2. 扬州大学附属医院儿科, 扬州 225001
2. Department of Pediatrics, Affiliated Hospital of Yangzhou University, Yangzhou 225001, Jiangsu, China
Toll样受体9(Toll-like receptor 9,TLR9)可以识别细菌和病毒DNA中的非甲基化CpG基序,在机体清除细菌和病毒的防御机制中发挥重要作用,但过度的TLR9应答会对机体造成损害[1-2]。TLR9与系统性红斑狼疮(system lupus erythmatosis,SLE)等自身免疫病的发生和发展密切相关[3],如SLE患者外周血中B淋巴细胞表达高水平的TLR9且与血清中抗双链DNA抗体水平呈正相关[4];特异性阻断狼疮小鼠模型中TLR9的表达,导致血清中抗双链DNA抗体和抗Sm抗体的水平均降低[5]。因此,负向调节B淋巴细胞上的TLR9信号可能为自身免疫性疾病的治疗提供新思路。
Fcγ段受体(Fcγ receptor,FcγR)Ⅱb是免疫球蛋白(immune globulin,Ig)G的FcγR中唯一的抑制性受体[6]。免疫复合物(immune complex,IC)与B淋巴细胞表面的FcγRⅡb交联可以抑制B淋巴细胞活化及产生抗体[7]。我们前期研究发现,IC还能够通过FcγRⅡb抑制TLR9介导的免疫应答,如体外实验发现IC通过FcγRⅡb抑制B淋巴细胞TLR9配体CpG寡脱氧核苷酸(oligodeoxynucleotide,ODN)刺激的CD40和CD80高表达[8]。本研究进一步探讨IC对TLR9的这一负向调节作用在体内实验中是否同样存在,以及IC通过抑制哪些TLR9信号通路从而抑制CD40和CD80的表达。
1 材料和方法 1.1 材料与试剂C57BL/6小鼠购自扬州大学实验动物中心;背景品系为C57BL/6的FcγRⅡb缺陷小鼠购自美国杰克逊实验室,由扬州大学医学院转化医学研究院保种[实验动物使用许可证号:SYXK(苏)2012-0029]。CpG ODN由生工生物工程(上海)股份有限公司合成,序列:5' -TCC ATG ACG TTC CTG ACG TT-3' ,序列全部硫代化,经去内毒素纯化后使用;抗小鼠CD19磁珠购自美国美天旎生物技术有限公司;抗CD19、抗CD40和抗CD80的荧光标记抗体均购自美国Biolegend公司;细胞外调节蛋白激酶(extracellular regulated protein kinase,ERK)抑制剂PD98059、c-Jun氨基末端蛋白激酶(c-Jun N-terminal protein kainse,JNK)抑制剂SP600125和p38抑制剂SB203580均购自上海碧云天生物技术有限公司(货号分别为S1805、S1876、S1863);兔抗p-JNK单克隆抗体、兔抗p-ERK单克隆抗体、兔抗p-p38单克隆抗体和兔抗β-actin单克隆抗体均购自美国Cell Signaling公司(货号分别为4671、4370、4511、4970)。
1.2 IC的制备参照文献[8]方法进行,简述如下:IC由卵清蛋白(ovalbumin,OVA)抗原与抗OVA按1:10的质量比混合,37 ℃孵育1 h后形成。体外刺激B淋巴细胞的IC为10 μg/mL OVA抗原和100 μg/mL抗OVA混合形成;体内实验中每只小鼠的IC为100 μg OVA抗原和1 mg抗OVA混合形成。
1.3 IC和CpG ODN体内注射小鼠腹腔注射IC 24 h后腹腔注射10 μg CpG ODN,注射CpG ODN 24 h后取小鼠脾脏,用免疫磁珠法分选获得脾脏CD19+ B淋巴细胞[9],并检测其表面CD40和CD80的表达。
1.4 脾脏B淋巴细胞的分选于无菌条件下取小鼠脾脏,研磨后收集单细胞悬液,用Tris-NH4Cl溶液裂解红细胞,PBS洗涤1次,用抗CD19磁珠分选后,流式细胞术测定CD19+ B淋巴细胞纯度为95%左右。
1.5 流式细胞术检测B淋巴细胞表面CD40和CD80表达待检细胞中加入异硫氰酸荧光素(fluorescein isothiocyanate,FITC)-抗CD19和藻红蛋白(P-phycoerythrin,PE)-抗CD40或PE-抗CD80,抗体的终浓度均为1 μg/mL,于4 ℃中放置20 min后用PBS洗涤,重悬于300 μL PBS中,上机检测并分析。
