mg/kg MP for 3 d; Smoke+MP (7D) group: Smoke+4 mg/kg MP for 7 d. Original magnification: ×200 (A, B). **P < 0.01 vs control group; △P < 0.05, △△P < 0.01 vs smoke group; ▲P < 0.05, ▲▲P < 0.01 vs smoke+MP (1D) group. n=6, x±s |
天狼星红染色结果(图 2B、2D)显示,烟雾吸入7 d时各组大鼠肺组织染色的光密度无显著差异;14 d时各组光密度增加,smoke组高于对照组(P<0.01),但与3个治疗组之间差异无统计学意义(P均>0.05);28 d时的smoke组高于smoke+MP(3D)和smoke+MP(7D)组(P=0.004,P=0.001),smoke+MP(1D)组也高于smoke+MP(3D)和smoke+MP(7D)组(P=0.022,P=0.007),但smoke+MP(3D)和smoke+MP(7D)组间差异无统计学意义((P>0.05)。
2.3 MP对烟雾吸入后大鼠肺组织中TGF-β1、α-SMA mRNA表达的影响图 3A显示,烟雾吸入后7 d时各组大鼠肺组织中TGF-β1 mRNA表达水平均升高,smoke组与3个治疗组之间差异无统计学意义(P均>0.05);14 d时smoke组TGF-β1 mRNA表达水平进一步升高,而3个治疗组无明显升高,smoke组与3个治疗组之间的差异均有统计学意义(P=0.012,P=0.001,P=0.001);28 d时smoke组TGF-β1 mRNA表达水平高于3个治疗组(P=0.006,P=0.001,P<0.001),smoke+MP(1D)组高于smoke+MP(3D)组和smoke+MP(7D)组(P=0.011,P=0.002),smoke+MP(3D)组和smoke+MP(7D)组之间差异无统计学意义((P>0.05)。图 3B显示,烟雾吸入后7 d各组大鼠肺组织中α-SMA mRNA的表达水平差异无统计学意义(P均>0.05);14 d时smoke组α-SMA mRNA的表达水平高于smoke+MP(7D)组(P=0.026);28 d时smoke组α-SMA mRNA的表达水平继续升高,高于smoke+MP(3D)组和smoke+MP(7D)组(P=0.002,P<0.001),smoke+MP(1D)组也高于smoke+MP(3D)组和smoke+MP(7D)组(P=0.022,P=0.007),smoke+MP(3D)组和smoke+MP(7D)组之间差异无统计学意义((P>0.05)。
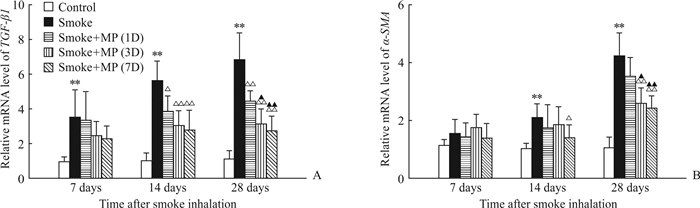
|
图 3 烟雾吸入后各组大鼠肺组织中TGF-β1、α-SMA mRNA的表达 Fig 3 mRNA expression levels of TGF-β1 and α-SMA in lung tissues of rats in each group after smoke inhalation A: TGF-β1 mRNA expression in each group; B: α-SMA mRNA expression in each group. Smoke+MP (1D) group: Smoke+4 mg/kg methylprednisolone (MP) for 1 d; Smoke+MP (3D) group: Smoke+4 mg/kg MP for 3 d; Smoke+MP (7D) group: Smoke+4 mg/kgMP for 7 d. TGF-β1: Transforming growth factor β1; α-SMA: α-Smooth muscle actin. **P < 0.01 vs control group; △P < 0.05, △△P < 0.01 vs smoke group; ▲P < 0.05, ▲▲P < 0.01 vs smoke+MP (1D) group. n=6, x±s |
2.4 烟雾吸入后各组大鼠肺组织中各炎性因子的变化
图 4显示,与smoke组相比,各治疗组在28 d时CTGF表达水平均降低(P均<0.01),其中smoke+MP(7D)组降低最显著。Smoke组的HMGB1表达水平高于各治疗组(P均<0.01),各治疗组间差异无统计学意义(P均>0.05)。IL-6表达水平在smoke组与smoke+MP(1D)组之间差异无统计学意义((P>0.05);而smoke+MP(3D)和smoke+MP(7D)组较smoke组和smoke+MP(1D)组均降低(P均<0.01),smoke+MP(3D)组和smoke+MP(7D)组之间差异无统计学意义((P>0.05)。与smoke组相比,MPO表达水平在3个治疗组也降低(P<0.05,P<0.01),smoke+MP(3D)和smoke+MP(7D)组较smoke+MP(1D)组更低(P均<0.01),smoke+MP(3D)组和smoke+MP(7D)组之间差异无统计学意义((P>0.05)。

|
图 4 烟雾吸入后各组大鼠在28 d时肺组织中MPO、CTGF、HMGB1、IL-6的蛋白表达 Fig 4 Protein expression levels of MPO, CTGF, HMGB1, IL-6 in lung tissues of rats in each group at 28 d after smoke inhalation Smoke+MP (1D) group: Smoke+4 mg/kg methylprednisolone (MP) for 1 d; Smoke+MP (3D) group: Smoke+4 mg/kg MP for 3 d; Smoke+MP (7D) group: Smoke+4 mg/kg MP for 7 d. MPO: Myeloperoxidase; CTGF: Connective tissue growth factor; HMGB1: High mobility group box 1; IL-6: Interleukin 6. **P < 0.01 vs control group; △P < 0.05, △△P < 0.01 vs smoke group; ▲▲P < 0.01 vs smoke+MP (1D) group. n=6, x±s |
