2. 海军军医大学(第二军医大学)长海医院病理科, 上海 200433;
3. 上海中医药大学附属龙华医院肿瘤科, 上海 200032
2. Department of Pathology, Changhai Hospital, Navy Medical University(Second Military Medical University), Shanghai 200433, China;
3. Department of Oncology, Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China
胆管癌(bile duct carcinoma)是一类恶性程度非常高的肿瘤,预后极差,5年生存率低于20%,其发病因素复杂,包括原发性胆汁性肝硬化、肝炎、先天性胆管扩张和致癌物的刺激等[1-2]。由于胆管癌的基础研究相对缓慢,目前缺乏新的治疗手段和方法。因此,预防胆管癌的发生尤为重要。化学预防是利用已知的小分子化合物或天然分子干预高风险人群,以减少肿瘤的发病率[3-4]。目前已知的化学预防药物包括二甲双胍、阿司匹林和中药等,其中阿司匹林长期应用可降低心血管疾病和结直肠癌的发病率[5],其与胆管癌发病风险的相关性受到广泛关注[6],但仍缺乏其体内抑制胆管癌发生的直观体现。本研究构建了胆管癌诱导成瘤模型,并利用阿司匹林干预该模型,旨在探讨阿司匹林能否降低胆管癌的发生率。
1 材料和方法 1.1 动物与试剂6个月龄SD雄性大鼠购于上海斯莱克实验动物有限公司[动物生产许可证号:SCXK(沪)2012-0002,使用许可证号:SYXK(沪)2012-0002],饲养于同济大学附属东方医院实验动物中心。硫代乙酰胺(thioacetamide,TAA)购于常州航宇医药技术开发有限公司(CAS号:27366-72-9);阿司匹林购自生工生物工程(上海)股份有限公司(MDL编号:MFCD00002430)。胆管癌细胞QBC939为本实验室保存。碳酸酐酶2(carbonic anhydrase 2,CA-2)抗体(PB1045)和辣根过氧化物酶标记的羊抗兔IgG抗体(BA1054)购自武汉博士德生物工程有限公司;SP免疫组织化学试剂盒(KIT-9720)购自福州迈新生物技术开发有限公司。RIPA裂解液(P0013B)购自于上海碧云天生物技术有限公司。
1.2 胆管癌动物模型的建立选取15只SD雄性大鼠,每天予以饮用含TAA浓度为300 mg/L的饮用水,饲养于SPF级动物房,分别于喂养第8、12、16周处死2只大鼠,手术切取肝脏,肉眼观察肝脏表面和切面情况,随后石蜡包埋、固定、苏木精-伊红(hematoxylin-eosin staining,H-E)染色、病理切片,显微镜(OLYMPUS,BX53)下观察肝脏病变情况;第20周时处死剩余大鼠,处理方式同前,观察肝脏病变情况。
1.3 阿司匹林干预肝内胆管癌的发生根据文献[7]和本研究可确定第12~16周是胆管癌发生的关键节点,在此节点予以干预可有助于阻断胆管癌的演变,故采用阿司匹林在此节点加以干预。选取20只SD雄性大鼠,300 mg/L TAA饲养12周后停用,将大鼠分为对照组和阿司匹林处理组,每组10只。阿司匹林处理组大鼠予以含有阿司匹林(250 mg/kg)的饮用水灌胃饲养,对照组予以常规饮用水。饲养3个月后,各组分别处死3只大鼠取肝脏,肉眼和显微镜下观察肝脏及切面的肿瘤形成情况;第6个月时处死剩余大鼠,行H-E染色观察肝脏表面及切面的肿瘤形成情况。
1.4 免疫组化实验取各组大鼠肝脏,根据SP免疫组织化学试剂盒说明书操作检测肝脏病变组织CA-2的表达情况,以PBS替代一抗为阴性对照。CA-2阳性染色为棕黄色或棕褐色,定位于细胞质膜。选取5个以上高倍镜视野计数阳性细胞所占比例,<5%定义为阴性,≥5%为阳性。
1.5 细胞培养和蛋白质印迹实验取胆管癌细胞QBC939以1×106/mL的密度种植于6孔板,予以阿司匹林5 mmol/L处理,对照组加入等量无血清DMEM,培养48 h后用RIPA裂解液裂解细胞并收集蛋白,BCA法测定蛋白浓度,取50 μg,变性后行十二烷(基)硫酸钠-聚丙烯酰胺凝胶电泳。电泳后的蛋白转移至PVDF膜,封闭液封闭过夜(4 ℃),分别加入抗CA-2一抗(1:500)和抗β-actin一抗(1:1 000)室温孵育3 h;Tris缓冲生理盐水(TBS)洗涤3次,分别加入相应二抗(1:2 000)室温孵育2 h,TBS洗涤3次,X线曝光显色。
1.6 统计学处理采用SPSS 11.0软件行数据处理。所有资料以x±s表示,组间比较采用两样本均数比较的t检验。检验水准(α)为0.05。
