引用本文

易扬, 路建饶, 吴好, 马骏, 赵颖丹, 宣怡, 刘文瑞. 慢性肾脏病患者冠状动脉钙化影响因素分析[J]. 第二军医大学学报, 2018, 39(6): 621-626

YI Yang, LU Jian-rao, WU Hao, MA Jun, ZHAO Ying-dan, XUAN Yi, LIU Wen-rui. Analysis of influencing factors of coronary artery calcification in patients with chronic kidney disease[J]. Academic Journal of Second Military Medical University, 2018, 39(6): 621-626 (in Chinese with English abstract)

慢性肾脏病患者冠状动脉钙化影响因素分析
易扬
1
, 路建饶
2, 吴好
1, 马骏
1

, 赵颖丹
1, 宣怡
1, 刘文瑞
2
1. 复旦大学附属上海市静安区中心医院肾内科, 上海 200040;
2. 上海中医药大学附属第七人民医院肾病科, 上海 200137
收稿日期: 2017-12-28 接受日期: 2018-03-28
基金项目: 上海市卫生和计划生育委员会科研基金面上项目(201440543),上海市静安区卫生和计划生育委员会第三批“十百千人才”培养基金(JWRC2014G05).
摘要:
目的
探讨慢性肾脏病(CKD)患者发生冠状动脉钙化(CAC)的影响因素。方法
选择行冠状动脉多层螺旋计算机断层扫描检查的CKD患者181例,根据美国国家肾脏病基金会制定的肾脏病患者生存质量指导(K/DOQI)指南标准将患者为分为CKD 1期、CKD 2~3期、CKD 4~5期和维持性血液透析(MHD)4组。利用Agatston评分方法评估CAC评分,CAC评分≤10为无CAC、11~100为轻度CAC、101~400为中度CAC、>400为重度CAC。采用Spearman线性回归分析研究CKD患者发生CAC的相关因素,用多因素Cox回归分析研究CKD患者发生CAC的独立影响因素。结果
181例患者中CKD 1期44例、CKD 2~3期36例、CKD 4~5期25例、MHD 76例。CKD患者CAC发生率为55.2%(100/181),其中MHD患者CAC发生率为80.3%(61/76)。随着肾功能损害的加重,CKD患者的CAC评分增加(r=0.526,P < 0.001)。CKD患者的CAC程度与其年龄、血液透析龄、平均动脉压、血尿素氮、血清肌酐、C-反应蛋白、总胆固醇、血钙、血磷、钙磷乘积、血清成纤维细胞生长因子23(FGF23)水平呈正相关(r=0.135、0.525、0.139、0.141、0.824、0.135、0.140、0.138、0.391、0.453、0.326,P均 < 0.05),与估算的肾小球滤过率(eGFR)、胎球蛋白A、25-羟维生素D3呈负相关(r=-0.871、-0.135、-0.138,P均 < 0.05);与血红蛋白、血糖、三酰甘油、碱性磷酸酶、全段甲状旁腺激素均无明显相关性(P均>0.05)。多因素Cox回归分析显示,患者的年龄、eGFR、血磷、钙磷乘积和血清FGF23水平是CKD患者发生CAC的独立影响因素[比值比(95%置信区间)分别为3.723(2.521~8.363)、0.582(0.415~0.724)、5.252(3.912~10.327)、11.243(10.185~16.836)和2.469(1.141~5.362)。结论
年龄、eGFR、血磷、钙磷乘积和血清FGF23水平是CKD患者发生CAC的独立影响因素。
关键词:
慢性肾脏病
冠状动脉钙化
钙磷乘积
成纤维细胞生长因子23
Analysis of influencing factors of coronary artery calcification in patients with chronic kidney disease
YI Yang
1
, LU Jian-rao
2, WU Hao
1, MA Jun
1

, ZHAO Ying-dan
1, XUAN Yi
1, LIU Wen-rui
2
1. Department of Nephrology, Shanghai Jing'an District Central Hospital Affiliated to Fudan University, Shanghai 200040, China;
2. Department of Nephrology, the 7th People's Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 200137, China
Supported by General Program of Scientific Research Fund of Shanghai Health and Family Planning (201440543) and Third Batch of "Ten Thousand Talents" Training Fund of Shanghai Jing'an District Health and Family Planning Committee (JWRC2014G05).
Abstract:
Objective
To investigate the influencing factors of coronary artery calcification (CAC) in patients with chronic kidney disease (CKD).