1.6 蛋白质印迹法检测蛋白磷酸化将B淋巴细胞按2×106/mL的密度铺入细胞培养板,用IC和(或)CpG ODN(终浓度0.3 μmol/L)处理30 min,用蛋白裂解液提取细胞总蛋白,聚丙烯酰胺凝胶电泳分离蛋白,后转移至硝酸纤维素膜上,用5%脱脂奶粉溶液室温封闭2 h,加入p-JNK、p-ERK、p-p38和β-actin一抗稀释液(稀释比例均为1:1 000),4 ℃静置孵育过夜,PBST洗涤后加入相应二抗,室温孵育2 h,PBST洗涤后用增强化学发光法显色。
1.7 抑制剂处理细胞将B淋巴细胞按2×105/孔的密度铺至96孔板,分别加入10 μmol/L PD98059、50 μmol/L SP600125和20 mg/L SB203580,同时设加DMSO作为对照组,处理细胞1 h后,再用0.3 μmol/L CpG ODN刺激细胞24 h,收集细胞用流式细胞术检测其表面CD40和CD80的表达情况。
1.8 统计学处理采用SPSS 12.0软件对数据进行录入和处理,正态分布且方差齐性的计量资料以x±s表示,组间两两比较采用t检验。检验水准(α)为0.05。
2 结果 2.1 IC抑制CpG ODN活化B淋巴细胞表面CD40和CD80的表达结果如图 1所示,体内注射CpG ODN能够上调B淋巴细胞表面CD40和CD80的表达(P均<0.05),IC预处理可抑制CpG ODN活化的B淋巴细胞表面CD40和CD80表达上调(P均<0.05)。
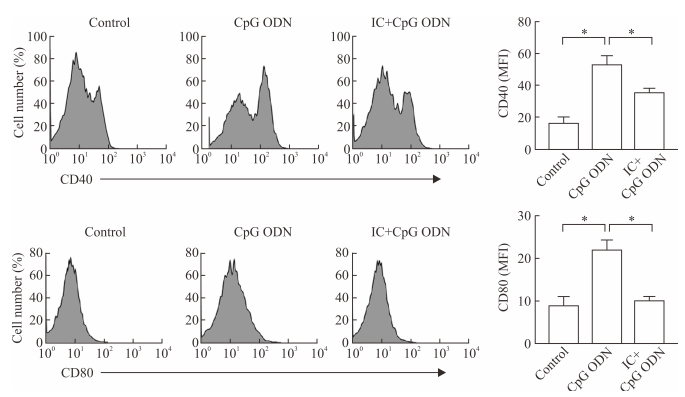
|
图 1 IC体内抑制CpG ODN活化B淋巴细胞表面CD40和CD80表达上调 Fig 1 IC inhibits the up-regulated CD40 and CD80 expressions on CpG ODN-activated B lymphocytes in vivo IC: Immune complex; ODN: Oligodeoxynucleotide; MFI: Median fluorescent intensity. *P < 0.05. n=3, x±s |
2.2 IC抑制CpG ODN活化B淋巴细胞JNK和p38的磷酸化
CpG ODN活化B淋巴细胞后不能明显诱导ERK磷酸化,但可以诱导JNK和p38磷酸化。而IC能够抑制CpG ODN活化B淋巴细胞JNK和p38磷酸化(图 2)。
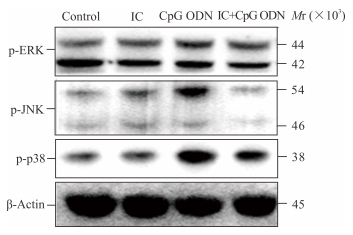
|
图 2 IC抑制CpG ODN活化B淋巴细胞JNK和p38磷酸化 Fig 2 IC inhibits JNK and p38 phosphorylation in CpG ODN-activated B lymphocytes IC: Immune complex; ODN: Oligodeoxynucleotide |
2.3 IC通过FcγRⅡb抑制CpG ODN活化B淋巴细胞JNK和p38磷酸化
结果如图 3所示,IC对FcγRⅡb缺陷小鼠来源的CpG ODN活化B淋巴细胞JNK和p38磷酸化水平无明显抑制作用,提示IC抑制CpG ODN活化B淋巴细胞JNK和p38的磷酸化依赖于FcγRⅡb。
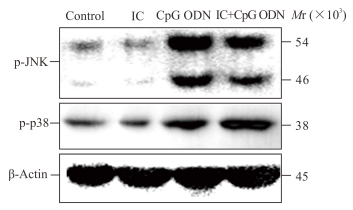
|
图 3 IC对FcγRⅡb缺陷小鼠来源CpG ODN活化B淋巴细胞JNK和p38磷酸化的作用 Fig 3 Effects of IC on JNK and p38 phosphorylation in CpG ODN-activated B lymphocytes from FcγRⅡb-/- mice IC: Immune complex; ODN: Oligodeoxynucleotide; FcγRⅡb: Fcγ receptor Ⅱb |
2.4 JNK和p38抑制剂下调CpG ODN活化B淋巴细胞表面CD40和CD80的表达
结果如图 4所示,JNK抑制剂(SP600125)和p38抑制剂(SB203580)均下调CpG ODN活化B淋巴细胞表面CD40和CD80的表达(P均<0.01),提示B淋巴细胞内CpG ODN通过JNK和p38通路调控CD40和CD80的表达。而ERK抑制剂(PD98059)未见相同作用。

|