2 结果 2.1 肝内胆管癌动物模型的建立TAA喂养8周后,大鼠肝脏形态仍正常,镜下未见明显异常;12周时大鼠肝脏形态大体正常,镜下可见散在纤维组织增生;16周时大鼠肝脏形态发生变化,表面出现黑斑,可见肿瘤形成,切面可见大量纤维组织增生和胆管癌形成;20周时大鼠肝脏体积缩小,颜色变为枯黄色,所有大鼠肝脏表面和切面均形成肉眼可见的肿瘤,镜下可见大量汇管区结缔组织增生、胆管细胞异型增生和肿瘤形成(图 1A、1B)。与第8周时相比,第20周时大鼠肝内肿瘤数目增加,差异有统计学意义(P<0.05,图 1C)。
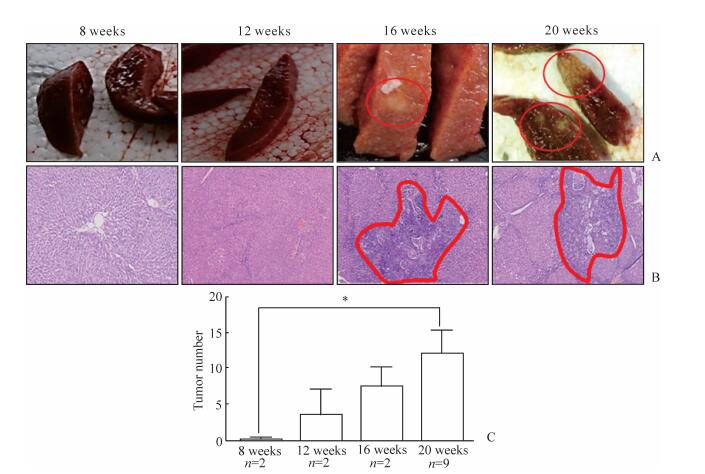
|
图 1 TAA诱导大鼠肝内胆管癌的发生过程 Fig 1 Thioacetamide (TAA) induces the development of intrahepatic bile duct carcinoma in rats A: Liver surface and cut surface of rats exposed to TAA at different time points (red circles show tumor); B: H-E staining revealing liver lesions induced by TAA at different time points (red circles show tumor, original magnification:×100); C: The average number of tumor nodules in livers (*P<0.05. x±s) |
2.2 阿司匹林中断胆管癌的发生进程
饲养3个月后,对照组大鼠肝脏出现少许纤维组织增生和个别疑似肿瘤,而阿司匹林处理组大鼠肝脏未见明显异常。第6个月时,对照组所有大鼠肝脏切面可见胆管扩张、纤维组织增生以及恶性肿瘤和疑似肿瘤的形成,成瘤率为100%(7/7);而阿司匹林处理组中只有2只大鼠肝脏出现少许纤维组织增生和疑似肿瘤,成瘤率为28.6%(2/7),见图 2。阿司匹林处理过程中未见药物所致的大鼠死亡。
2.3 阿司匹林降低肝脏病变组织和胆管癌细胞中CA-2的表达CA-2阳性染色为棕黄色或棕褐色,定位于细胞质。TAA喂养8周后,大鼠肝脏病变组织中CA-2的表达较低,而随着病情进展,CA-2的表达逐渐升高,第12、16、20周时TAA诱导形成的肝脏肿瘤组织中CA-2均呈阳性表达(图 3A)。饲养3个月后,对照组大鼠肝脏肿瘤组织中存在CA-2的过度表达,而阿司匹林处理组大鼠肝脏组织中CA-2的表达减弱(图 3B)。
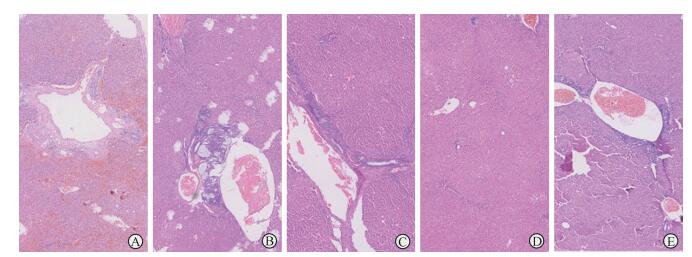
|
图 2 阿司匹林中断或延缓胆管癌进展 Fig 2 Aspirin interrupts or delays the progress of bile duct carcinoma A-C: A large number of bile duct dilation (A), tumor formation (B) and fibrosis (C) were found in livers of rats in control group; D-E: No apparent abnormality (D) was found in livers in aspirin treatment group, except for two cases of visible tumor (E). H-E staining. Original magnification: ×200 |
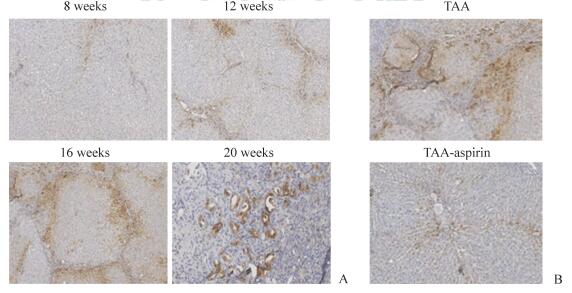
|
图 3 阿司匹林抑制TAA诱导的肝脏病变组织中CA-2的表达 Fig 3 Aspirin inhibits expression of CA-2 in liver lesions induced by thioacetamide (TAA) A: Liver lesions of rats exposed to TAA at different time points; B: Liver lesions of rats treated with aspirin for 3 months after exposure to TAA for 12 weeks. CA-2: Carbonic anhydrase 2. Immunohistochemical staining. Original magnification: ×200 |
蛋白质印迹结果(图 4)显示,5 mmol/L阿司匹林处理胆管癌细胞48 h后,细胞中CA-2的表达下调,与未处理对照组相比差异有统计学意义(P<0.01)。
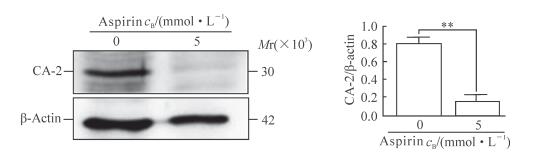
|
