2. 海军军医大学(第二军医大学)东方肝胆外科医院肝外四科, 上海 200438
2. Department of Hepatic Surgery(Ⅳ), Eastern Hepatocellular Surgery Hospital, Navy Medical University(Second Military Medical University), Shanghai 200438, China
原发性肝癌是全球最常见的恶性肿瘤之一,高居肿瘤致死原因第3位[1-2]。肝细胞癌是最重要的肝癌病理学分型,占原发性肝癌的85%~90%,全球超过一半的肝细胞癌病例发生在中国[1]。已有大量研究表明,术前血清甲胎蛋白(α-fetoprotein,AFP)水平是影响肝细胞癌切除术后及肝移植术后患者预后的重要因素[3-8]。但我们在临床实践中观察到,术前AFP水平相近的患者其预后同样存在很大差异,需要另外的指标进一步区分,以更好地判断预后。糖类抗原19-9(carbohydrate antigen 19-9,CA19-9)是一种常用的肿瘤学标志物,既往研究证明血清CA19-9水平升高与肝细胞癌患者不良预后有关[9]。新近研究发现,术前CA19-9水平可作为AFP阴性肝细胞癌患者术后预后的指标[10],并且可增强AFP对肝细胞癌切除术后预后的预测效能[11]。本研究拟探讨术前血清CA19-9水平对不同AFP水平肝细胞癌患者术后预后的影响。
1 资料和方法 1.1 病例选择前瞻性收集2008年1月4日至2010年12月31日在我院因肝细胞癌首次接受肝切除术治疗的6 713例患者的临床资料。病例纳入标准:(1)首次因肝细胞癌接受根治性肝细胞癌切除术;(2)术前未接受抗肿瘤治疗;(3)无其他恶性肿瘤史;(4)肿瘤无肝外转移;(5)术中未见大血管及胆管癌栓;(6)术后病理提示肿瘤切缘阴性且病理报告为肝细胞癌;(7)非围手术期死亡;(8)无胆囊炎、胆结石、胆道结石。本研究通过我院伦理委员会批准,患者及家属知情同意并签署知情同意书。
1.2 资料收集与随访患者于术后每个月门诊复查血清AFP和CA19-9水平、肝功能、病毒指标,每4个月复查超声、肝脏增强计算机断层扫描(computed tomography,CT)或增强磁共振成像(magnetic resonance imaging,MRI)、胸部CT或X线片检查。收集患者的年龄、性别、饮酒史、乙型肝炎病毒抗原和抗体检查结果、乙型肝炎病毒DNA载量(HBV-DNA)、肝硬化、术前肝功能、术中肝门阻断时间、术中出血量等基本资料;从术后大体标本中获得切除肿瘤的相关指标,包括肿瘤最大径、肿瘤数目、是否有包膜、是否有微血管侵犯(microvascular invasion,MVI)等。根据美国肝病研究协会(American Association for the Study of Liver Disease,AASLD)临床实践指南[12]中的诊断标准确诊肝细胞癌及肝细胞癌复发。患者的死亡信息主要来自我院术后随访办公室,随访终点为患者死亡或2014年4月28日。
1.3 统计学处理采用X-tile软件确定CA19-9的最佳截断值。采用SPSS 23.0软件进行数据分析。计数资料以例数和百分数表示,组间比较采用χ2检验。计量资料以x±s表示,组间比较采用方差分析。应用Kaplan-Meier法绘制总生存(overall survival,OS)和无瘤生存(disease free survival,DFS)曲线,应用log-rank检验比较组间差异。应用Cox回归模型对可能影响OS和DFS的因素进行单因素和多因素分析。检验水准(α)为0.05。
2 结果 2.1 临床病理学特征最终3 791例患者纳入研究。CA19-9水平为32 U/mL时2组患者进行术后生存期比较的P值最小(Pmin=0.00 013),低于临床中以37 U/mL为截断值时的P值(P=0.03 654),因此CA19-9的最佳截断值取32 U/mL。根据文献[13]取血清AFP截断值为400 ng/mL。根据上述截断值,双阳性(double positive,DP)组纳入患者427例,AFP单阳性[single positive(AFP),SP(AFP)]组869例,CA19-9单阳性[single positive(CA19-9),SP(CA19-9)]组875例,双阴性(double negative,DN)组1 620例。纳入患者的临床病理学特征见表 1。4组患者的平均年龄、肝硬化患者比例差异有统计学意义(P < 0.01,P < 0.05)。DP组患者的平均年龄最大,SP(AFP)组最小;DP组术后病理提示肝硬化的患者比例最高(63.9%),SP(CA19-9)组最低(55.0%)。4组患者的性别、饮酒史、乙型肝炎病毒表面抗原阳性率、HBV-DNA、乙型肝炎病毒e抗原阳性率、丙型肝炎病毒抗体阳性率、丙氨酸转氨酶水平、白蛋白、血小板计数、术中出血量、术中输血量、肝门阻断时间、肿瘤包膜、Child-Pugh分级差异均无统计学意义,具有可比性。