图 4 JNK和p38对CpG ODN活化B淋巴细胞表面CD40和CD80表达的影响 Fig 4 Effects of JNK and p38 on CD40 and CD80 expressions in CpG ODN-activated B lymphocytes A: Flow cytometry results; B: Quantitative analysis. PD98059: ERK inhibitor, 10 μmol/L; SP600125: JNK inhibitor, 50 μmol/L; SB203580: p38 inhibitor, 20 mg/L. ODN: Oligodeoxynucleotide; DMSO: Dimethyl sulphoxide; MFI: Median fluorescent intensity. **P < 0.01. n=3, x±s |
3 讨论
静脉注射IgG治疗多种自身免疫性疾病已取得良好的疗效[10],IgG与可溶性抗原形成可溶性IC后能模拟静脉注射IgG的效应用于治疗某些自身免疫性疾病[11]。静脉注射Ig能负向调节树突状细胞表面CD80和CD86表达[12],体外用IC可以抑制CpG ODN活化B淋巴细胞表面CD40和CD80表达上调[8]。本研究结果显示,体内注射IC同样能抑制CpG ODN活化B淋巴细胞表面CD40和CD80表达。有文献报道,B淋巴细胞和T淋巴细胞表面CD40与CD40配体(CD40L)相互作用对SLE自身抗体的产生有重要作用[13],抗CD40L能阻断SLE小鼠自身抗体的产生[14],提示阻断CD40-CD40L通路可能对治疗SLE有重要作用。
所有B淋巴细胞亚群均表达TLR9且能被TLR9配体活化[15]。TLR9不仅能被病毒和细菌DNA非甲基化CpG基序活化,同样也能被人工合成短的单链ODN活化[16]。因而,无论来源如何,所有富含CpG基序均能活化B淋巴细胞。B淋巴细胞TLR9介导的信号通路与树突状细胞类似,即TLR9配体作用于TLR9后引起丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)和NF-κB途径活化[17]。本实验发现,CpG ODN活化B淋巴细胞后引起NF-κB途径(数据未显示)以及JNK和p38-MAPK途径的活化,但不能明显诱导ERK-MAPK途径的活化;IC作用后CpG ODN诱导的NF-κB活化并没有受到明显影响(数据未显示),而JNK和p38的活化被明显抑制,提示IC并不是通过抑制NF-κB通路抑制CD40和CD80的表达,而有可能通过抑制JNK和p38-MAPK途径从而对CD40和CD80的表达发挥抑制作用。本实验结果表明,JNK和p38抑制剂能下调CpG ODN活化B淋巴细胞表面CD40和CD80的表达,提示JNK和p38-MAPK途径确实参与了对CD40和CD80表达的调控。
IC抑制TLR9通路的机制目前还不清楚。前期检测了IC刺激后的B淋巴细胞内某些蛋白激酶的磷酸化水平,发现IC刺激不引起脾酪氨酸激酶和Bruton酪氨酸激酶的活化,但能使酪氨酸激酶Lyn活化。抑制Lyn会导致TLR4介导的CD40表达进一步增加,提示IC通过活化Lyn抑制TLR4介导的CD40高表达[18]。有文献报道,Lyn可以磷酸化接头蛋白Dok,维持含SH2区域肌醇5'磷酸酶1的活化,从而抑制NF-κB通路的活化[19-20]。此外,前期研究还发现激活B淋巴细胞Lyn可以抑制NF-κB通路的活化[21]。那么IC能否通过活化Lyn抑制TLR9通路中的JNK和p38-MAPK信号通路,从而抑制CD40和CD80的表达,仍需要进一步的实验研究。
综上所述,本研究发现IC与B淋巴细胞的FcγRⅡb结合抑制TLR9激动剂CpG ODN诱导的JNK和p38-MAPK途径,从而抑制B淋巴细胞表面CD40和CD80的表达。本实验结果有助于更全面地认识IC对TLR介导的免疫应答负向调节机制,也为治疗B淋巴细胞内过度TLR9应答提供了线索。
| [1] | HAMERMAN J A, POTTLE J, NI M, HE Y, ZHANG Z Y, BUCKNER J H. Negative regulation of TLR signaling in myeloid cells-implications for autoimmune diseases[J]. Immunol Rev, 2016, 269: 212–227. DOI: 10.1111/imr.2016.269.issue-1 |
