2. 解放军 123 医院重症医学科, 蚌埠 233015
2. Intensive Care Unit, No. 123 Hospital of PLA, Bengbu 233015, Anhui, China
脓毒症是指由严重感染引起的宿主免疫功能紊乱,即全身炎症反应综合征(systemic inflammatory response syndrome,SIRS),其可导致危及生命的多器官功能障碍。在脓毒症导致功能障碍的多器官中,肺脏易受累,约50%~55%的重度脓毒症患者可并发急性呼吸窘迫综合征(acute respiratory distress syndrome,ARDS)[1-2]。ARDS一旦发生,将会出现严重缺氧,同时加重其他器官功能障碍,这是脓毒症患者的主要死因[3]。肺泡上皮细胞损伤是脓毒症ARDS的发病机制之一[4]。右美托咪定(dexmedetomidine,DEX)是一种α2-肾上腺素受体激动剂,具有镇静、抗焦虑的生物学特点[5]。有研究表明DEX具有调控免疫功能的作用[6],而这种作用如何影响免疫功能紊乱的脓毒症小鼠尚不清楚。基于此,本研究设计离体和在体实验以探究DEX对脓毒症小鼠全身炎性反应和肺泡上皮细胞的影响。
1 材料和方法 1.1 细胞培养与主要试剂小鼠肺泡上皮细胞MLE12由海军军医大学(第二军医大学)基础医学院医学免疫学教研室从小鼠肺组织中分选而来,于海军军医大学(第二军医大学)长海医院中心实验室保存并培养。肺泡上皮细胞MLE12用含10%胎牛血清(FBS)、1%青/链霉素的RPMI 1640培养液于37 ℃、5% CO2培养箱中培养,待细胞融合度达到80%时进行传代。RPMI 1640培养液和10% FBS均购自美国Gibco公司;脂多糖(lipopolysaccharide,LPS)、DEX购自美国Sigma公司;ELISA试剂盒、Pierce蛋白定量专用试剂盒、ECL显影液购自美国eBioscience公司;兔抗细胞外信号调节激酶(extracellular signal-regulated kinase,ERK)1/2、磷酸化细胞外信号调节激酶(phosphorylated extracellular signal-regulated kinase,p-ERK)、c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)、磷酸化c-Jun氨基末端激酶(phosphorylated c-Jun N-terminal kinase,p-JNK)抗体均购自美国Cell Signaling Technology公司。
1.2 盲肠结扎穿孔(cecal ligation and puncture,CLP)模型的构建雄性C57BL/6小鼠,20~25 g,共96只,购自海军军医大学(第二军医大学)动物实验中心[实验动物生产许可证号:SCXK(沪)2017-0002],术前适应性饲养1周。探究DEX对脓毒症小鼠存活率的影响时,将小鼠随机分为CLP组、CLP+DEX组和假手术组(Sham组),每组8只。探究DEX对脓毒症小鼠全身炎症反应的作用时,将小鼠随机分为CLP组、CLP+DEX组,每组36只。CLP组和CLP+DEX组小鼠吸入异氟烷维持麻醉,同时吸氧,消毒,沿腹中线切开腹膜,暴露盲肠,用丝线在回盲肠联合部结扎盲肠,18号针头对盲肠穿孔,然后将盲肠放回腹腔,依次缝合。Sham组小鼠异氟烷吸入麻醉后,沿腹中线切开腹膜,暴露盲肠,取出后重新放回腹腔,缝合。缝合后小鼠均保温和正常饲养。CLP+DEX组小鼠麻醉前15 min腹腔注射DEX(50 μg/kg),Sham组和CLP组小鼠在麻醉前15 min腹腔注射1 mL无菌生理盐水。
1.3 在体实验与样本收集记录CLP术后24 h内各组小鼠的存活率。在CLP术后0、6、12、24 h时行小鼠眼球取血,4 ℃ 398.25×g离心2 min,收集血清样本于-80 ℃冰箱内保存;同时提取肺泡灌洗液置于-80 ℃冰箱保存。
1.4 体外实验与样本收集取MLE12细胞进行传代和培养,待细胞融合度达到80%时以2×106/孔的密度接种于6孔板,将细胞分为两组:LPS组(1 μg/mL LPS处理)、LPS+DEX组(1 μg/mL LPS+0.2 μg/mL DEX处理),每组设双复孔。处理0、6、12、24 h时提取各组细胞上清液,加入含蛋白酶抑制剂的RIPA蛋白裂解液裂解细胞提取总蛋白,用Pierce蛋白定量专用试剂盒、BCA定量法进行蛋白定量,后放于沸水中煮5 min使蛋白变性。将提取的细胞上清液和变性蛋白置于-80 ℃冰箱保存,蛋白质印迹法检测MLE12细胞中ERK1/2、p-ERK1/2、JNK、p-JNK的表达。
1.5 ELISA法检测小鼠血清、肺泡灌洗液及细胞上清液中炎性因子的表达采用高黏合96孔板,分别用白细胞介素(interleukin,IL)-6、IL-1β、肿瘤坏死因子α(tumor necrosis factor α,TNF-α)ELISA试剂盒包被相应的ELISA板,包被完成后分别作ELISA标准曲线。取小鼠血清、肺泡灌洗液和MLE12细胞上清液于室温放置30 min,分别上样于包被好抗体的96孔板,密封样板,4 ℃孵育过夜,按照ELISA试剂盒说明书操作行ELISA检测。