Methods
A total of 181 CKD patients undergoing multi-slice spiral computed tomography for coronary artery were selected. The patients were divided into four groups of CKD 1 stage, CKD 2-3 stage, CKD 4-5 stage and maintenance hemodialysis (MHD) according to the kidney disease outcome quality initiative (K/DOQI) guidelines established by the National Kidney Foundation. According to the CAC scores assessed using the Agatston scoring method, the patients were divided into non-CAC group (CAC score ≤ 10), mild CAC group (CAC score 11-100), moderate CAC group (CAC score 101-400), and severe CAC group (CAC score>400). The related factors and independent influencing factors of CAC in CKD patients were analyzed by Spearman linear regression analysis and multivariate Cox regression analysis, respectively.
Results
Of the 181 patients, 44 were CKD 1, 36 were CKD 2-3, 25 were CKD 4-5, and 76 were MHD. The incidence of CAC in the CKD patients and MHD patients was 55.3% (100/181) and 80.3% (61/76), respectively. The CAC score of CKD patients was significantly increased with the aggravation of renal impairment (r=0.526, P < 0.001). The degree of CAC in the CKD patients was positively correlated to age, dialysis duration, average arterial pressure, blood urea nitrogen, serum creatinine, C-reactive protein, total cholesterol, serum calcium, serum phosphorus, calcium-phosphorus product, and serum fibroblast growth factor 23 (FGF23) level (r=0.135, 0.525, 0.139, 0.141, 0.824, 0.135, 0.140, 0.138, 0.391, 0.453, and 0.326; all P < 0.05), and negatively correlated to estimated glomerular filtration rate (eGFR), fetuin A, and 25-hydroxy vitamin D3 (r=-0.871, -0.135, and -0.138; all P < 0.05); the degree of CAC was not significantly correlated with hemoglobin, blood glucose, triglyceride, alkaline phosphatase, or intact parathyroid hormone (all P>0.05). Multivariate Cox regression analysis showed that age, eGFR, serum phosphorus, calcium-phosphorus product and serum FGF23 level were the independent influencing factors of CAC in CKD patients (OR[95% CI]:3.723[2.521-8.363], 0.582[0.415-0.724], 5.252[0.415-0.724], 11.243[10.185-16.836], and 2.469[1.141-5.362]).
Conclusion
Age, eGFR, serum phosphorus, calciumphosphorus product and serum FGF23 level are independent influencing factors of CAC in CKD patients.
Key words:
chronic kidney disease
coronary artery calcification
calcium-phosphorus product
fibroblast growth factor 23
我国慢性肾脏病(chronic kidney disease,CKD)的发生率约为10.8%[1],CKD和终末期肾脏病(end stage renal disease,ESRD)患者心血管疾病(cardiovascular disease,CVD)的患病率和死亡率为普通人群的10~30倍[2]。CVD占CKD和ESRD患者全因死亡的30%~50%[3]。究其原因,主要与CKD患者心血管钙化高发密切相关;血管钙化已成为CKD和ESRD患者CVD发病率和死亡率的独立危险因素[2-5]。本研究通过多层螺旋计算机断层扫描(multi-slice spiral computed tomography,MSCT)检测CKD患者冠状动脉钙化(coronary artery calcification,CAC)的发生情况,并探讨其影响因素。