|
|
表 1 各组患者临床病理学特征的比较 Tab 1 Comparison of clinicopathological characteristics of patients among four groups |
2.2 4组患者肿瘤特征的比较
4组患者的肿瘤学特征见表 2。与DN组相比,SP(AFP)组患者的肿瘤最大径更大(P < 0.01)、病理Edmondson-Steiner分级为Ⅲ~Ⅳ级的患者比例更高(P < 0.01)、MVI发生率更高(P < 0.01);SP(CA19-9)组患者的肿瘤最大径更小(P < 0.05),而多发肿瘤的比例更高(P < 0.01);DP组患者的肿瘤最大径更大(P < 0.01)、多发肿瘤比例更高(P < 0.05)、病理Edmondson-Steiner分级为Ⅲ~Ⅳ级的患者比例和MVI发生率更高(P < 0.01)。
|
|
表 2 各组患者的肿瘤学特征比较 Tab 2 Comparison of tumor charactersitics of patients among four groups |
2.3 术后OS和DFS
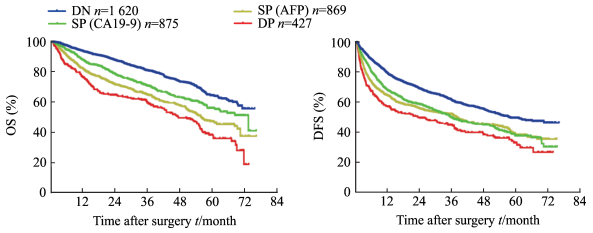
中位随访时间为38.70个月,截至随访结束,1 270例(33.50%)患者死亡,1 777例(46.8%)患者复发。所有入组患者的1年、3年和5年OS率分别为89.6%、74.2%、56.5%,1年、3年和5年DFS率分别为71.7%、55.7%、43.3%。4组患者的1年、3年和5年OS率差异均有统计学意义,按DN组、SP(CA19-9)组、SP(AFP)组和DP组的顺序,患者的1年、3年和5年OS率均依次降低(P均 < 0.01,图 1A)。DP组患者的1年、3年和5年DFS率均低于其他3组(P均 < 0.01),DN组患者的1年、3年和5年DFS率均高于其他3组患者(P均 < 0.01),而SP(CA19-9)组与SP(AFP)组患者的1年、3年和5年DFS率差异无统计学意义(图 1B)。
|
图 1 行肝切除术后的各组肝细胞癌患者的OS和DFS Fig 1 OS and DFS of hepatocellular carcinoma patients after hepatectomy The cut-off values o The cut-off values of AFP and CA19-9 are 400 ng/mL and 32 U/mL, respectively. DN: Double negative group; SP (AFP): Single positive (AFP) group; SP (CA19-9): Single positive (CA19-9) group; DP: Double positive group. AFP: α-Fetoprotein; CA19-9: Carbohydrate antigen 19-9; OS: Overall survival; DFS: Disease free survival |
2.4 AFP水平分层分析结果
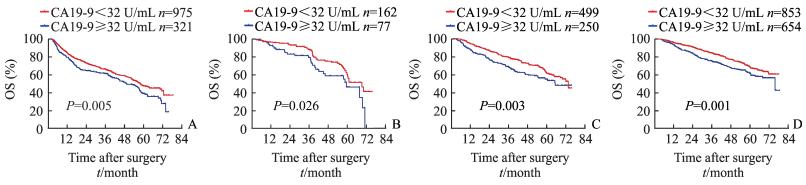
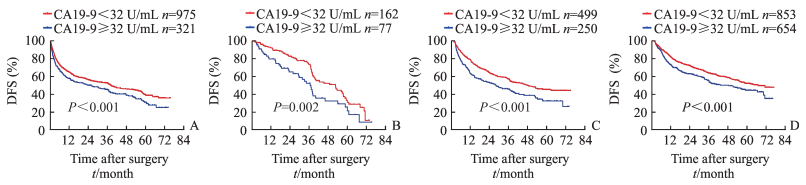
根据术前AFP水平将患者分为≥400 ng/mL、200~399 ng/mL、20~199 ng/mL和 < 20 ng/mL 4层,再将各分层患者分为CA19-9≥32 U/mL组和CA19-9 < 32 U/mL组2个亚组,分析不同AFP水平分层患者的OS和DFS。结果(图 2、3)显示,4个分层中的CA19-9 < 32 U/mL组患者的1年、3年和5年OS率以及1年、3年和5年DFS率均高于CA19-9≥32 U/mL组患者(P < 0.05)。
|
图 2 不同AFP分层患者的CA19-9水平与术后OS率的关系 Fig 2 Relationship between CA19-9 level and OS rate of patients with different AFP levels A: AFP≥400 ng/mL; B: AFP 200-399 ng/mL; C: AFP 20-199 ng/mL; D: AFP < 20 ng/mL. AFP: α-Fetoprotein; CA19-9: Carbohydrate antigen 19-9; OS: Overall survival |