1.6 蛋白质印迹法检测细胞中ERK1/2、p-ERK1/2、JNK、p-JNK的表达采用聚丙烯酰胺凝胶电泳法分离目的蛋白,电泳转移2 h后,用湿转法将蛋白转移至硝酸纤维素膜上,以5%脱脂牛奶封闭,用兔抗ERK1/2、p-ERK1/2、JNK、p-JNK抗体(1:2 000)在摇床上孵育2 h,4 ℃孵育过夜,后用相应的羊抗兔二抗(1:2 000)孵育1.5 h,将膜放入显影箱,用ECL显影液显色曝光。
1.7 统计学处理所有实验均重复3次,应用GraphPad Prism 6.0软件行数据处理与分析。数据以x±s或百分数表示,组间比较采用单因素方差分析(ANOVA)。检验水准(α)为0.05。
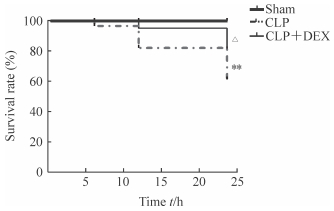
2 结果 2.1 DEX提高脓毒症小鼠的存活率结果(图 1)显示,Sham组、CLP组和CLP+DEX组小鼠6 h时的存活率分别为100.0%、97.5%和100.0%,12 h时分别为100.0%、83.5%和95.0%,24 h时分别为100.0%、58.5%和80.0%。与Sham组比较,CLP组小鼠CLP术后24 h内的存活率下降,差异有统计学意义(P<0.01);与CLP组比较,CLP+DEX小鼠CLP术后24 h内的组存活率升高,差异有统计学意义(P<0.05)。
|
图 1 DEX提高脓毒症小鼠的存活 Fig 1 DEX improves survival rate of sepsis mice Sham group: Mice were treated with sterile normal saline (1 mL) by intraperitoneal injection without CLP; CLP group: Mice were treated with sterile normal saline (1 mL) by intraperitoneal injection before CLP; CLP+DEX group: Mice were treated with DEX (50 μg/kg) by intraperitoneal injection before CLP. DEX: Dexmedetomidine; CLP: Cecal ligation and puncture.**P < 0.01 vs Sham group; △P < 0.05 vs CLP group |
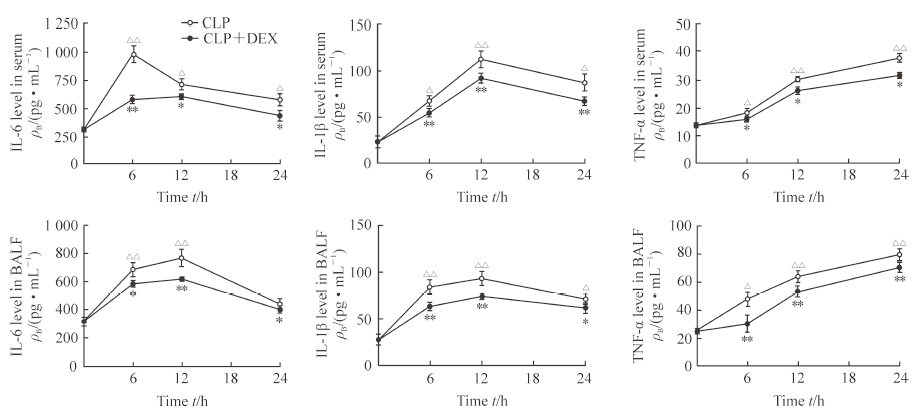
2.2 小鼠血清和肺泡灌洗液中IL-6、IL-1β、TNF-α的水平
ELISA检测结果(图 2)显示,CLP术后6、12、24 h时CLP组小鼠血清和肺泡灌洗液中IL-6、IL-1β、TNF-α的水平相比0 h时均升高,差异有统计学意义(P<0.05,P<0.01);与CLP组相比,CLP术后6、12、24 h时CLP+DEX组小鼠血清和肺泡灌洗液中IL-6、IL-1β、TNF-α的水平均降低,差异有统计学意义(P<0.05,P<0.01)。
|
图 2 各组小鼠血清和BALF中IL-6、IL-1β、TNF-α的表达 Fig 2 Levels of IL-6, IL-1β and TNF-α in serum and BALF of mice in each group ELISA results. CLP group: Mice were treated with sterile normal saline (1 mL) by intraperitoneal injection before CLP; CLP+DEX group: Mice were treated with DEX (50 μg/kg) by intraperitoneal injection before CLP. IL: Interleukin; TNF-α: Tumor necrosis factor α; BALF: Bronchoalveolar lavage fluid; CLP: Cecal ligation and puncture; DEX: Dexmedetomidine. *P < 0.05, **P < 0.01 vs CLP group at same time point; △P < 0.05, △△P < 0.01 vs CLP group at 0 h. n=3, x±s |
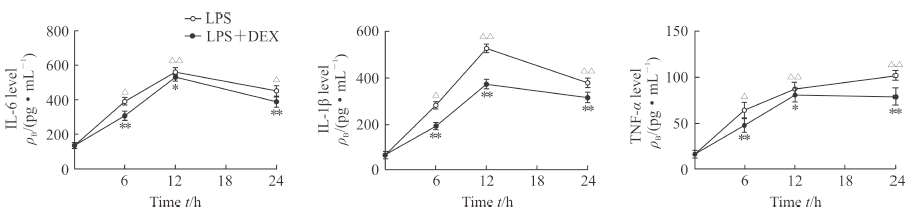
2.3 MLE12细胞上清液中IL-6、IL-1β、TNF-α 的水平
ELISA检测结果(图 3)显示,处理MLE12细胞6、12、24 h时LPS组细胞上清液中IL-6、IL-1β和TNF-α的水平相比0 h时均升高,差异有统计学意义(P<0.05,P<0.01),IL-6、IL-1β在12 h时表达水平最高,TNF-α在24 h时表达水平最高。与LPS组相比,处理MLE12细胞6、12、24 h时LPS+DEX组细胞上清液中IL-6、IL-1β、TNF-α的水平均降低,差异有统计学意义(P<0.05,P<0.01)。