1 资料和方法
1.1 病例来源
纳入2015年3月至2017年2月行冠状动脉MSCT血管成像检查的住院治疗的CKD患者181例,其中120例来源于复旦大学附属上海市静安区中心医院、61例来源于上海中医药大学附属第七人民医院。纳入标准:(1)年龄 > 18岁;(2)符合CKD诊断和分期标准[6];(3)ESRD维持性血液透析(maintenance hemodialysis,MHD)患者血液透析时间 > 3个月,病情稳定。排除标准:(1)妊娠或患精神疾病、自身免疫性疾病;(2)1个月内有感染、手术、外伤或使用激素、免疫抑制剂等;(3)严重内分泌紊乱;(4)1个月内发生急性心脑血管事件;(5)合并其他严重疾病,如恶性肿瘤、严重吸收不良等。本研究通过复旦大学附属上海市静安区中心医院和上海中医药大学附属第七人民医院伦理委员会审批。
1.2 临床分组
根据美国国家肾脏病基金会(National Kidney Foundation,NKF)制定的肾脏病患者生存质量指导(kidney disease outcome quality initiative,K/DOQI)指南诊断标准[6]将患者分为CKD 1期[估算的肾小球滤过率(estimated glomerular filtration rate,eGFR)≥90 mL/(min • 1.73 m2)]、CKD 2~3期[eGFR为30~89 mL/(min • 1.73 m2)]、CKD 4~5期[eGFR < 30 mL/(min • 1.73 m2)且未行血液透析]和MHD [eGFR < 10 mL/(min • 1.73 m2)且维持性血液透析 > 3个月] 4组。
1.3 研究方法
1.3.1 肾功能评估
根据简化肾脏病膳食改良试验(modification of diet in renal disease,MDRD)公式计算患者的eGFR,评估入组患者的肾功能情况。eGFR [mL/(min • 1.73 m2)]=186.3×血清肌酐(serum creatinine,SCr)-1.154×年龄-0.203×1.212(如果患者为黑人)×0.742(如果患者为女性)。
1.3.2 校正钙的计算
根据公式计算校正钙:校正钙(mmol/L)=血清总钙(mmol/L)+0.02×[47-白蛋白(g/L)]。
1.3.3 冠状动脉MSCT检查
采用德国Siemens Somatom Definition AS MSCT扫描仪,扫描时设为心电信号窗,显像管电流为160 mA,峰电压为120 kV,探测器宽度为128×0.6 mm。CAC评分(Agatston法[7])由1位固定的影像科主治医师按盲法并采用德国Siemens CaScoring软件进行评估,CAC分值为钙化斑块的面积乘以固定系数(由最大像素密度决定)。钙化斑块定义为CT值≥130 HU且面积≥1 mm2的病变组织;评估的血管包括左冠状动脉主干、左前降支、左回旋支、右冠状动脉、主动脉瓣环和基底部、升主动脉壁、左房室瓣环。独立评估每张断层图像,每例所有断层图像的分值相加得到的总分即为此患者的CAC评分,表示该患者以上评估血管的总钙化负荷。CAC评分≤10为无CAC,11~100为轻度CAC,101~400为中度CAC,> 400为重度CAC。
1.3.4 实验室检查
采用美国R & D公司酶联免疫吸附试验(enzyme linked immunosorbent assay,ELISA)试剂盒,利用双抗体夹心法测定血清全段甲状旁腺激素(intact parathyroid hormone,iPTH)。采用检验科常规设备及试剂盒检测患者血尿素氮(blood urea nitrogen,BUN)、SCr、血钙(calcium,Ca)、血磷(phosphorus,P)、碱性磷酸酶(alkaline phosphatase,ALP)、血红蛋白(hemoglobin,Hb)、血糖(glucose,Glu)、C-反应蛋白(C-reactive protein,CRP)、总胆固醇(total cholesterol,TC)、三酰甘油(triglyceride,TG)、25-羟维生素D3 [25-hydroxy vitamin D3,25(OH)VitD3]。采用日本Kainos公司检测试剂盒用酶联免疫法测定血清成纤维细胞生长因子23(fibroblast growth factor 23,FGF23)。采用捷克BioVendor公司血清胎球蛋白A(fetuin A,FA)ELISA试剂盒测定FA浓度。
1.3.5 临床资料收集
采用专用调查表调查登记患者年龄、性别、平均动脉压(mean arterial pressure,MAP)、原发病、是否患糖尿病、是否行血液透析和血液透析龄等。
1.4 统计学处理
采用美国IBM公司SPSS 19.0软件进行统计学分析。计量资料若呈正态分布以x±s表示,组间比较采用两样本均数比较的t检验;若呈偏态分布则以中位数(下四分位数,上四分位数)表示。计数资料以例数和百分数表示,组间比较采用χ2检验。采用Spearman线性回归分析研究CKD患者发生CAC的相关因素;以CAC为终点事件,采用多因素Cox回归分析研究其独立影响因素。检验水准(α)为0.05。
2 结果
2.1 CKD分期与各因素的相关性分析
181例患者中CKD 1期44例、CKD 2~3期36例、CKD 4~5期25例、MHD 76例,CKD患者CAC发生率为55.2%(100/181),其中MHD患者CAC发生率为80.3%(61/76)。Spearman线性回归分析结果(表 1)显示,CKD分期与MAP、CAC评分、BUN、SCr、CRP、P、钙磷乘积(Ca×P)、iPTH、FGF23均呈正相关(P均 < 0.05),与eGFR、Hb、Ca、FA和25(OH)VitD3均呈负相关(P均 < 0.05),与年龄、Glu、TG、TC和ALP均无明显相关性(P均 > 0.05)。
