头颈肿瘤在世界范围内常见肿瘤中居于第6位[1],下咽癌是头颈肿瘤中恶性程度较高的一种,据美国癌症组织预测,2018年美国将新增下咽癌患者2 500例[2]。下咽癌易发生早期转移[3],这不仅因为头颈部淋巴组织丰富,还与该肿瘤细胞的生物学特性、所处微环境等有关。尽管手术治疗、放射治疗、化学治疗等单一或联合应用的治疗手段在不断进步,但一旦肿瘤发生转移则意味着预后差[4],因此利用合适的动物模型深入研究肿瘤转移的机制尤为迫切。目前常用的肿瘤转移机制研究模型有尾静脉注射肿瘤细胞肺转移模型和局部接种肿瘤细胞全身转移模型,其中局部接种模型常见的接种部位有肩背部和腋下区。下咽癌原发部位在头颈部,肩背部和腋下区血供、淋巴回流情况及局部组织层次等均与头颈部不同,而颏下区与下咽癌的在体微环境接近。基于上述情况,本研究采取在颈部紧靠下咽的颏下区接种下咽癌细胞株建立裸鼠下咽癌模型,并与传统的腋下区接种模型进行比较,观察模型裸鼠局部成瘤情况、颈部淋巴结转移情况和远处转移情况。
1 材料和方法 1.1 下咽癌细胞株RPMI 1640培养液、DMEM培养液、胎牛血清(FBS)均购自美国Gibco公司;0.25%胰酶购自美国Life Technologies公司。人下咽癌细胞株FADU购自中国科学院细胞所;HN31为人下咽癌细胞[5],由美国韦恩州立大学惠赠。细胞常规培养、传代,至状态良好、融合度达90%时PBS洗涤3次,胰酶消化后吹打成单细胞悬液,计数后将细胞重悬于不完全培养液中,调整细胞密度为1×106/mL。
1.2 动物饲养与建模选取在无特定病原体(specific pathogen free,SPF)级动物饲养环境中繁殖、生长的5周龄雄性BALB/c裸鼠48只,培养温度为26~28 ℃,相对湿度保持在40%~60%。每日维持10 h光照、14 h无光的明暗周期。将裸鼠分为颏下组和腋下组2组,每组24只;2组均分别接种FADU及HN31细胞,即为FADU颏下组、HN31颏下组、FADU腋下组、HN31腋下组4个亚组,每亚组12只。使用胰岛素注射器以皮下注射法接种肿瘤细胞。颏下组裸鼠在左侧颈根部进针,朝向颏下中线与左下颌骨水平支围成的三角区域方向,皮下潜行约5 mm后接种细胞100 μL;腋下组于双腋窝连线与右腋前线交叉点稍偏左进针,朝向腋窝方向,皮下潜行约5 mm后接种细胞100 μL。注意进针时需皮下潜行约5 mm,避免渗漏[6]。
1.3 接种后观察观察各组裸鼠的成瘤时间。接种7 d后有裸鼠局部见肿瘤形成,第13天时半数以上裸鼠可见瘤体形成。此后每日测量裸鼠体质量和瘤体体积(长径×短径×短径),直至有裸鼠死亡。观察并记录裸鼠生存与死亡情况,直至第8周。
1.4 超声与病理检查将8周后仍生存的裸鼠行全身麻醉(采用2%异氟烷吸入麻醉,异氟烷购自深圳市瑞沃德生命科技有限公司),使用意大利Esaote MyLabTM One/Touch B型超声机(探头型号为SL3116,频率22 MHz)检查局部肿瘤生长、浸润情况,以及肝、脾、肾等器官转移情况。采用颈椎脱臼法处死裸鼠,取肝、脾、肺组织行常规H-E染色和免疫组织化学染色,观察肿瘤在组织中的转移情况,验证超声检查结果。
1.5 统计学处理采用SPSS 22.0软件和GraphPad Prism 5.0软件进行数据分析。符合正态分布的计量资料以x±s表示,组间比较采用两样本均数比较的t检验;计数资料的组间比较采用χ2检验。采用Kaplan-Meier法计算裸鼠生存率,并行log-rank检验比较组间差异。检验水准(α)为0.05。
2 结果 2.1 成瘤时间与瘤体体积变化接种后第7天颏下组裸鼠颏下区即有可见的局部肿块,第9天腋下组裸鼠腋下区开始出现可见的肿块,至第13天半数以上裸鼠均已出现肿块。各亚组裸鼠的瘤体体积变化如图 1A、1B所示。第33天时腋下组成瘤裸鼠的瘤体体积均大于颏下组,其中FADU颏下组与FADU腋下组、HN31颏下组与HN31腋下组差异均有统计学意义(P < 0.05,P < 0.01)。

|
图 1 接种不同细胞颏下组与腋下组裸鼠瘤体体积(A、B)和体质量(C、D)的比较 Fig 1 Comparison of tumor volume (A, B) and body mass (C, D) of nude mice inoculated with different cells between submental and axillary groups *P < 0.05, **P < 0.01 vs FADU/HN31 submental group at same time point. n=12, x±s |