| [2] | LIEW F Y, XU D, BRINT E K, O'NEILL L A. Negative regulation of Toll-like receptor-mediated immune responses[J]. Nat Rev Immunol, 2005, 5: 446–458. DOI: 10.1038/nri1630 |
| [3] | CELHAR T, MAGALHÃES R, FAIRHURST A M. TLR7 and TLR9 in SLE:when sensing self goes wrong[J]. Immunol Res, 2012, 53: 58–77. DOI: 10.1007/s12026-012-8270-1 |
| [4] | PAPADIMITRAKI E D, CHOULAK C, KOUTALA E, BERTSIAS G, TSATSANIS C, GERGIANAKI I, et al. Expansion of Toll-like receptor 9-expressing B lymphocytes in active systemic lupus erythematosus:implications for the induction and maintenance of the autoimmune process[J]. Arthritis Rheum, 2006, 54: 3601–3611. DOI: 10.1002/(ISSN)1529-0131 |
| [5] | PAWAR R D, RAMANJANEYULU A, KULKAMI O P, LECH M, SEGERER S, ANDERS H J. Inhibition of Toll-like receptor-7(TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus[J]. J Am Soc Nephrol, 2007, 18: 1721–1731. DOI: 10.1681/ASN.2006101162 |
| [6] | SMITH K G, CLATWORTHUL M R. FcγRⅡB in autoimmunity and infection:evolutionary and therapeutic implications[J]. Nat Rev Immunol, 2010, 10: 328–343. DOI: 10.1038/nri2762 |
| [7] | WENINK M H, SANTEGOETS K C, ROELOFS M F, HUIJBENS R, KOENEN H J, VAN BEEK R, et al. The inhibitory FcγRⅡb receptor dampens TLR4-mediated immune responses and is selectively up-regulated on dendritic cells from rheumatoid arthritis patients with quiescent disease[J]. J Immunol, 2009, 183: 4509–4520. DOI: 10.4049/jimmunol.0900153 |
| [8] | QIAN L, CHEN W, QIN H, RUI C, JIA X, FU Y, et al. Immune complex negatively regulates Toll-like receptor 9-mediated immune responses in B lymphocytes through the inhibitory Fc-γ receptor Ⅱb[J]. Microbiol Immunol, 2015, 59: 142–151. DOI: 10.1111/mim.v59.3 |
| [9] |
钱莉, 佟大可, 潘兴元, 田芳, 龚卫娟, 季明春. 脂多糖对B细胞的活化作用及机制的初步研究[J]. 第二军医大学学报, 2011, 32: 1231–1234.