表 1
(Tab 1)
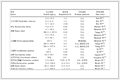
表 1 CKD分期与各相关因素的Spearman线性回归分析
Tab 1 Spearman linear regression analysis of CKD stage and related factors
| Characteristic |
CKD 1 stage
n=44 |
CKD 2-3 stage
n=36 |
CKD 4-5 stage
n=25 |
MHD
n=76 |
r value |
P value |
| Age (year), x±s |
56.2±6.9 |
55.5±7.2 |
56.7±6.4 |
55.8±6.3 |
0.046 |
0.257 |
| MAP p/mmHg, x±s |
86.7±9.4 |
90.0±8.3 |
108.4±10.2 |
112.3±13.0 |
0.145 |
0.026 |
| CAC score M (QL, QU) |
3 (2, 5) |
15 (13, 23) |
126 (51, 225) |
408 (260, 662) |
0.526 |
< 0.001 |
| BUN cB/(mmol • L-1), x±s |
4.1±1.3 |
14.6±3.3 |
21.8±6.6 |
27.3±11.2 |
0.643 |
< 0.001 |
| SCr cB/(μmol • L-1), x±s |
73.4±6.4 |
115.2±12.6 |
315.3±116.9 |
728.1±242.5 |
0.725 |
< 0.001 |
| eGFRa x±s |
90.3±6.1 |
42.6±5.2 |
18.3±6.2 |
5.3±2.2 |
-0.832 |
< 0.001 |
| Hb ρB/(g • L-1), x±s |
136.5±11.4 |
101.1±2.3 |
90.2±2.1 |
92.6±2.4 |
-0.146 |
0.024 |
| CRP ρB/(mg • L-1), x±s |
3.2±1.2 |
4.8±2.3 |
8.1±3.7 |
12.4±5.3 |
0.138 |
0.031 |
| Glu cB/(mmol • L-1), x±s |
5.2±0.5 |
5.6±1.2 |
5.7±1.1 |
5.6±0.9 |
0.059 |
0.162 |
| TG cB/(mmol • L-1), x±s |
1.6±0.3 |
1.7±0.5 |
1.7±0.3 |
1.8±0.4 |
0.052 |
0.183 |
| TC cB/(mmol • L-1), x±s |
4.5±1.1 |
5.0±0.9 |
4.9±0.8 |
5.2±1.0 |
0.031 |
0.625 |
| Ca cB/(mmol • L-1), x±s |
2.3±0.2 |
2.2±0.2 |
2.1±0.2 |
2.3±0.4 |
-0.129 |
0.042 |
| P cB/(mmol • L-1), x±s |
1.3±0.3 |
1.5±0.3 |
1.6±0.3 |
2.1±0.3 |
0.214 |
0.005 |
| Ca×P (mmol2 • L-2), x±s |
3.1±0.2 |
3.2±0.4 |
3.4±0.4 |
4.7±0.5 |
0.372 |
< 0.001 |
| ALP zB/(U • L-1), x±s |
52.6±10.1 |
88.6±21.6 |
104.5±51.2 |
111.9±46.1 |
0.086 |
0.082 |
| iPTH ρB/(pg • mL-1), M (QL, QU) |
30.7 (22.4, 42.8) |
52.0 (37.2, 62.3) |
147.0 (101.9, 201.5) |
225.5 (140.5, 303.7) |
0.438 |
< 0.001 |
| FGF23 ρB/(mg • L-1), M (QL, QU) |
36.7 (21.8, 48.9) |
95.9 (72.2, 115.4) |
168.2 (90.0, 207.6) |
351.5 (242.2, 484.7) |
0.393 |
< 0.001 |
| FA ρB/(mg • L-1), x±s |
126.2±42.8 |
98.4±36.5 |
80.2±30.1 |
71.2±20.3 |
-0.149 |
0.021 |
| 25(OH)VitD3 ρB/(pg • L-1), x±s |
42.6±18.2 |
36.1±15.8 |
15.4±10.1 |
9.5±6.4 |
-0.150 |
0.016 |
| a: mL/(min • 1.73 m2). 1 mmHg=0.133 kPa. CKD: Chronic kidney disease; MAP: Mean arterial pressure; CAC: Coronary artery calcification; BUN: Blood urea nitrogen; SCr: Serum creatine; eGFR: Estimated glomerular filtration rate; Hb: Hemoglobin; CRP: C-reactive protein; Glu: Glucose; TG: Triglyceride; TC: Total cholesterol; Ca: Calcium; P: Phosphorus; Ca×P: Calcium-phosphorus product; ALP: Alkaline phosphatase; iPTH: Intact parathyroid hormone; FGF23: Fibroblast growth factor 23; FA: Fetuin A; 25(OH)VitD3: 25-Hydroxy vitamin D3; M (QL, QU): Median (lower quartile, upper quartile) |