2.2 裸鼠体质量变化
接种初期,各组裸鼠体质量均缓慢增加,不同接种部位组间裸鼠体质量差异无统计学意义。至第17~19天时各组裸鼠体质量基本达到峰值。此后,颏下组裸鼠体质量逐步减轻,日渐消瘦,精神萎靡,呈恶病质状态(图 2A、2B);腋下组裸鼠精神好,无明显消瘦(图 2C)。第33天时FADU颏下组与FADU腋下组、HN31颏下组与HN31腋下组间裸鼠体质量差异均有统计学意义(P均 < 0.01,图 1C、1D)。

|
图 2 不同模型裸鼠的大体观察 Fig 2 Gross observation of nude mice in each group Gross observation of nude mice in each group A: HN31 submental group, the mouse had facial and eyelid swelling; B: FADU submental group, the mouse had lighter body mass and less subcutaneous fat, and was cachexia; C: FADU axillary group, the mouse had bigger tumor volume and the tumor was cystic |
2.3 各组裸鼠生存与死亡情况
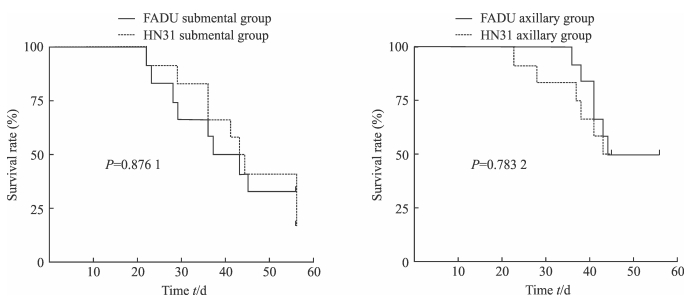
接种后第22天时FADU颏下组开始出现裸鼠死亡,死亡时裸鼠体质量低于平均体质量,肿瘤局部破溃,皮下脂肪薄,呈恶病质状态。第23天时腋下组开始出现裸鼠死亡,死于肿瘤消耗与浸润。至第8周结束时,FADU颏下组死亡8只,HN31颏下组死亡10只,颏下组共存活6只;FADU腋下组死亡6只,HN31腋下组死亡6只,腋下组共存活12只;两组间裸鼠死亡率差异无统计学意义。接种部位相同、但接种肿瘤细胞不同的各组间裸鼠死亡率差异无统计学意义。各亚组裸鼠的生存曲线见图 3。
|
图 3 各组模型裸鼠的生存率比较 Fig 3 Comparison of survival rate of nude mice in each group |
2.4 淋巴转移与远处转移情况
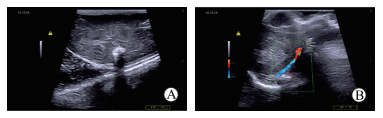
第8周时超声检查结果显示,颏下组存活裸鼠局部瘤体的深部已突破肌肉层,呈深度浸润,并有钙化;其中4只肝内可见大小不同、回声高低不一的占位,有钙化(图 4A),考虑为转移灶。腋下组存活裸鼠背部肌肉层均有肿瘤侵犯,但没有大面积突破;此外,可见肿瘤挤压腋动脉,但尚未侵犯(图 4B)。颏下组和腋下组裸鼠超声检查均未发现有脾脏转移灶。
|
图 4 超声检查成瘤裸鼠肿瘤局部生长、浸润情况 Fig 4 Tumor growth and infiltration in nude mice detected by ultrasound A: Submental group, the metastatic tumor and calcified plaque were observed in liver; B: Axillary group, the tumor squeezed the axillary artery, but it had not been violated |
病理检查结果显示,颏下组存活裸鼠中有4只发现颈部淋巴结转移及肝脏转移灶,腋下组仅有1只发现肝脏转移灶,两组间差异有统计学意义(P < 0.05)。颏下组存活裸鼠中有2只发现肺部转移灶,腋下组有1只,两组间差异无统计学意义。颏下组有1只裸鼠发现脾脏转移灶,腋下组无裸鼠存在脾脏转移灶,两组间差异无统计学意义。所有裸鼠均未发现肾脏转移灶。
