系统性红斑狼疮(systemic lupus erythematosus,SLE)是一种累及多器官、多系统的慢性自身免疫性疾病,其病理特点为自身抗体大量产生,并与体内相应的自身抗原形成免疫复合物,沉积于组织和器官,从而导致损伤[1]。SLE的发病机制尚不清楚,但目前认为其主要与T淋巴细胞、B淋巴细胞功能紊乱导致的免疫稳态失衡有关[1]。
滤泡辅助性T细胞(follicular helper T cell,Tfh)是CD4+ T淋巴细胞的一个亚群,在二级淋巴器官生发中心为B淋巴细胞提供活化信号,促进体细胞高频突变及抗体类型转换[2]。Tfh通过表达转录因子B细胞淋巴瘤6(B cell lymphoma 6,BCL-6)、C-X-C趋化因子受体(C-X-C chemokine receptor,CXCR)5、诱导型共刺激分子(inducible costimulator,ICOS)及程序性死亡蛋白1(programmed death 1,PD-1)等分子调节生发中心的B淋巴细胞的发育、增殖、分化,促进抗体分泌细胞形成[3-4]。Tfh还可以通过分泌白细胞介素(interleukin,IL)21(IL-21)促进生发中心形成、B淋巴细胞增殖和分化以及大量抗体的产生[5]。
Tfh不仅存在于生发中心,亦存在于外周血中。外周血Tfh亚群紊乱与多种自身免疫性疾病有关,但其表型特征、临床意义以及在自身免疫性疾病发病中的作用仍然不清楚。研究发现,在小鼠和人体内可根据C-C趋化因子受体7(C-C chemokine receptor 7,CCR7)和PD-1的表达量,将Tfh分为2种功能状态:CCR7hiPD-1lo(CCR7高表达、PD-1低表达)静息型Tfh和CCR7loPD-1hi(CCR7低表达、PD-1高表达)效应型Tfh,其中CCR7loPD-1hi Tfh的比例增加提示Tfh活化[6]。本研究通过检测SLE患者外周血单个核细胞(peripheral blood mononuclear cell,PBMC)中CCR7loPD-1hi Tfh的比例,分析其与临床指标和成浆细胞的相关性,从而揭示其在SLE发病机制中的作用。
1 资料和方法 1.1 研究对象纳入2018年2月至2018年7月在我院风湿免疫科就诊的31例SLE患者,均符合1997年美国风湿病协会(American College of Rheumatology,ACR)的SLE诊断标准[7],且均行系统性红斑狼疮疾病活动指数(systemic lupus erythematosus disease activity index,SLEDAI)测评。纳入同期在我院风湿免疫科就诊的类风湿关节炎(rheumatoid arthritis,RA)患者29例(均符合1987年ACR的RA诊断标准[8])、干燥综合征(Sj?gren’s syndrome,SS)患者12例(均符合2002年ACR的SS诊断标准[9]),以及在我院体检中心行健康体检的健康对照37名。本研究经我院医学伦理委员会审批,所有受试者均签署知情同意书。
1.2 主要试剂与仪器活细胞染料、别藻蓝蛋白-花青素7(allophycocyanin-cyanine 7,APC-cy7)标记的抗CD4、异硫氰酸荧光素(fluorescein isothiocyanate,FITC)标记的抗CD45RA、AlexFlour 647标记的抗CXCR5、Brilliant VioletTM 421标记的抗CCR7、多甲藻黄素叶绿素蛋白-花青素5.5(peridinin chlorophyll protein-cyanine 5.5,PerCP-cy5.5)标记的抗PD-1、藻红蛋白(phycoerythrin,PE)标记的抗信号淋巴细胞激活分子家族成员5(signaling lymphocytic activation molecule family member 5,SLAMF5)、PE-CFTM594标记的抗CXCR3、藻红蛋白-花青素7(phycoerythrin-cyanine 7,PE-cy7)标记的抗ICOS、别藻蓝蛋白(allophycocyanin,APC)标记的抗CD19、PerCP-cy5.5标记的抗CD27、PE-cy7标记的抗CD38和PE标记的抗免疫球蛋白(immunoglobulin,Ig)D(IgD)抗体均购自美国Biolegend公司。人淋巴细胞分离液购自美国Sigma公司。使用美国BD公司LSRFortessa型流式细胞仪进行检测。