|
表 1 CKD分期与各相关因素的Spearman线性回归分析
Tab 1 Spearman linear regression analysis of CKD stage and related factors
|
2.2 无CAC组和CAC组患者临床资料和实验室检查结果的比较
无CAC组(CAC评分≤10)和CAC组(CAC评分 > 10)患者分别为81例和100例。无CAC组CKD患者的年龄、血液透析占比、MAP、BUN、SCr、CRP、TC、Ca、P、Ca×P、ALP、iPTH和FGF23均低于CAC组,而eGFR、Hb、FA和25(OH)VitD3均高于CAC组,差异均有统计学意义(P均 < 0.01),见表 2。
表 2
(Tab 2)

表 2 无CAC组和CAC组CKD患者临床资料和实验室检查结果的比较
Tab 2 Comparison of clinical data and laboratory examinations between CKD patients with or without CAC
| Characteristic |
Non-CAC group N=81 |
CAC group N=100 |
t/χ2 value |
P value |
| Age (year), x±s |
54.2±9.4 |
58.1±10.8 |
3.127 |
0.004 |
| Dialysis number n (%) |
15 (18.5) |
61 (61.0) |
10.277 |
0.009 |
| MAP p/mmHg, x±s |
88.3±8.2 |
114.6±12.8 |
10.251 |
< 0.001 |
| BUN cB/(mmol • L-1), x±s |
10.1±3.1 |
23.6±10.2 |
11.623 |
< 0.001 |
| SCr cB/(μmol • L-1), x±s |
103.8±2.3 |
426.6±217.9 |
12.852 |
< 0.001 |
| eGFRa x±s |
50.4±38.6 |
18.6±15.3 |
9.237 |
< 0.001 |
| Hb ρB/(g • L-1), x±s |
111.1±12.1 |
110.2±12.3 |
3.142 |
0.003 |
| CRP ρB/(mg • L-1), x±s |
3.7±1.1 |
13.3±7.8 |
12.856 |
< 0.001 |
| Glu cB/(mmol • L-1), x±s |
5.5±0.9 |
5.6±0.7 |
1.032 |
0.341 |
| TG cB/(mmol • L-1), x±s |
1.7±0.4 |
1.7±0.3 |
1.341 |
0.196 |
| TC cB/(mmol • L-1), x±s |
4.5±1.7 |
5.5±2.1 |
4.237 |
< 0.001 |
| Ca cB/(mmol • L-1), x±s |
2.0±0.2 |
2.3±0.3 |
6.521 |
< 0.001 |
| P cB/(mmol • L-1), x±s |
1.5±0.2 |
1.8±0.3 |
7.683 |
< 0.001 |
| Ca×P (mmol2 • L-2), x±s |
2.9±0.6 |
4.1±0.7 |
12.943 |
< 0.001 |
| ALP zB/(U • L-1), x±s |
52.6±20.1 |
70.5±33.3 |
5.591 |
< 0.001 |
| iPTH ρB/(pg • mL-1), x±s |
67.5±42.2 |
148.3±125.5 |
7.635 |
< 0.001 |
| FGF23 ρB/(mg • L-1), x±s |
85.3±33.7 |
201.6±149.3 |
8.294 |
< 0.001 |
| FA ρB/(mg • L-1), x±s |
92.9±38.1 |
80.5±32.4 |
3.282 |
0.001 |
| 25(OH)VitD3 ρB/(pg • L-1), x±s |
32.1±25.5 |
22.8±18.1 |
3.256 |
0.002 |
| a: mL/(min • 1.73 m2). 1 mmHg=0.133 kPa. CAC: Coronary artery calcification; CKD: Chronic kidney disease; MAP: Mean arterial pressure; BUN: Blood urea nitrogen; SCr: Serum creatine; eGFR: Estimated glomerular filtration rate; Hb: Hemoglobin; CRP: C-reactive protein; Glu: Glucose; TG: Triglyceride; TC: Total cholesterol; Ca: Calcium; P: Phosphorus; Ca×P: Calcium-phosphorus product; ALP: Alkaline phosphatase; iPTH: Intact parathyroid hormone; FGF23: Fibroblast growth factor 23; FA: Fetuin A; 25(OH)VitD3: 25-Hydroxy vitamin D3 |
|
表 2 无CAC组和CAC组CKD患者临床资料和实验室检查结果的比较
Tab 2 Comparison of clinical data and laboratory examinations between CKD patients with or without CAC
|
2.3 CAC程度与各因素的相关性分析