|
图 3 不同AFP分层的CA19-9水平与患者术后DFS率的关系 Fig 3 Relationship between CA19-9 level and DFS rate of patients with different AFP levels A: AFP≥400 ng/mL; B: AFP 200-399 ng/mL; C: AFP 20-199 ng/mL; D: AFP < 20 ng/mL. AFP: α-Fetoprotein; CA19-9: Carbohydrate antigen 19-9; DFS: Disease free survival |
2.5 OS和DFS的危险因素分析
单因素分析结果显示,AFP≥400 ng/mL、CA19-9≥32 U/mL、术中出血量 > 600 mL、肿瘤最大径≥5 cm,肿瘤多发、肿瘤包膜缺如、MVI、Edmondson-Steiner分级为Ⅲ~Ⅳ级是OS和DFS的危险因素(P < 0.05);HBsAg(+)是影响DFS的危险因素。多因素分析结果显示,AFP≥400 ng/mL、CA19-9≥32 U/mL、术中出血≥600 mL、肿瘤最大径≥5 cm、肿瘤多发、肿瘤包膜缺如、MVI、Edmondson-Steiner分级为Ⅲ~Ⅳ级是影响OS的独立危险因素(P < 0.05);HBsAg(+)、AFP≥400 ng/mL、CA19-9≥32 U/mL、肿瘤最大径≥5 cm、肿瘤多发、肿瘤包膜缺如、MVI是影响DFS的独立危险因素(P < 0.05)。见表 3。
|
|
表 3 影响OS和DFS的多因素Cox回归分析 Tab 3 Multivariable Cox analysis of OS and DFS in hepatocellular carcinoma patients |
3 讨论
AFP是肝细胞癌患者术后的重要预后指标,多项研究已表明术前AFP升高的肝细胞癌患者预后差[3-8]。本研究发现,与DN组相比,SP(AFP)组患者的肿瘤最大径更大、病理Edmondson-Steiner分级为Ⅲ~Ⅳ级的患者比例更高、MVI发生率更高。我们在临床实践中观察到,术前AFP水平相近的患者预后亦存在很大的差异,因此需要其他指标进一步区分预后。
血清CA19-9是重要的肿瘤标志物,其临床应用广泛。近年来研究表明,术前血清CA19-9水平也是影响肝细胞癌患者术后OS的独立危险因素。Zhang等[14]研究指出术前血清CA19-9水平升高是影响肝细胞癌切除术后患者OS的独立危险因素,术前血清CA19-9水平可影响肝切除术后患者的预后。Chen等[9]的研究也得出相似结论,显示术前血清CA19-9水平升高的患者预后差。Wan等[15]对肝移植患者的研究亦指出,术前CA19-9水平能预测肝细胞癌患者肝移植术后的预后。本研究结果也得出相同的结论,与术前血清CA19-9 < 32 U/mL的患者相比,术前血清CA19-9≥32 U/mL的患者预后差,且术前血清CA19-9水平是影响肝细胞癌患者术后OS的独立危险因素。本研究还发现,术前CA19-9水平对患者的DFS亦有显著影响。生存分析结果显示,DN组患者的1年、3年和5年DFS率均高于SP(CA19-9)组。多因素回归分析结果也表明,术前CA19-9水平是影响患者DFS的独立危险因素。
为进一步验证CA19-9在肝细胞癌中的预后作用,本研究根据患者术前AFP水平将患者分为≥400 ng/mL、200~399 ng/mL、20~199ng/mL和 < 20 ng/mL 4层,分层分析结果表明,CA19-9 < 32 U/mL组患者的1年、3年和5年OS率以及1年、3年和5年DFS率均高于CA19-9≥32 U/mL组患者,进一步证明了术前CA19-9水平与肝细胞癌患者术后预后相关,术前CA19-9水平是影响肝细胞癌患者术后预后的独立危险因素。
本研究还发现,DP组患者具有独特的肿瘤学特征,与DN组相比,其肿瘤最大径更大、多发肿瘤比例更高、病理Edmondson-Steiner分级为Ⅲ~Ⅳ级的比例和MVI发生率更高。并且DP患者的预后较其他组差,这可能与肝细胞癌的变异有关。Lu等[16]研究指出可能存在同时表达肝细胞癌表型和肝内胆管细胞癌表型的双表型肝细胞癌(dual-phenotype hepatocellular carcinoma,DPHCC)。与混合型肝细胞癌不同,DPHCC的同一个细胞上可以同时表达肝细胞癌表型和肝内胆管细胞癌表型;与经典肝细胞癌相比,DPHCC具有更高的侵袭性和更差的预后。本研究中DP组患者同时高表达AFP和CA19-9,术后病理结果已经排除了混合性肝细胞癌的可能性,能够导致患者术前CA19-9水平升高的常见原因在入组时也均已排除,推测DP组患者的肝癌组织中可能混杂了DPHCC细胞,从而导致不良预后。Hsu等[17]研究表明,术前CA19-9水平升高的肝细胞癌患者预后差;免疫组织化学结果显示,在伴有CA19-9水平升高的肝细胞癌患者正常肝组织中可以观察到祖细胞样细胞和表达中间肝胆管细胞表型的细胞,据此推测高水平CA19-9患者的预后差可能与非肿瘤肝实质内的祖细胞样细胞及表达中间肝胆管细胞表型的细胞有关。这些推测均需进一步研究证实。