|
图 3 各组MLE12细胞上清液中IL-6、IL-1β、TNF-α的表达 Fig 3 Levels of IL-6, IL-1β and TNF-α in MLE12 cell supernatants of each group ELISA results. LPS group: LPS (1 μg/mL); LPS+DEX group: LPS (1 μg/mL)+DEX (0.2 μg/mL). IL: Interleukin; TNF-α: Tumor necrosis factor α; LPS: Lipopolysaccharide; DEX: Dexmedetomidine. *P < 0.05, **P < 0.01 vs LPS group at same time point. △P < 0.05, △△P < 0.01 vs LPS group at 0 h. n=3, x±s |
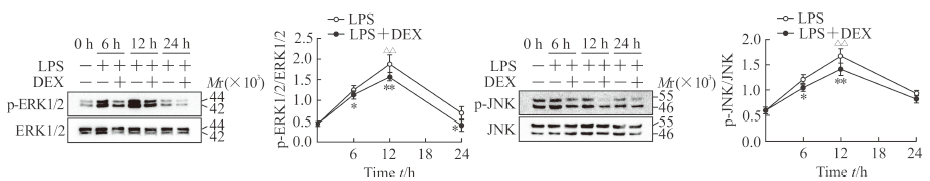
2.4 DEX抑制ERK1/2、JNK信号通路的激活
蛋白质印迹结果(图 4)显示,处理MLE12细胞12 h时LPS组细胞p-ERK1/2、p-JNK的表达相比0 h时均升高,差异有统计学意义(P<0.01)。与LPS组相比,LPS+DEX组MLE12细胞中p-ERK1/2的表达在6、12、24 h时下降,差异有统计学意义(P<0.05,P<0.01);p-JNK的表达在6、12 h时下降,差异有统计学意义(P<0.05,P<0.01)。
|
图 4 各组MLE12细胞中p-ERK1/2、p-JNK的表达 Fig 4 Expressions of p-ERK1/2 and p-JNK in MLE12 cells in each group Western blotting results. LPS group: LPS (1 μg/mL); LPS+DEX group: LPS (1 μg/mL)+DEX (0.2 μg/mL). ERK: Extracellular signalregulated kinase; p-ERK: Phosphorylated extracellular signal-regulated kinase; JNK: c-Jun N-terminal kinase; p-JNK: Phosphorylated c-Jun N-terminal kinase; LPS: Lipopolysaccharide; DEX: Dexmedetomidine. *P < 0.05, **P < 0.01 vs LPS group at same time point; △△P < 0.01 vs LPS group at 0 h. n=3, x±s |
3 讨论
脓毒症是重症监护病房(intensive care unit,ICU)患者死亡的主要原因,其病理过程涉及免疫紊乱,最终导致多器官功能衰竭[7]。肺脏是脓毒症所致多器官功能障碍中首先受累的脏器[8],易引发ARDS。肺泡上皮细胞在ARDS中对维护肺血屏障的完整性和修复受损屏障有重要作用,是肺内一种具有自我更新能力的干细胞[9]。免疫修复有利于损伤肺上皮细胞的功能恢复,有效的肺泡上皮修复有益于脓毒症ARDS患者的肺功能,但是脓毒症时肺泡上皮细胞的损伤机制目前尚不清楚。因此探寻肺泡上皮细胞参与脓毒症免疫调控的分子机制显得至关重要。
DEX是目前常用的围手术期和ICU患者的镇静药。研究表明,DEX不仅能通过调节机体内源性儿茶酚胺释放产生镇静、抗焦虑、镇痛、拮抗阿片类药物等作用,还可以调节免疫、减轻炎性反应等[10-13]。DEX的临床应用及研究主要集中于手术麻醉及术后镇静、镇痛,其药理作用主要表现在对神经系统、免疫系统、机体炎症、器官功能保护等方面,可通过降低小神经胶质细胞刺激引起的前列腺素E2(prostaglandin E2,PGE2)、一氧化氮(nitric oxide,NO)、诱导型一氧化氮合酶(inducible nitric oxide synthase,iNOS)、IL-1β、TNF-α等促炎因子的表达起到神经保护作用[14-15]。机体在感染状态时,血浆中IL-6和TNF-α的表达水平可作为预测病死率的重要指标之一[16],临床数据显示DEX比丙泊酚、咪达唑仑等镇静药物能更显著地降低ICU患者谵妄及其他并发症的发生率[17-18],并且能通过兴奋迷走神经和抑制高迁移率簇蛋白B 1(high mobility group protein B1,HMGB1)/核因子κB(nuclear factor κB,NF-κB)/Toll样受体(Toll-like receptor,TLR)信号通路,抑制机体炎性反应[19-20]。此外,DEX能抑制疼痛状态下炎症引起的自然杀伤细胞活性,对固有免疫功能具有保护效应,从而降低创伤和脓毒症患者和动物的病死率[21-22]。尽管近年来关于DEX抗炎作用的研究越来越多,但有关DEX对脓毒症小鼠肺泡上皮细胞发挥免疫调控的作用未见报道,而肺泡上皮细胞在肺部天然免疫效应中具有重要作用。