3 讨论头颈部鳞状细胞癌治疗失败的原因之一是肿瘤局部复发或远处转移。下咽癌具有高转移和复发率,早期即可发生跳跃式转移,是头颈部鳞状细胞癌中预后较差的一种。肿瘤发生远处转移需经过多个步骤,受多种因素的制约,其中每一步都受肿瘤自身条件和局部环境的制约,要求肿瘤细胞具备一些特定能力[7],趋利避害,适时进行自我改造[8],例如与周围环境中的间充质干细胞相互作用[9],或分泌外泌体抑制宿主免疫[10],并为转移创造微环境[11]。此中很多机制尚不明确,需要建立一种能真实模拟下咽癌生长、转移特点的动物模型。目前常用的肿瘤转移模型有尾静脉注射肿瘤细胞肺转移模型和局部接种肿瘤细胞全身转移模型。尾静脉注射模型的肿瘤细胞并未经历肿瘤细胞前期从原发灶脱落的步骤,而是直接进入静脉系统,经右心很快进入肺循环,因此无法全程模拟体内肿瘤转移情况[12],仅模拟了肿瘤转移的后半程[13],不适于研究转移启动和初级阶段。局部接种肿瘤细胞全身转移模型可以观察肿瘤对局部组织的浸润、破坏、淋巴转移和其他脏器转移情况,相比肺转移模型更接近肿瘤在体情况。局部接种模型常用的接种部位有肩背部和腋下区。这些部位的优点是成瘤部位皮肤厚,周围无重要器官、血管,不影响小鼠的日常生活;缺点是不能提供不同来源的肿瘤特异性的生存环境。另一种相对成熟的头颈部鳞状细胞癌动物模型是Dunne等[14]建立的新西兰大白兔VX2肿瘤模型,该模型成瘤速度快,且淋巴结转移率与人类相当,但缺点是在耳部注射。下咽癌原发部位在头颈部,若在肩背部、腋下区或耳部接种,其血供情况、淋巴回流情况、局部组织构成及层次等均与原发部位不同。而颏下区与下咽癌原发部位的在体微环境接近,接近人下咽癌细胞的在体情况,可模拟肿瘤局部微环境,重演肿瘤转移过程中发生的事件。为此,本研究建立了颏下区接种下咽癌细胞株的裸鼠成瘤模型。
此前虽有学者研究颏下区成瘤[15],但未对比颏下区成瘤与其他常规区域成瘤的优劣。Dinesman等[16]比较了口底肌肉内成瘤与背部成瘤的区别,但考虑口底注射对组织的损伤,以及背部血供、淋巴回流情况与口底迥异,该研究可比性并不强。因此,本研究选择以腋下区为对照与颏下区成瘤进行比较。腋下区是常用的成瘤部位,其淋巴管网丰富,更具有可比性。结果发现颏下区血供丰富导致肿瘤生长快、易成瘤,故成瘤时间短,同时因肿瘤快速生长引起占位效应,影响进食吞咽、淋巴静脉回流,故裸鼠头面部肿胀;此外,因头面部神经较丰富,癌性疼痛影响进食,故造成恶病质,导致后期裸鼠体质量较轻。腋下区肿瘤生长稍慢,成瘤时间晚于颏下区。但颏下皮肤较薄,肿瘤侵犯皮肤后易破溃形成溃疡;而腋下区及周围胸背部皮肤较坚韧,成瘤后不易破溃,故后期瘤体体积较大。
本研究发现,FADU与HN31细胞相同注射部位的裸鼠组间死亡率差异无统计学意义,提示两种下咽癌细胞的生物学特性在肿瘤致死性方面无明显差异。同一种下咽癌细胞、不同注射部位的裸鼠组间死亡率差异也无统计学意义,提示不同部位接种并不改变肿瘤细胞的恶性行为。
本研究结果显示,颏下组裸鼠的淋巴结转移及肝脏转移率高于腋下组,表明不同区域淋巴回流、末梢循环的特点不一样,颏下区成瘤更适合模拟下咽癌细胞转移的情景。此外,本研究发现裸鼠颈部淋巴结有转移,腋窝淋巴结未发现转移,提示不同组织来源的肿瘤细胞有组织特异性,与环境中的间充质干细胞、基质等相互作用才能形成转移[9]。颏下区的血供、淋巴系统结构更接近下咽肿瘤细胞的在体情况,能更好地模拟肿瘤在人体中的生长和转移情况。
本研究还应用超声检查观察了小动物脏器转移情况,超声检查基于声波在软组织传播而成像,其因所达到组织深度的限制和成像质量易受骨组织或空气的影响而应用受限[17-18]。但其具有无辐射、操作简单(特别是在浅表大血管成像方面)、设备价格便宜等特点,因此在小动物研究中有一定的价值。超声检查分辨组织层次的能力非常实用,本研究应用超声检查观察肿瘤的局部浸润、破坏和探测远处实性器官转移情况,效果满意。相较于MRI、CT等检查,超声检查更经济、便捷,但由于解剖等原因,其在探查脾脏病变时敏感性稍差。
综上所述,颏下区成瘤与腋下区成瘤模型各有优缺点。颏下区成瘤模型因瘤体生长快、局部侵犯仿真度高,且早期即出现淋巴结转移,适用于研究肿瘤(特别是头颈部肿瘤)的局部浸润生长、转移情况。腋下区无重要器官,故腋下成瘤模型对裸鼠的生存威胁小,生存期长,且移植瘤会保留肿瘤原有特性[19],故适用于肿瘤自身特性的研究,如耐药性等方面的研究。
| [1] | DUVVURI U, MYERS J N. Cancer of the head and neck is the sixth most common cancer worldwide[J]. Curr Probl Surg, 2009, 46: 114–117. DOI: 10.1067/j.cpsurg.2008.10.002 |