本研究通过多因素分析还发现,肿瘤最大径≥5 cm和多发肿瘤是影响肝细胞癌术后DFS和OS的独立危险因素,与既往研究结果[18-20]一致。MVI也是影响肝癌患者预后的独立危险因素,大量研究表明MVI是影响肝癌早期复发及远期生存的重要危险因素[21-24]。术中出血≥600 mL亦是影响肝癌患者预后的独立危险因素。张业繁等[25]研究805例行根治性肝切除术的巴塞罗那分期B期肝细胞癌患者发现,术中出血≥400 mL是影响DFS和OS的独立危险因素,这可能与术中出血增加肿瘤术中血行转移和腹腔转移风险有关;此外,出血可引起组织局部缺氧和缺氧再灌注损伤,影响免疫功能,进而促进肿瘤的复发和进展。
本研究是一项前瞻性队列研究,与既往研究相比纳入病例数更多,且研究对象均为经病理结果验证为肝细胞癌的患者,研究结果可信。本研究为单中心研究,可能存在混杂偏倚的影响,而且在收集是否存在MVI的病理信息时,有78例(2.05%)患者的MVI信息缺失,因此实验结论有待多中心随机对照试验进一步验证。
综上所述,术前血清AFP≥400 ng/mL和术前血清CA19-9≥32 U/mL是影响肝细胞癌患者OS和DFS的独立危险因素,术前CA19-9水平可以用于进一步评估不同AFP水平肝细胞癌患者预后。AFP和CA19-9双阳性患者的预后最差,并有侵袭性肝细胞癌干细胞的特性,可能需要针对性靶向治疗改善预后。
| [1] | EL-SERAG H B, RUDOLPH K L. Hepatocellular carcinoma:epidemiology and molecular carcinogenesis[J]. Gastroenterology, 2007, 132: 2557–2576. DOI: 10.1053/j.gastro.2007.04.061 |
| [2] | SIEGEL R L, MILLER K D, JEMAL A. Cancer Statistics, 2017[J]. CA Cancer J Clin, 2017, 67: 7–30. DOI: 10.3322/caac.21387 |
| [3] | FORNER A, LLOVET J M, BRUIX J. Hepatocellular carcinoma[J]. Lancet, 2012, 379: 1245–1255. DOI: 10.1016/S0140-6736(11)61347-0 |
| [4] | TATEISHI R, YOSHIDA H, MATSUYAMA Y, MINE N, KONDO Y, OMATA M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma:a systematic review[J]. Hepatol Int, 2008, 2: 17–30. DOI: 10.1007/s12072-007-9038-x |
| [5] | TSUKUMA H, HIYAMA T, TANAKA S, NAKAO M, YABUUCHI T, KITAMURA T, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease[J]. N Engl J Med, 1993, 328: 1797–1801. DOI: 10.1056/NEJM199306243282501 |
| [6] | MA W J, WANG H Y, TENG L S. Correlation analysis of preoperative serum alpha-fetoprotein level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy[J]. World J Surg Oncol, 2013, 11: 212–218. DOI: 10.1186/1477-7819-11-212 |