本研究通过在体实验探讨DEX对脓毒症小鼠炎性反应的影响。在CLP所致脓毒症小鼠中,DEX可抑制IL-6、IL-1β、TNF-α等炎性因子的产生,提高CLP所致脓毒症小鼠的存活率,表明DEX对脓毒症小鼠具有抗炎作用。离体实验初步探讨了DEX对LPS刺激肺泡上皮细胞MLE12后的炎性调控作用,发现LPS刺激6、12、24 h后MLE12细胞中IL-6、IL-1β、TNF-α的表达水平升高,而给予DEX处理后IL-6、IL-1β、TNF-α的表达水平均下降,表明DEX对脓毒症小鼠肺泡上皮细胞炎性反应具有一定的抑制效应,可起到保护肺泡上皮细胞的作用。LPS能激活多种细胞内信号分子,包括丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)家族,如ERK1/2、JNK和p38蛋白激酶[23]。本研究的蛋白质印迹结果显示DEX可抑制炎性信号通路中ERK1/2、JNK的激活,提示DEX对脓毒症小鼠的抗炎作用可能与ERK1/2、JNK信号通路有关,为今后机制研究提供了一定的方向。然而,还需要进一步研究DEX作为辅助治疗ARDS的潜在临床运用。
脓毒症引起的免疫失衡导致IL-6、IL-1β、TNF-α等炎性因子过度释放[24-25],最终导致ARDS乃至多器官功能衰竭。寻求调控脓毒症引起的促炎与抗炎失衡是治疗脓毒症的关键。目前针对脓毒症ARDS的治疗,临床多采用保护性机械通气、限制性液体疗法、体外膜肺氧合、抗生素和血管活性药物等进行有效的支持治疗和对症治疗[26]。尽管理论模型和体外实验均证实了这些方法的临床疗效,但仍然缺乏有效的针对宿主免疫失衡的分子治疗机制[27]。本研究为探寻脓毒症的治疗干预靶点继而降低病死率提供了一定实验依据。
| [1] | MATTHAY M A, ZEMANS R L. The acute respiratory distress syndrome:pathogenesis and treatment[J]. Annu Rev Pathol, 2011, 28: 147–163. |
| [2] | SADOWITZ B, ROY S, GATTO L A, HABASHI N, NIEMAN G. Lung injury induced by sepsis:lessons learned from large animal models and future directions for treatment[J]. Expert Rev Anti Infect Ther, 2011, 9: 1169–1178. DOI: 10.1586/eri.11.141 |
| [3] | STAPLETON R D, WANG B M, HUDSON L D, RUBENFELD G D, CALDWELL E S, STEINBERG K P. Causes and timing of death in patients with ARDS[J]. Chest, 2005, 128: 525–532. DOI: 10.1378/chest.128.2.525 |
| [4] | WHITSETT J A, ALENGHAT T. Respiratory epithelial cells orchestrate pulmonary innate immunity[J]. Nat Immunol, 2015, 16: 27–35. DOI: 10.1038/ni.3045 |
| [5] | KEATING G M. Dexmedetomidine:a review of its use for sedation in the intensive care setting[J]. Drugs, 2015, 75: 1119–1130. DOI: 10.1007/s40265-015-0419-5 |
| [6] | McMURPHY R M, FELS R J, KENNEY M J. Dexmedetomidine and regulation of splenic sympathetic nerve discharge in aged F344 rats[J]. Auton Neurosci, 2015, 190: 53–57. DOI: 10.1016/j.autneu.2015.03.001 |
| [7] | GU J, SUN P, ZHAO H, WATTS H R, SANDERS R D, TERRANDO N, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice[J]. Crit Care, 2011, 15: R153. DOI: 10.1186/cc10283 |