3 讨论
SI-ALI常常导致急性呼吸窘迫综合征(acute respiratory distress syndrome,ARDS)的发生,其特点是:(1)组织炎症导致的血管通透性增加、黏附因子释放、炎性细胞侵袭;(2)凝血系统紊乱导致血管内微血栓形成、血管外纤维素沉积、纤溶活性受抑制;(3)组织修复的过程中发生纤维细胞增殖及细胞外基质的沉积[2]。ARDS的纤维增殖期即肺纤维化的发生也是多种类型肺损伤可能导致的共同结局,特别是ARDS导致的肺纤维化预后差,其治疗仍是一个难题[5]。巨噬细胞的极化作用是导致肺纤维化发生、发展及加重的重要因素[6],错误的M2方向极化可引起错误的修饰过程,导致纤维化的产生[2, 7]。对肺损伤进行早期干预,预防控制ARDS的发生、发展,是抑制后期纤维化发生的重要手段。
MP作为一种糖皮质激素,在多种炎症性疾病中均有应用,其不但能抑制炎性因子的产生,也可调节树突状细胞、巨噬细胞、调节性T细胞参与的多种固有或继发免疫反应。对于糖皮质激素在急性肺损伤及ARDS中的应用一直存在争议[8-9]。目前认为短期大剂量糖皮质激素在ARDS或脓毒症治疗中并无显著效果,临床证据表明ARDS早期启动长时程低剂量MP治疗(每天1 mg/kg)合并缓慢减量可以改善ARDS的预后[10],也有证据表明中小剂量糖皮质激素的短期应用可改善脓毒症的病死率[9]。糖皮质激素是否会影响急性肺损伤后期的肺纤维化的研究仍不充分。
本研究显示,烟雾吸入后早期可导致大鼠较高的死亡率,急性期后大鼠生存率未发生明显改变;烟雾吸入后给予MP可降低大鼠早期死亡率,不同应用时程之间的28 d生存率差异无统计学意义,提示早期给予糖皮质激素产生了有效的救治作用。
马松三色染色和天狼星红染色是目前判断纤维化病理进展的重要手段,通过对7、14、28 d的肺组织进行马松三色染色及天狼星红染色发现,烟雾吸入后导致的大鼠肺组织胶原沉积是一个逐渐加重的过程,28 d达到高峰。对不同时程糖皮质激素的应用效果分析显示,早期单次给予MP对晚期纤维化进展的抑制作用并不显著,而3 d及7 d的治疗方案可以有效抑制胶原的沉积,但是这两种治疗方案之间差异无统计学意义。TGF-β1是一种与肺纤维化显著相关的细胞因子,主要由M2型巨噬细胞分泌[11]。本研究发现烟雾吸入后第7天大鼠肺组织中TGF-β1水平升高,随着时间的推移,smoke组呈现持续升高的趋势;smoke+MP(1D)组也呈现时间相关的升高趋势,但是较smoke组下降;smoke+MP(3D)组和smoke+MP(7D)组下降的趋势更显著,但是smoke+MP(3D)组和smoke+MP(7D)组之间差异无统计学意义,提示短期应用MP可以缓解纤维化的进展,而延长应用时程并不会进一步改善纤维化过程。既往曾有多项研究表明,长时程或高剂量糖皮质激素在急性肺损伤的治疗中并无显著效果,而对于不同时程中低剂量糖皮质激素在肺损伤及ARDS中的应用目前仍有诸多争议[12-14],本研究发现,单剂量或延长治疗时间并不会对远期肺损伤有显著改善。
CTGF是TGF-β1发挥促纤维化作用的下游因子。有研究表明中和细胞中的CTGF可以降低纤维化过程[15]。本研究显示,烟雾吸入后28 d大鼠肺组织中的CTGF表达处于较高水平,而不同治疗组的CTGF表达有不同程度的降低,这也提示糖皮质激素对后期纤维化的改善作用。对28 d时各组大鼠肺组织中的炎性因子MPO、HMGB1、IL-6的检测结果显示,smoke+MP(3D)组及smoke+MP(7D)组的糖皮质激素治疗可以降低后期肺内炎症反应,但2组之间差异无统计学意义。糖皮质激素作为一种有效的炎症抑制剂,可以抑制急性肺损伤各类炎性因子的分泌,而这些炎性因子是导致后期纤维化的重要因素,因此早期短时程的激素治疗改善纤维化和炎症反应的重要原因是有效抑制了早期炎症反应。
本研究存在一定的局限性:首先,临床上评估肺纤维化程度一般是通过影像学特别是高分辨率计算机断层扫描检查,虽然本研究通过病理分析来判断纤维化进展,但是受限于大鼠体型及实验设备的客观情况,未对肺部进行影像学评估;其次,本研究采用MP单药治疗,目前临床上对于烟雾吸入性损伤多采用雾化吸入及全身药物综合治疗手段,下一步我们将研究联合治疗方案是否可降低近远期肺损伤程度。
综上所述,烟雾吸入后早期给予短时程MP可提高大鼠生存率、改善后期纤维化程度,但长时程治疗无法取得更多益处。
| [1] |
DE CARVALHO F O, SILVA É R, FELIPE F A, TEIXEIRA L G B, ZAGO L B S, NUNES P S, et al. Natural and synthetic products used for the treatment of smoke inhalation:a patent review[J]. Expert Opin Ther Pat, 2017, 27: 877-886. DOI:10.1080/13543776.2017.1339790 |
| [2] |
THOMPSON B T, CHAMBERS R C, LIU K D. Acute respiratory distress syndrome[J]. N Engl J Med, 2017, 377: 562-572. DOI:10.1056/NEJMra1608077 |
| [3] |
ZHU F, QIU X, WANG J, JIN Y, SUN Y, LV T, et al. A rat model of smoke inhalation injury[J]. Inhal Toxicol, 2012, 24: 356-364. DOI:10.3109/08958378.2012.673179 |
| [4] |