图 4 阿司匹林抑制胆管癌细胞QBC939中CA-2的表达 Fig 4 Aspirin inhibits expression of CA-2 in bile duct carcinoma QBC939 cell lines CA-2: Carbonic anhydrase 2. **P < 0.01. n=3, x±s |
3 讨论
近年来胆管癌的发病率逐年增高,除炎症等因素外,化学致癌物的持续刺激是重要诱因[8]。化学致癌物诱发的肿瘤突变谱系明显,以T>C和C>T突变为主[9],容易构建相关模型,在该模型上进行预防实验和干预实验能很好地模拟人体肿瘤特点。本研究成功构建了TAA诱导的胆管癌模型,并在此模型基础上实现了阿司匹林的肿瘤预防实验。
胆管癌常见动物模型包括裸鼠皮下成瘤模型、原位种植模型和药物诱导模型[7, 10-11]。基于裸鼠的成瘤模型成瘤时间短,便于观察基因或药物的功效,但其缺乏细胞免疫,生存周期短,不利于长期观察,难以行干预性研究。TAA是一种化学制剂,广泛用于生产催化剂、稳定剂、阻聚剂、电镀添加剂、照相药品、农药、染色助剂和选矿剂等,其长期蓄积易损伤肝脏。腹腔注射或口服一定剂量的TAA后,大鼠肝硬化的发生率高达80%,其特征表现为中央静脉周围炎性细胞浸润、肝细胞气球样变、肝细胞变性坏死、纤维组织增生且向肝小叶内延伸,最终形成纤维间隔;而随着TAA用药时间的延长,最终导致肝癌和(或)胆管癌的发生[12]。TAA诱导的肝纤维化/肿瘤模型的机制可能为TAA影响了肝细胞DNA、RNA和蛋白质合成,致使肝代谢紊乱、毒性蓄积,进而导致肝纤维化以及肿瘤的发生[13]。Yeh等[7]通过改变TAA的给药剂量、方式和时间,建立了稳定的胆管癌动物模型;TAA饮用9周时大鼠出现胆管上皮异型增生,16周时出现侵袭性肠型胆管癌,22周时所有大鼠均出现肠型胆管癌,成瘤率高达100%。本研究中TAA诱导12周时有大鼠出现胆管上皮增生和肝内局灶性纤维化,16周时大鼠肝内出现大量纤维化和非侵袭性胆管癌,20周时所有大鼠出现侵袭性肠型胆管癌,成功复制了胆管癌大鼠模型,而且TAA诱导的胆管癌与人类肝门部胆管癌极为类似,肿瘤细胞分化较好,形成腺腔,大量纤维组织增生和炎性细胞侵犯。该模型为胆管癌的基础和临床研究提供了理想平台。
化学预防是降低肿瘤发病率的必要手段[14]。用于化学预防的药物要求安全、无毒副作用、可长期服用,目前常用的药物包括阿司匹林、二甲双胍和中药等,其中对阿司匹林的研究最深入细致、跨度最广。阿司匹林预防结直肠癌的作用已得到验证,其对其他恶性肿瘤发病风险的防控作用也逐渐受到关注[5]。Choi等[6]调查发现阿司匹林的长期应用使胆管癌的发生率降低了2.7~3.6倍。高风险人群是指接触化学致癌物一段时间后的人群,即使脱离化学致癌物,也仍然易患肿瘤,因此提前予以化学药物干预疾病进展尤为必要。本研究结果显示TAA诱导的模型中第12周是胆管癌发生的关键节点,如果此时加以干预可以模拟上述环境,对于中断或延缓胆管癌的发生具有重要指导意义,因此在TAA处理大鼠12周后给予阿司匹林预防。结果发现经过6个月的干预,阿司匹林处理组大鼠的肿瘤发病率仅为28.6%(2/7),而对照组所有大鼠均形成了镜下可见的肿瘤,表明阿司匹林能在一定程度上中断TAA诱发的胆管癌的发生。
阿司匹林抗肿瘤机制的研究较为深入,其可以抑制环氧合酶、核因子κB和Bcl-2基因活性,上调Bax基因表达,激活死亡受体和抑制血小板聚集等[15]。此外,阿司匹林还能调控多种肿瘤代谢通路,发挥其抗肿瘤和延缓衰老的作用[16-18]。CA是一种含锌酶,参与多种生理病理过程,如尿素生成、糖异生和脂肪生成等,部分CA在肿瘤的发生和发展过程中也起到重要作用[19]。CA-2是CA家族成员之一,其在鼻咽癌和膀胱癌组织中高表达[20-21]。本研究发现在病变肝脏和TAA诱导的胆管癌组织中CA-2均呈现高表达,提示CA-2可能参与了TAA诱导的胆管癌的发生。CA-2与血栓性疾病中阿司匹林的耐药相关[22],而本研究发现在肿瘤细胞中阿司匹林也能降低CA-2的表达,动物研究也证实阿司匹林降低肿瘤发生的同时降低了CA-2的表达,提示CA-2可能是阿司匹林的潜在作用靶点,阿司匹林通过降低CA-2的表达延缓TAA诱导的胆管癌的发生。
综上所述,TAA能成功诱导胆管癌的发生,其构建的胆管癌模型是研究胆管癌预防和治疗的理想平台。阿司匹林能中断或延缓胆管癌的发生,其作用可能是通过降低CA-2的表达实现的。
| [1] | BERGQUIST A, VON SETH E. Epidemiology of cholangiocarcinoma[J]. Best Pract Res Clin Gastroenterol, 2015, 29: 221–232. DOI: 10.1016/j.bpg.2015.02.003 |
| [2] | RAZUMILAVA N, GORES G J. Cholangiocarcinoma[J]. Lancet, 2014, 383: 2168–2179. DOI: 10.1016/S0140-6736(13)61903-0 |