| [7] | KOELINK C J, VAN HASSELT P, VAN DER PLOEG A, VAN DEN HEUVEL-EIBRINK M M, WIJBURG F A, BIJLEVELD C M, et al. Tyrosinemia type Ⅰ treated by NTBC:how does AFP predict liver cancer?[J]. Mol Genet Metab, 2006, 89: 310–315. DOI: 10.1016/j.ymgme.2006.07.009 |
| [8] | IOANNOU G N, PERKINS J D, CARITHERS R L Jr. Liver transplantation for hepatocellular carcinoma:impact of the MELD allocation system and predictors of survival[J]. Gastroenterology, 2008, 134: 1342–1351. |
| [9] | CHEN Y L, CHEN C H, HU R H, HO M C, JENG Y M. Elevated preoperative serum CA19-9 levels in patients with hepatocellular carcinoma is associated with poor prognosis after resection[J/OL]. ScientificWorldJournal, 2013, 2013: 380797. doi: 10.1155/2013/380797. |
| [10] | LU L H, ZHANG Y F, WEI W, SHI M, GUO R P. Preoperative carbohydrate antigen 19-9:its neglected role in alpha-fetoprotein-negative hepatocellular carcinoma patients[J]. J Gastrointest Surg, 2017, 21: 2025–2032. DOI: 10.1007/s11605-017-3528-5 |
| [11] | ZHOU L, RUI J A, WANG S B, CHEN S G, QU Q. Carbohydrate antigen 19-9 increases the predictive efficiency of α-fetoprotein for prognosis of resected hepatocellular carcinoma[J]. Am Surg, 2018, 84: 80–85. |
| [12] | BRUIX J, SHERMAN M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma:an update[J]. Hepatology, 2011, 53: 1020–1022. DOI: 10.1002/hep.24199 |
| [13] | HUANG S, JIANG F, WANG Y, YU Y, REN S, WANG X, et al. Diagnostic performance of tumor markers AFP and PIVKA-Ⅱin Chinese hepatocellular carcinoma patients[J/OL]. Tumour Biol, 2017, 39: 1010428317705763. doi: 10.1177/1010428317705763. |
| [14] | ZHANG J, HUANG T, ZHANG F, XU J, CHEN G, WANG X, et al. Prognostic role of serum carbohydrate antigen 19-9 levels in patients with resectable hepatocellular carcinoma[J]. Tumour Biol, 2015, 36: 2257–2261. DOI: 10.1007/s13277-014-2435-6 |
| [15] | WAN P, ZHANG J, LONG X, LI Q, XU N, ZHANG M, et al. Serum levels of preoperative alpha-fetoprotein and CA19-9 predict survival of hepatic carcinoma patients after liver transplantation[J]. Eur J Gastroenterol Hepatol, 2014, 26: 553–561. DOI: 10.1097/MEG.0000000000000070 |