| [8] | ZHOU P, MA B, XU S, ZHANG S, TANG H, ZHU S, et al. Knockdown of Burton's tyrosine kinase confers potent protection against sepsis-induced acute lung injury[J]. Cell Biochem Biophys, 2014, 70: 1265–1275. DOI: 10.1007/s12013-014-0050-1 |
| [9] | BARKAUSKAS C E, CRONCE M J, RACKLEY C R, BOWIE E J, KEENE D R, STRIPP B R, et al. Type 2 alveolar cells are stem cells in adult lung[J]. J Clin Invest, 2013, 123: 3025–3036. DOI: 10.1172/JCI68782 |
| [10] | GELOEN A, CHAPELIER K, CIVIDJIAN A, DANTONY E, RABILLOUD M, MAY C N, et al. Clonidine and dexmedetomidine increase the pressor response to norepinephrine in experimental sepsis:a pilot study[J]. Crit Care Med, 2013, 41: 431–438. DOI: 10.1097/CCM.0b013e3182986248 |
| [11] | WU Y, LIU Y, HUANG H, ZHU Y, ZHANG Y, LU F, et a1. Dexmedetomidine inhibits inflammatory reaction in lung tissues of septic rats by suppressing TLR4/NF-κB pathway[J/OL]. Mediators Inflamm, 2013, 2013: 562154. doi: 10.1155/2013/562154. |
| [12] | XIANBAO L, HONK Z, XU Z, CHUNFANG Z, DUNJIN C. Dexmedetomidine reduced cytokine release during postpartum bleeding-induced multiple organ dysfunction syndrome in rats[J/OL]. Mediators Inflamm, 2013, 2013: 627831. doi: 10.1155/2013/627831. |
| [13] | JIANG L, LI L, SHEN J, QI Z, GUO L. Effect of dexmedetomidine on lung ischemia-reperfusion injury[J]. Mol Med Rep, 2014, 9: 419–426. DOI: 10.3892/mmr.2013.1867 |
| [14] | HAYAMA H R, DRUMHELLER K M, MASTROMONACO M, REIST C, CAHILL L F, ALKIRE M T. Event-related functional magnetic resonance imaging of a low dose of dexmedetomidine that impairs long-term memory[J]. Anesthesiology, 2012, 117: 981–995. DOI: 10.1097/ALN.0b013e31826be467 |
| [15] | PENG M, WANG Y L, WANG C Y, CHEN C. Dexmedetomidine attenuates lipopolysaccharide-induced proinflammatory response in primary microglia[J]. J Surg Res, 2013, 179: 219–225. |
| [16] | TUGLU D, YUVANC E, YILMAZ E, GENCAY I Y, ATASOY P, KISA U, et al. The antioxidant effect of dexmedetomidine on testicular ischemia-reperfusion injury[J]. Acta Cir Bras, 2015, 30: 414–421. DOI: 10.1590/S0102-865020150060000007 |
| [17] | FAN T W, TI L K, ISLAM I. Comparison of dexmedetomidine and midazolam for conscious sedation in dental surgery monitored by bispectral index[J]. Br J Oral Maxillofac Surg, 2013, 51: 428–433. DOI: 10.1016/j.bjoms.2012.08.013 |