| [3] | CUZICK J. Preventive therapy for cancer[J/OL]. Lancet Oncol, 2017, 18: e472-e482. doi: 10.1016/S1470-2045(17)30536-3. |
| [4] | ALBINI A, DECENSI A, CAVALLI F, COSTA A. Cancer prevention and interception:a new era for chemopreventive approaches[J]. Clin Cancer Res, 2016, 22: 4322–4327. DOI: 10.1158/1078-0432.CCR-16-0695 |
| [5] | RICHMAN I B, OWENS D K. Aspirin for primary prevention[J]. Med Clin North Am, 2017, 101: 713–724. DOI: 10.1016/j.mcna.2017.03.004 |
| [6] | CHOI J, GHOZ H M, PEERAPHATDIT T, BAICHOO E, ADDISSIE B D, HARMSEN W S, et al. Aspirin use and the risk of cholangiocarcinoma[J]. Hepatology, 2016, 64: 785–796. DOI: 10.1002/hep.v64.3 |
| [7] | YEH C N, MAITRA A, LEE K F, JAN Y Y, CHEN M F. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat:an animal model recapitulating the multi-stage progression of human cholangiocarcinoma[J]. Carcinogenesis, 2004, 25: 631–636. |
| [8] | PLENTZ R R, MALEK N P. Clinical presentation, risk factors and staging systems of cholangiocarcinoma[J]. Best Pract Res Clin Gastroenterol, 2015, 29: 245–252. DOI: 10.1016/j.bpg.2015.02.001 |
| [9] | ALEXANDROV L B, NIK-ZAINAL S, WEDGE D C, APARICIO S A, BEHJATI S, BIANKIN A V, et al. Signatures of mutational processes in human cancer[J]. Nature, 2013, 500: 415–421. DOI: 10.1038/nature12477 |
| [10] | YU G, YU W, JIN G, XU D, CHEN Y, XIA T, et al. PKM2 regulates neural invasion of and predicts poor prognosis for human hilar cholangiocarcinoma[J/OL]. Mol Cancer, 2015, 14: 193. doi: 10.1186/s12943-015-0462-6. |
| [11] | CHEN Y, CHA Z, FANG W, QIAN B, YU W, LI W, et al. The prognostic potential and oncogenic effects of PRR11 expression in hilar cholangiocarcinoma[J]. Oncotarget, 2015, 6: 20419–20433. |
| [12] | Becker F F. Thioacetamide hepatocarcinogenesis[J]. J Natl Cancer Inst, 1983, 71: 553–558. |
| [13] | AN J H, SEONG J, OH H, KIM W, HAN K H, PAIK Y H. Protein expression profiles in a rat cirrhotic model induced by thioacetamide[J]. Korean J Hepatol, 2006, 12: 93–102. |