100例有CAC的CKD患者中,轻度CAC 51例、中度CAC 34例、重度CAC 15例。Spearman相关分析结果显示,CAC程度与患者年龄、血液透析龄、MAP、BUN、SCr、CRP、TC、Ca、P、Ca×P、FGF23均呈正相关(P均 < 0.05),与eGFR、FA、25(OH)VitD3均呈负相关(P均 < 0.05),与Hb、Glu、TG、ALP、iPTH均无明显相关性(P均 > 0.05),见表 3。
表 3
(Tab 3)

表 3 181例CKD患者CAC程度与各相关因素的Spearman线性回归分析
Tab 3 Spearman linear regression analysis of related factors of CAC degree in 181 CKD patients
| x±s |
| Characteristic |
Non-CAC
n=81 |
Mild CAC
n=51 |
Moderate CAC
n=34 |
Severe CAC
n=15 |
r value |
P value |
| Age (year) |
54.2±9.4 |
57.2±8.2 |
58.5±13.6 |
61.1±16.4 |
0.135 |
0.036 |
| Dialysis age t/month |
5.4±1.1 |
12.2±10.5 |
21.4±14.8 |
38.9±19.3 |
0.525 |
< 0.001 |
| MAP p/mmHg |
86.3±6.2 |
103.6±7.1 |
112.4±10.2 |
121.5±10.8 |
0.139 |
0.014 |
| CAC score |
3.2±2.7 |
51.4±23.6 |
287.5±101.8 |
873.4±262.5 |
0.893 |
< 0.001 |
| BUN cB/(mmol • L-1) |
10.1±3.1 |
12.5±4.9 |
16.2±4.1 |
24.9±5.6 |
0.141 |
0.012 |
| SCr cB/(μmol • L-1) |
103.8±15.4 |
356.3±121.6 |
515.3±252.7 |
852.8±216.4 |
0.824 |
< 0.001 |
| eGFRa |
50.4±23.6 |
21.6±13.2 |
16.2±5.8 |
8.1±3.5 |
-0.871 |
< 0.001 |
| Hb ρB/(g • L-1) |
111.1±12.1 |
108.3±11.7 |
101.4±12.1 |
103.2±12.4 |
0.021 |
0.625 |
| CRP ρB/(mg • L-1) |
3.7±1.1 |
6.2±4.7 |
11.9±6.1 |
18.6±10.2 |
0.135 |
0.036 |
| Glu cB/(mmol • L-1) |
5.5±0.9 |
5.6±0.7 |
5.6±0.6 |
5.7±0.7 |
0.039 |
0.132 |
| TG cB/(mmol • L-1) |
1.6±0.3 |
1.7±0.4 |
1.7±0.3 |
1.7±0.3 |
0.032 |
0.241 |
| TC cB/(mmol • L-1) |
4.5±1.7 |
5.2±1.8 |
5.8±2.1 |
6.6±2.3 |
0.140 |
0.013 |
| Ca cB/(mmol • L-1) |
2.0±0.15 |
2.3±2.5 |
2.4±0.2 |
2.6±0.3 |
0.138 |
0.032 |
| P cB/(mmol • L-1) |
1.4±0.2 |
1.6±0.2 |
1.8±0.2 |
2.2±0.2 |
0.391 |
< 0.001 |
| Ca×P (mmol2 • L-2) |
2.7±0.6 |
3.7±0.3 |
4.3±0.3 |
6.1±0.4 |
0.453 |
< 0.001 |
| ALP zB/(U • L-1) |
52.6±10.1 |
68.4±21.6 |
74.5±24.2 |
71.9±26.1 |
0.086 |
0.082 |
| iPTH ρB/(pg • mL-1) |
67.5±42.2 |
124.6±131.8 |
141.3±122.9 |
236.1±182.4 |
0.101 |
0.071 |
| FGF23 ρB/(mg • L-1) |
85.3±23.7 |
162.1±94.0 |
212.2±102.3 |
326.2±227.3 |
0.326 |
< 0.001 |
| FA ρB/(mg • L-1) |
92.9±38.1 |
83.1±39.1 |
82.6±31.8 |
76.8±30.7 |
-0.135 |
0.036 |
| 25(OH)VitD3 ρB/(pg • L-1) |
32.1±25.5 |
25.3±16.6 |
22.2±17.1 |
19.2±15.9 |
-0.138 |
0.035 |
| a: mL/(min • 1.73 m2). 1 mmHg=0.133 kPa. CKD: Chronic kidney disease; CAC: Coronary artery calcification; MAP: Mean arterial pressure; BUN: Blood urea nitrogen; SCr: Serum creatine; eGFR: Estimated glomerular filtration rate; Hb: Hemoglobin; CRP: C-reactive protein; Glu: Glucose; TG: Triglyceride; TC: Total cholesterol; Ca: Calcium; P: Phosphorus; Ca×P: Calcium-phosphorus product; ALP: Alkaline phosphatase; iPTH: Intact parathyroid hormone; FGF23: Fibroblast growth factor 23; FA: Fetuin A; 25(OH)VitD3: 25-Hydroxy vitamin D3 |