1.3 实验方法 1.3.1 PBMC的制备用乙二胺四乙酸(ethylenediaminetetraacetic acid,EDTA)抗凝管采集所有研究对象外周静脉血各5 mL,加入5 mL磷酸盐缓冲液(phosphate buffer saline,PBS)稀释、混匀,然后加至含5 mL人淋巴细胞分离液的离心管中,1 100×g离心23 min。吸取中间白膜层,PBS洗涤2次,加入含0.5%胎牛血清的1 mL流式染色缓冲液重悬细胞,即得PBMC悬液。
1.3.2 细胞表面染色调整PBMC悬液细胞密度为1×107/mL,取100 μL(1×106个细胞)细胞悬液于流式管中,分别加入活细胞染料和抗CD4、抗CD45RA、抗CXCR5、抗CXCR3、抗CCR7、抗ICOS、抗PD-1、抗SLAMF5抗体。另取1×106个细胞,分别加入活细胞染料和抗CD19、抗CD27、抗IgD、抗CD38抗体。4 ℃孵育30 min,PBS洗涤2次,加入300 μL流式染色缓冲液重悬后使用流式细胞仪进行检测,并采用FlowJo软件进行数据分析。
1.4 统计学处理应用GraphPad Prism 7.0软件进行统计学分析。呈正态分布且方差齐性的计量资料以x±s表示,组间比较采用t检验或方差分析;偏态分布的计量资料以中位数(下四分位数,上四分位数)表示,两组间比较采用Mann-Whitney U检验;计数资料以例数和百分数表示,组间比较采用χ2检验;相关性分析采用Spearman相关分析。检验水准(α)为0.05。
2 结果 2.1 各组的基线资料见表 1。健康对照37名,男4名、女33名,年龄(42.49±11.29)岁;SLE患者31例,男3例、女28例,年龄为(40.29±13.06)岁;RA患者29例,男3例、女26例,年龄(63.10±10.95)岁;SS患者12例,男1例、女11例,年龄(50.75±11.80)岁。各组间的性别构成差异无统计学意义(χ2=0.07,P>0.05);SLE、SS患者年龄与健康对照差异均无统计学意义(t=-0.77、2.12,P均>0.05),而RA患者年龄大于健康对照,差异有统计学意义(t=7.09,P<0.01)。RA患者C3、C4、IgM和IgE水平均高于SLE患者(t=5.12、4.66,U=207.00、198.50;P均<0.01)。SS患者红细胞沉降率(erythrocyte sedimentation rate,ESR)、C反应蛋白(C-reactive protein,CRP)、C4、IgA水平低于RA患者(U=78.50、73.50,t=-2.53,U=68.50;P<0.05,P<0.01)。各组间病程、白细胞计数、淋巴细胞计数、IgG水平差异均无统计学意义(H=4.04、F=0.04、F=0.50、H=3.07,P均>0.05)。
|
|
表 1 1各组临床资料比较 Tab 1 Comparison of clinical characteristics among different groups |
2.2 CCR7loPD-1hi Tfh与CCR7hiPD-1lo Tfh表面分子的表达情况
根据CCR7和PD-1的表达情况,可将健康对照外周血CD4+CD45RA-CXCR5+Tfh分为CCR7loPD-1hi Tfh和CCR7hiPD-1lo Tfh(图 1A)。相比CCR7hiPD-1lo Tfh,CCR7loPD-1hi Tfh表面CXCR3、ICOS和SLAMF5表达水平较高(t=3.73、5.06、8.27,P均<0.01;图 1B)。
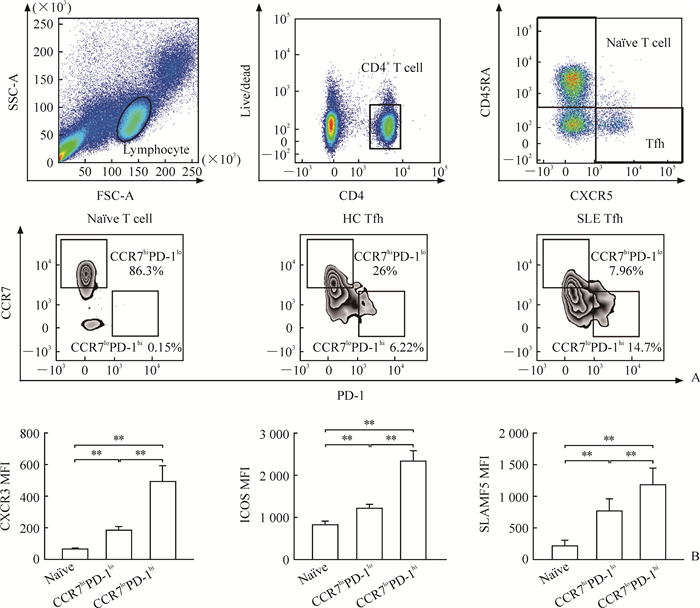
|
图 1 CCR7hiPD-1lo Tfh与CCR7loPD-1hi Tfh设门方法及表面分子表达差异 Fig 1 Gate strategy and distinct phenotypes of CCR7hiPD-1lo Tfh and CCR7loPD-1hi Tfh subsets A: Gate strategy to identify CCR7hiPD-1lo Tfh and CCR7loPD-1hi Tfh subsets in Tfh; B: MFI of CXCR3, ICOS and SLAMF5 expression levels on CCR7hiPD-1lo Tfh and CCR7loPD-1hi Tfh subsets. Tfh: Follicular helper T cell; CCR7: C-C chemokine receptor 7; PD-1: Programmed death 1; hi: High expression; lo: Low expression; HC: Healthy control; SLE: Systemic lupus erythematosus; CXCR: C-X-C chemokine receptor; ICOS: Inducible costimulatory; SLAMF5: Signaling lymphocytic activation molecule family member 5; MFI: Mean fluorescence intensity. **P < 0.01. n=12, x±s |