| [14] | BODE A M, DONG Z, WANG H. Cancer prevention and control:alarming challenges in China[J]. Natl Sci Rev, 2016, 3: 117–127. DOI: 10.1093/nsr/nwv054 |
| [15] | BRUNO A, DOVIZIO M, TACCONELLI S, PATRIGNANI P. Mechanisms of the antitumoural effects of aspirin in the gastrointestinal tract[J/OL]. Best Pract Res Clin Gastroenterol, 2012, 26: e1-e13. doi: 10.1016/j.bpg.2012.10.001. |
| [16] | HUANG X B, MU X H, WAN Q L, HE X M, WU G S, LUO H R. Aspirin increases metabolism through germline signalling to extend the lifespan of Caenorhabditis elegans[J/OL]. PLoS One, 2017, 12: e0184027. doi: 10.1371/journal.pone.0184027. |
| [17] | YANG G, WANG Y, FENG J, LIU Y, WANG T, ZHAO M, et al. Aspirin suppresses the abnormal lipid metabolism in liver cancer cells via disrupting an NFκB-ACSL1 signaling[J]. Biochem Biophys Res Commun, 2017, 486: 827–832. DOI: 10.1016/j.bbrc.2017.03.139 |
| [18] | AI G, DACHINENI R, KUMAR D R, ALFONSO L F, MARIMUTHU S, BHAT G J. Aspirin inhibits glucose-6-phosphate dehydrogenase activity in HCT 116 cells through acetylation:identification of aspirin-acetylated sites[J]. Mol Med Rep, 2016, 14: 1726–1732. DOI: 10.3892/mmr.2016.5449 |
| [19] | NÓGRÁDI A. The role of carbonic anhydrases in tumors[J]. Am J Pathol, 1998, 153: 1–4. DOI: 10.1016/S0002-9440(10)65537-X |
| [20] | LUO Y, MOK T S, LIN X, ZHANG W, CUI Y, GUO J, et al. SWATH-based proteomics identified carbonic anhydrase 2 as a potential diagnosis biomarker for nasopharyngeal carcinoma[J/OL]. Sci Rep, 2017, 7: 41191. doi: 10.1038/srep41191. |
| [21] | TACHIBANA H, GI M, KATO M, YAMANO S, FUJIOKA M, KAKEHASHI A, et al. Carbonic anhydrase 2 is a novel invasion-associated factor in urinary bladder cancers[J]. Cancer Sci, 2017, 108: 331–337. DOI: 10.1111/cas.13143 |
| [22] | JAKUBOWSKI M, DEBSKI J, SZAHIDEWICZ-KRUPSKA E, TUREK-JAKUBOWSKA A, GAWRYS J, GAWRYS K, et al. Platelet carbonic anhydrase Ⅱ, a forgotten enzyme, may be responsible for aspirin resistance[J/OL]. Oxid Med Cell Longev, 2017, 2017: 3132063. doi: 10.1155/2017/3132063. |
 2018, Vol. 39
2018, Vol. 39