| [18] |
王婧, 奚望, 殷亮, 李伟, 申华, 王志农. 右美托咪定与丙泊酚对心脏瓣膜术后机械通气患者镇静效果及血流动力学的影响[J]. 第二军医大学学报, 2017, 38: 563–569.
WANG J, XI W, YIN L, LI W, SHEN H, WANG Z N. Effect of dexmedetomidine and propofol on sedation and hemodynamics of patients undergoing mechanical ventilation after cardiac valve surgery[J]. Acad J Sec Mil Med Univ, 2017, 38: 563–569. |
| [19] | XIANG H, HU B, LI Z, LI J. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway[J]. Inflammation, 2014, 37: 1763–1770. DOI: 10.1007/s10753-014-9906-1 |
| [20] | 陈裕洁, 龚楚链, 谭芳, 周少丽. 右美托咪定预处理对脓毒症肾损伤大鼠炎性因子和氧化应激的影响[J]. 南方医科大学学报, 2015, 35: 1472–1475. DOI: 10.3969/j.issn.1673-4254.2015.10.20 |
| [21] | TANIGUCHI T, KURITA A, KOBAYASHI K, YAMAMOTO K, INABA H. Dose and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats[J]. J Anesth, 2008, 22: 221–228. DOI: 10.1007/s00540-008-0611-9 |
| [22] | TASDOGAN M, MEMIS D, SUT N, YUKSEL M. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis[J]. J Clin Anesth, 2009, 21: 394–400. DOI: 10.1016/j.jclinane.2008.10.010 |
| [23] | SHIMAMOTO A, POHLMAN T H, SHOMURA S, TARUKAWA T, TAKAO M, SHIMPO H. Toll-like receptor 4 mediates lung ischemia-reperfusion injury[J]. Ann Thorac Surg, 2006, 82: 2017–2023. DOI: 10.1016/j.athoracsur.2006.06.079 |
| [24] | CASEY L C, BALK R A, BONE R C. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome[J]. Ann Intern Med, 1993, 119: 771–778. DOI: 10.7326/0003-4819-119-8-199310150-00001 |
| [25] | DAMAS P, REUTER A, GYSEN P, DEMONTY J, LAMY M, FRANCHIMONT P. Tumor necrosis factor and interleukin-1 serum levels during severe sepsis in humans[J]. Crit Care Med, 1989, 17: 975–978. DOI: 10.1097/00003246-198910000-00001 |
| [26] | FINFER S. Clinical controversies in the management of critically ill patients with severe sepsis:resuscitation fluids and glucose control[J]. Virulence, 2014, 5: 200–205. DOI: 10.4161/viru.25855 |
| [27] | SHUKLA P, RAO G M, PANDEY G, SHARMA S, MITTAPELLY N, SHEGOKAR R, et al. Therapeutic interventions in sepsis:current and anticipated pharmacological agents[J]. Br J Pharmacol, 2014, 171: 5011–5031. |
 2018, Vol. 39
2018, Vol. 39