QIAN L, TONG D K, PAN X Y, TIAN F, GONG W J, JI M C. Lipopolysaccharide activates B lymphocytes and the underlying mechanisms[J]. Acad J Sec Mil Med Univ, 2011, 32: 1231–1234. |
| [10] | SCHWAB I, NIMMERJAHN F. Intravenous immuno-globulin therapy:how does IgG modulate the immune system?[J]. Nat Rev Immunol, 2013, 13: 176–189. DOI: 10.1038/nri3401 |
| [11] | SIRAGAM V, BRINC D, CROW A R, SONG S, FREEDMAN J, LAZARUS A H. Can antibodies with specificity for soluble antigens mimic the therapeutic effects of intravenous IgG in the treatment of autoimmune disease?[J]. J Clin Invest, 2005, 115: 155–160. DOI: 10.1172/JCI200522753 |
| [12] | BAYRY J, LACROIX-DESMAZES S, CARBONNEIL C, MISRA N, DONKOVA V, PASHOV A, et al. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin[J]. Blood, 2003, 101: 758–765. DOI: 10.1182/blood-2002-05-1447 |
| [13] | NÉRON S, BOIRE G, DUSSAULT N, RACINE C, DE BRUM-FERNANDES A J, CÔTÉ S, et al. CD40-activated B lymphocytes from patients with systemic lupus erythematosus can be modulated by therapeutic immunoglobulins in vitro[J]. Arch Immunol Ther Exp (Warsz), 2009, 57: 447–458. DOI: 10.1007/s00005-009-0048-3 |
| [14] | DRIVER C B, ISHIMORI M, WEISMAN M H. The B lymphocyte in systemic lupus erythematosus:a rational target for more effective therapy[J]. Ann Rheum Dis, 2008, 67: 1374–1381. DOI: 10.1136/ard.2007.076745 |
| [15] | RAWLINGS D J, SCHWARTZ M A, JACKON S W, MEYER-BAHLBURG A. Integration of B lymphocyte responses through Toll-like receptors and antigen receptors[J]. Nat Rev Immunol, 2012, 12: 282–294. DOI: 10.1038/nri3190 |
| [16] | BLASIUS A L, BEUTLER B. Intracellular Toll-like receptors[J]. Immunity, 2010, 32: 305–315. DOI: 10.1016/j.immuni.2010.03.012 |
| [17] | PENG S L. Signaling in B lymphocytes via Toll-like receptors[J]. Curr Opin Immunol, 2005, 17: 230–236. DOI: 10.1016/j.coi.2005.03.003 |
| [18] | 钱莉, 王少卿, 刘阳, 陈文艳, 龚卫娟, 田芳, 等. FcγRⅡb抑制B细胞内TLR4介导的CD40和CD80表达[J]. 扬州大学学报(农业与生命科学版), 2016, 37: 15–19. |
| [19] | HUGHAN S C, SPRING C M, SCHOENWAELDER S M, STURGEON S, ALWIS I, YUAN Y, et al. Dok-2 adaptor protein regulates the shear-dependent adhesive function of platelet integrin αⅡbβ3 in mice[J]. J Biol Chem, 2014, 289: 5051–5060. DOI: 10.1074/jbc.M113.520148 |
| [20] | NG C H, XU S, LAM K P. Dok-3 plays a nonredundant role in negative regulation of B-cell activation[J]. Blood, 2007, 110: 259–266. DOI: 10.1182/blood-2006-10-055194 |
| [21] | QIAN L, CHEN W, WANG S, LIU Y, JIA X, FU Y, et al. FcγRⅡb attenuates TLR4-mediated NF-κB signaling in B lymphocytes[J]. Mol Med Rep, 2017, 16: 5693–5698. DOI: 10.3892/mmr.2017.7269 |
 2018, Vol. 39
2018, Vol. 39