| [2] | PDQ Adult Treatment Editorial Board. Hypopharyngeal Cancer Treatment (Adult) (PDQ®): Patient Version. PDQ Cancer Information Summaries[EB/OL]. Bethesda (MD): National Cancer Institute (US). (2018-02-07)[2018-02-27]. https://www.ncbi.nlm.nih.gov/books/NBK65756/. |
| [3] | KUO P, CHEN M M, DECKER R H, YARBROUGH W G, JUDSON B L. Hypopharyngeal cancer incidence, treatment, and survival:temporal trends in the United States[J]. Laryngoscope, 2014, 124: 2064–2069. DOI: 10.1002/lary.24651 |
| [4] | MORRIS L G, CHANDRAMOHAN R, WEST L, ZEHIR A, CHAKRAVARTY D, PFISTER D G, et al. The molecular landscape of recurrent and metastatic head and neck cancers: insights from a precision oncology sequencing platform[J/OL]. JAMA Oncol, 2016. doi: 10.1001/jamaoncol.2016.1790. [Epub ahead of print] |
| [5] | ZHAO M, SANO D, PICKERING C R, JASSER S A, HENDERSON Y C, CLAYMAN G L, et al. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites[J/OL]. Clin Cancer Res, 2011, 17: 7248-7264. |
| [6] | LIN W Y, CHEN J, LIN Y, HAN K. Implantation of VX2 carcinoma into the liver of rabbits:a comparison of three direct-injection methods[J]. J Vet Med Sci, 2002, 64: 649–652. DOI: 10.1292/jvms.64.649 |
| [7] | GUPTA G P, MASSAGUÉ J. Cancer metastasis:building a framework[J]. Cell, 2006, 127: 679–695. DOI: 10.1016/j.cell.2006.11.001 |
| [8] | NIETO M A, HUANG R Y, JACKSON R A, THIERY J P. EMT:2016[J]. Cell, 2016, 166: 21–45. DOI: 10.1016/j.cell.2016.06.028 |
| [9] | WHITESIDE T L. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment[J]. Semin Immunol, 2018, 35: 69–79. DOI: 10.1016/j.smim.2017.12.003 |
| [10] | WHITESIDE T L. Tumor-derived exosomes and their role in tumor-induced immune suppression[J/OL]. Vaccines (Basel), 2016, 4. pii: E35. doi: 10.3390/vaccines4040035. |
| [11] | TKACH M, THÉRY C. Communication by extracellular vesicles:where we are and where we need to go[J]. Cell, 2016, 164: 1226–1232. DOI: 10.1016/j.cell.2016.01.043 |