|
表 3 181例CKD患者CAC程度与各相关因素的Spearman线性回归分析
Tab 3 Spearman linear regression analysis of related factors of CAC degree in 181 CKD patients
|
2.4 CKD患者发生CAC的影响因素
以CAC为终点事件,纳入以上对CAC影响差异有统计学意义的因素,采用多因素Cox回归分析寻找与CAC相关的危险因素,结果显示患者年龄、eGFR、P、Ca×P和FGF23是CKD患者发生CAC的独立影响因素(P均 < 0.01),见表 4。
表 4
(Tab 4)

表 4 CKD患者发生CAC影响因素的多因素Cox回归分析
Tab 4 Multivariate Cox regression analysis of influencing factors of CAC in CKD patients
| Variable |
B |
Wald χ2 |
OR (95% CI) |
P value |
| Age |
1.812 |
9.261 |
3.723 (2.521, 8.363) |
0.001 |
| eGFR |
-0.263 |
15.726 |
0.582 (0.415, 0.724) |
< 0.001 |
| P |
2.152 |
12.382 |
5.252 (3.912, 10.327) |
< 0.001 |
| Ca×P |
2.416 |
22.314 |
11.243 (10.185, 16.836) |
< 0.001 |
| FGF23 |
1.541 |
7.746 |
2.469 (1.141, 5.362) |
< 0.001 |
| CKD: Chronic kidney disease; CAC: Coronary artery calcification; eGFR: Estimated glomerular filtration rate; P: Phosphorus; Ca×P: Calcium-phosphorus product; FGF23: Fibroblast growth factor 23; B: Regression coefficient; OR: Odds ratio; CI: Confidence interval |
|
表 4 CKD患者发生CAC影响因素的多因素Cox回归分析
Tab 4 Multivariate Cox regression analysis of influencing factors of CAC in CKD patients
|
3 讨论
临床上血管钙化的诊断方法很多,其中以CT为基础的诊断技术被认为是检测心血管钙化的“金标准”。本研究回顾性分析了行冠状动脉MSCT检查的181例CKD患者的临床资料,发现CKD患者CAC发生率为55.2%(100/181),其中MHD患者CAC的发生率为80.3%(61/76),与文献报道[4-5]相似。随着肾功能损害的加重,CKD患者eGFR、Hb、Ca、FA、25(OH)VitD3降低,MAP、P、FGF23、ALP、iPTH等指标升高,符合CKD的临床表现和发展规律。根据CAC评分将患者分为CAC组和无CAC组进行比较,发现CAC组患者的年龄、血液透析患者占比、MAP、BUN、SCr、CRP、TC、Ca、P、Ca×P、ALP、iPTH、FGF23均高于无CAC组,而eGFR、Hb、FA和25(OH)VitD3均低于无CAC组;同时进行相关性分析发现,CKD患者的CAC评分与患者的年龄、血液透析龄、MAP、BUN、SCr、CRP、TC、Ca、P、Ca×P、FGF23呈正相关,与eGFR、FA和25(OH)VitD3呈负相关,进一步行多因素分析发现年龄、eGFR、P、Ca×P和FGF23是CKD患者发生CAC的独立影响因素,与文献报道[4-5]一致。
研究表明血磷水平每升高10 mg/L,CVD死亡风险上升50%,全因死亡风险上升26%[8]。高磷血症广泛存在于CKD患者,是其血管钙化的主要诱因和促进血管钙化的独立危险因素;血磷水平升高可抑制1,25-二羟维生素D3的合成,其与血管钙化和CVD有关[9]。同时,还有研究表明高磷血症诱导血管平滑肌细胞(vascular smooth muscle cell,VSMC)表达成骨细胞表型,且呈时间和剂量依赖性[10]。高磷还可通过诱导促进钙化细胞因子的表达从而促进血管钙化的发生和发展。
FGF23是一种与磷酸盐稳态密切相关的矿物质代谢调节因子。其对人体血磷的调节主要与以下环节有关:(1)通过刺激残余的肾单位排泄磷;(2)通过抑制1,25-二羟维生素D3合成减少饮食中磷的吸收[11]。研究表明CKD患者FGF23水平升高,且与肾功能异常、慢性肾脏病-矿物质和骨异常密切相关[12]。然而,FGF23对血管钙化的作用存有争议,争论的焦点在于FGF23是直接还是间接作用于VSMC,是促进还是抑制细胞基质钙化。同时,也有学者报道FGF23对培养的VSMC向成骨细胞表型分化有保护作用[13]。因此,仍需要更多的研究明确FGF23对血管钙化的影响是正面的还是负面的。
FA是一种最强大的循环羟基磷灰石形成抑制剂[14]。目前FA与血管钙化的关系争议较大,其抑制异位钙化的主要机制为:(1)FA作为拥有众多钙离子结合位点的高亲和力钙离子结合蛋白,其功能之一是与钙和磷形成可溶性的胶体微球,并增加FA溶解度,从而抑制血清过饱和的钙磷酸盐沉积;(2)FA可以通过与基质Gla蛋白、Ca、P结合抑制磷灰石前体的形成和沉淀,从而抑制组织钙化的发生和发展;(3)在局部组织抑制骨形成蛋白和转化生长因子β,从而抑制钙化形成[15]。
综上所述,CKD患者尤其是MHD患者CAC发生率高,年龄、eGFR、P、Ca×P和FGF23水平是CKD患者发生CAC的独立影响因素。但仍需多中心大样本长期随访的临床研究进一步证实CAC对患者心血管事件发生率和死亡率的影响。
参考文献
| [1] |
ZHANG L, WANG F, WANG L, WANG W, LIU B, LIU J, et al.