| [16] | LU X Y, XI T, LAU W Y, DONG H, ZHU Z, SHEN F, et al. Hepatocellular carcinoma expressing cholangiocyte phenotype is a novel subtype with highly aggressive behavior[J]. Ann Surg Oncol, 2011, 18: 2210–2217. DOI: 10.1245/s10434-011-1585-7 |
| [17] | HSU C C, GOYAL A, IUGA A, KRISHNAMOORTHY S, LEE V, VERNA E C, et al. Elevated CA19-9 is associated with increased mortality in a prospective cohort of hepatocellular carcinoma patients[J/OL]. Clin Transl Gastroenterol, 2015, 6: e74. doi: 10.1038/ctg.2014.22. |
| [18] | KUO Y H, WANG J H, HUNG C H, RAU K M, WU I P, CHEN C H, et al. Albumin-Bilirubin grade predicts prognosis of HCC patients with sorafenib use[J]. J Gastroenterol Hepatol, 2017, 32: 1975–1981. DOI: 10.1111/jgh.13783 |
| [19] | 周延岩, 许鑫森, 王志鑫, 苗润晨, 陈伟, 万永, 等. 肿瘤直径与肝细胞癌肝切除患者预后的关系[J]. 中华肝脏外科手术学电子杂志, 2015, 4: 227–231. DOI: 10.3877/cma.j.issn.2095-3232.2015.04.009 |
| [20] | 胡永浩. 143例中期肝细胞癌患者的预后分析[D]. 天津: 天津医科大学, 2017. |
| [21] | JIN L, LIU W R, TIAN M X, JIANG X F, WANG H, ZHOU P Y, et al. CCL24 contributes to HCC malignancy via RhoB-VEGFA-VEGFR2 angiogenesis pathway and indicates poor prognosis[J]. Oncotarget, 2017, 8: 5135–5148. |
| [22] | BERTUZZO V R, CESCON M, RAVAIOLI M, GRAZI G L, ERCOLANI G, DEL GAUDIO M, et al. Analysis of factors affecting recurrence of hepatocellular carcinoma after liver transplantation with a special focus on inflammation markers[J]. Transplantation, 2011, 91: 1279–1285. DOI: 10.1097/TP.0b013e3182187cf0 |
| [23] | HUANG Z Y, LIANG B Y, XIONG M, ZHAN D Q, WEI S, WANG G P, et al. Long-term outcomes of repeat hepatic resection in patients with recurrent hepatocellular carcinoma and analysis of recurrent types and their prognosis:a single-center experience in China[J]. Ann Surg Oncol, 2012, 19: 2515–2525. DOI: 10.1245/s10434-012-2269-7 |
| [24] | 白石磊, 杨平华, 李俊, 沈锋. 微血管侵犯对小肝细胞癌和大肝细胞癌术后远期预后的影响[J]. 中华肝脏外科手术学电子杂志, 2017, 6: 207–211. |
| [25] | 张业繁, 陈晓, 李智宇, 毕新宇, 赵建军, 周健国, 等. 术中出血400 ml以上是BCLC B期肝癌患者预后不良的独立危险因素[J]. 癌症进展, 2017, 15: 308–311. |
 2018, Vol. 39
2018, Vol. 39