| [12] | BACCELLI I, SCHNEEWEISS A, RIETHDORF S, STENZINGER A, SCHILLERT A, VOGEL V, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay[J]. Nat Biotechnol, 2013, 31: 539–544. DOI: 10.1038/nbt.2576 |
| [13] | AU S H, STOREY B D, MOORE J C, TANG Q, CHEN Y L, JAVAID S, et al. Clusters of circulating tumor cells traverse capillary-sized vessels[J]. Proc Natl Acad Sci USA, 2016, 113: 4947–4952. DOI: 10.1073/pnas.1524448113 |
| [14] | DUNNE A A, SCHMIDT A, KUROPKAT C, RAMASWAMY A, SCHULZ S, WERNER J A. The auricular VX2 carcinoma-an animal model for sentinel node concept[J]. In Vivo , 2003, 17: 457–461. |
| [15] | MERMOD M, HIOU-FEIGE A, BOVAY E, ROH V, SPONAROVA J, BONGIOVANNI M, et al. Mouse model of postsurgical primary tumor recurrence and regional lymph node metastasis progression in HPVrelated head and neck cancer[J]. Int J Cancer, 2018, 142: 2518–2528. DOI: 10.1002/ijc.v142.12 |
| [16] | DINESMAN A, HAUGHEY B, GATESG A, AUFDEMORTE T, VON HOFF D D. Development of a new in vivo model for head and neck cancer[J]. Otolaryngol Head Neck Surg, 1990, 103: 766–774. DOI: 10.1177/019459989010300517 |
| [17] | WALK E L, MCLAUGHLIN S L, WEED S A. Highfrequency ultrasound imaging of mouse cervical lymph nodes[J/OL]. J Vis Exp, 2015, 25: e52718. doi: 10.3791/52718. |
| [18] | WALK E L, MCLAUGHLIN S, COAD J, WEED S A. Use of high frequency ultrasound to monitor cervical lymph node alterations in mice[J/OL]. PLoS One, 2014, 9: e100185. doi: 10.1371/journal.pone.0100185. |
| [19] | PENG S, CREIGHTON C J, ZHANG Y, SEN B, MAZUMDAR T, MYERS J N, et al. Tumor grafts derived from patients with head and neck squamous carcinoma authentically maintain the molecular and histologic characteristics of human cancers[J/OL]. J Transl Med, 2013, 11: 198. doi: 10.1186/1479-5876-11-198. |
 2018, Vol. 39
2018, Vol. 39