Prevalence of chronic kidney disease in China:a cross-sectional survey[J]. Lancet, 2012, 379: 815–822.
DOI: 10.1016/S0140-6736(12)60033-6 |
| [2] |
COLLINS A J, FOLEY R N, GILBERTSON D T, CHEN S C.
United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease[J]. Kidney Int Suppl (2011), 2015, 5: 2–7.
DOI: 10.1038/kisup.2015.2 |
| [3] |
MASAKANE I, NAKAI S, OGATA S, KIMATA N, HANAFUSA N, HAMANO T, et al.
An overview of regular dialysis treatment in Japan (as of 31 December 2013)[J]. Ther Apher Dial, 2015, 19: 540–574.
DOI: 10.1111/1744-9987.12378 |
| [4] |
GÓRRIZ J L, MOLINA P, CERVERÓN M J, VILA R, BOVER J, NIETO J, et al.
Vascular calcification in patients with nondialysis CKD over 3 years[J]. Clin J Am Soc Nephrol, 2015, 10: 654–666.
DOI: 10.2215/CJN.07450714 |
| [5] |
VERVLOET M, COZZOLINO M.
Vascular calcification in chronic kidney disease:different bricks in the wall?[J]. Kidney Int, 2017, 91: 808–817.
DOI: 10.1016/j.kint.2016.09.024 |
| [6] |
National Kidney Foundation.
K/DOQI clinical practice guidelines for chronic kidney disease:evaluation, classification, and stratification[J]. Am J Kidney Dis, 2002, 39(2 Suppl): S1–S266.
|
| [7] |
AGATSTON A S, JANOWITZ W R, HILDNER F J, ZUSMER N R, VIAMONTE M Jr, DETRANO R.
Quantification of coronary artery calcium using ultrafast computed tomography[J]. J Am Coll Cardiol, 1990, 15: 827–832.
DOI: 10.1016/0735-1097(90)90282-T |
| [8] |
EDDINGTON H, HOEFIELD R, SINHA S, CHRYSOCHOU C, LANE B, FOLEY R N, et al.
Serum phosphate and mortality in patients with chronic kidney disease[J]. Clin J Am Soc Nephrol, 2010, 5: 2251–2257.
DOI: 10.2215/CJN.00810110 |
| [9] |
TANIGUCHI M, FUKAGAWA M, FUJⅡ N, HAMANO T, SHOJI T, YOKOYAMA K, et al.
Serum phosphate and calcium should be primarily and consistently controlled in prevalent hemodialysis patients[J]. Ther Apher Dial, 2013, 17: 221–228.
DOI: 10.1111/tap.2013.17.issue-2 |
| [10] |
CHEN N X, O'NEILL K D, DUAN D, MOE S M.
Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells[J]. Kidney Int, 2002, 62: 1724–1731.
DOI: 10.1046/j.1523-1755.2002.00625.x |
| [11] |
SAITO H, MAEDA A, OHTOMO S, HIRATA M, KUSANO K, KATO S, et al.
Circulating FGF-23 is regulated by 1α, 25-dihydroxyvitamin D3 and phosphorus in vivo[J]. J Biol Chem, 2005, 280: 2543–2549.
DOI: 10.1074/jbc.M408903200 |
| [12] |
SCIALLA J J, WOLF M.
Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease[J]. Nat Rev Nephrol, 2014, 10: 268–278.
DOI: 10.1038/nrneph.2014.49 |
| [13] |
ZHU D, MACKENZIE N C, MILLAN I L L A N J L, FARQUHARSON C, MACRAE V E.
A protective role for FGF-23 in local defence against disrupted arterial wall integrity?[J]. Mol Cell Endocrinol, 2013, 372(1/2): 1–11.
|
| [14] |
REYNOLDS J L, SKEPPER J N, MCNAIR R, KASAMA T, GUPTA K, WEISSBERG P L, et al.
Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification[J]. J Am Soc Nephrol, 2005, 16: 2920–2930.
DOI: 10.1681/ASN.2004100895 |
| [15] |
MOE S M, RESLEROVA M, KETTELER M, O'NEILL K, DUAN D, KOCZMAN J, et al.
Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD)[J]. Kidney Int, 2005, 67: 2295–2304.
DOI: 10.1111/j.1523-1755.2005.00333.x |
 2018, Vol. 39
2018, Vol. 39