HÜBNER R H, GITTER W, EL MOKHTARI N E, MATHIAK M, BOTH M, BOLTE H, et al. Standardized quantification of pulmonary fibrosis in histological samples[J]. Biotechniques, 2008, 44: 507-511, 514-517. DOI:10.2144/000112729 |
| [5] |
MEDURI G U, ELTORKY M A. Understanding ARDSassociated fibroproliferation[J]. Intensive Care Med, 2015, 41: 517-520. DOI:10.1007/s00134-014-3613-0 |
| [6] |
GHARIB S A, JOHNSTON L K, HUIZAR I, BIRKLAND T P, HANSON J, WANG Y, et al. MMP28 promotes macrophage polarization toward M2 cells and augments pulmonary fibrosis[J]. J Leukoc Biol, 2014, 95: 9-18. DOI:10.1189/jlb.1112587 |
| [7] |
ARORA S, DEV K, AGARWAL B, DAS P, SYED M A. Macrophages:their role, activation and polarization in pulmonary diseases[J]. Immunobiology, 2018, 223(4/5): 383-396. |
| [8] |
VENKATESH B, FINFER S, COHEN J, RAJBHANDARI D, ARABI Y, BELLOMO R, et al. Adjunctive glucocorticoid therapy in patients with septic shock[J]. N Engl J Med, 2018, 378: 797-808. DOI:10.1056/NEJMoa1705835 |
| [9] |
ANNANE D, RENAULT A, BRUN-BUISSON C, MEGARBANE B, QUENOT J P, SIAMI S, et al. Hydrocortisone plus fludrocortisone for adults with septic shock[J]. N Engl J Med, 2018, 378: 809-818. DOI:10.1056/NEJMoa1705716 |
| [10] |
MEDURI G U, BRIDGES L, SHIH M C, MARIK P E, SIEMIENIUK R A C, KOCAK M. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes:analysis of individual patients' data from four randomized trials and trial-level meta-analysis of the updated literature[J]. Intensive Care Med, 2016, 42: 829-840. DOI:10.1007/s00134-015-4095-4 |
| [11] |
CHEN C, WANG Y Y, WANG Y X, CHENG M Q, YIN J B, ZHANG X, et al. Gentiopicroside ameliorates bleomycin-induced pulmonary fibrosis in mice via inhibiting inflammatory and fibrotic process[J]. Biochem Biophys Res Commun, 2018, 495: 2396-2403. DOI:10.1016/j.bbrc.2017.12.112 |
| [12] |
THOMPSON B T. Glucocorticoids and acute lung injury[J]. Crit Care Med, 2003, 31(4 Suppl): S253-S257. |
| [13] |
MARIK P E, MEDURI G U, ROCCO P R, ANNANE D. Glucocorticoid treatment in acute lung injury and acute respiratory distress syndrome[J]. Crit Care Clin, 2011, 27: 589-607. DOI:10.1016/j.ccc.2011.05.007 |
| [14] |
YANG Z G, LEI X L, LI X L. Early application of lowdose glucocorticoid improves acute respiratory distress syndrome:a meta-analysis of randomized controlled trials[J]. Exp Ther Med, 2017, 13: 1215-1224. DOI:10.3892/etm.2017.4154 |
| [15] |
GIBSON D J, PI L, SRIRAM S, MAO C, PETERSEN B E, SCOTT E W, et al. Conditional knockout of CTGF affects corneal wound healing[J]. Invest Ophthalmol Vis Sci, 2014, 55: 2062-2070. DOI:10.1167/iovs.13-12735 |
 2019, Vol. 40
2019, Vol. 40