2.3 SLE、RA和SS患者外周血中CCR7loPD-1hi Tfh和CCR7hiPD-1lo Tfh的比例
SLE、RA和SS患者外周血中CD4+CD45RA-CXCR5+ Tfh的比例与健康对照相比差异均无统计学意义(P均>0.05,图 2A)。与健康对照相比,SLE患者外周血中CCR7loPD-1hi Tfh的比例增多[5.79%(2.97%,8.95%)vs 2.93%(1.63%,5.21%),U=314.5,P<0.01;图 2B],CCR7hiPD-1lo Tfh的比例降低[17.00%(10.50%,27.60%)vs 25.20%(16.55%,44.90%),U=341.5,P<0.01;图 2C]。RA患者CCR7loPD-1hi Tfh的比例与健康对照相比亦增加[4.57%(2.51%,7.60%)vs 2.93%(1.63%,5.21%),U=332.5,P<0.01;图 2B],而CCR7hiPD-1lo Tfh的比例与健康对照相比差异无统计学意义(U=413.5,P>0.05;图 2C)。SS患者CCR7loPD-1hi Tfh和CCR7hiPD-1lo Tfh的比例与健康对照相比差异均无统计学意义(U=183.0、208.0,P均>0.05;图 2B、2C)。
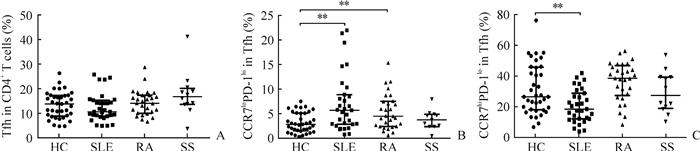
|
图 2 Tfh(A)、CCR7loPD-1hi Tfh(B)和CCR7hiPD-1lo Tfh(C)在HCs和SLE、RA、SS患者外周血中的比例 Fig 2 Proportions of Tfh (A), CCR7loPD-1hi Tfh (B) and CCR7hiPD-1lo Tfh (C) in peripheral blood of SLE, RA and SS patients and HCs Tfh: Follicular helper T cell; CCR7: C-C chemokine receptor 7; PD-1: Programmed death 1; lo: Low expression; hi: High expression; HC: Healthy control (n=37); SLE: Systemic lupus erythematosus (n=31); RA: Rheumatoid arthritis (n=29); SS: Sj?gren's syndrome (n=12). **P < 0.01 |
2.4 SLE患者外周血中CCR7loPD-1hi Tfh比例与临床指标及成浆细胞比例的相关性
SLE患者外周血中CCR7loPD-1hi Tfh的比例与患者年龄、病程无明显相关性(r=-0.041 2、0.123 3,P均>0.05),而与SLEDAI、抗双链DNA(double-stranded DNA,dsDNA)抗体滴度以及成浆细胞(CD19+IgD-CD38++)比例呈正相关(r=0.447 1、0.517 4、0.466 9,P均<0.05)。见图 3。

|
图 3 SLE患者外周血中CCR7loPD-1hi Tfh比例与临床指标及成浆细胞比例的相关性 Fig 3 Correlation analysis between clinical data, frequency of plasmablasts and percentage of CCR7loPD-1hi Tfh subset in peripheral blood of SLE patients SLE: Systemic lupus erythematosus; CCR7: C-C chemokine receptor 7; PD-1: Programmed death 1; lo: Low expression; hi: High expression; Tfh: Follicular helper T cell; SLEDAI: Systemic lupus erythematosus activity index; dsDNA: Double-stranded DNA |
3 讨论
Tfh的主要功能是辅助B淋巴细胞增殖、分化并促进抗体生成,调节体液免疫。因此,Tfh比例及功能失调与诸多自身免疫性疾病的发生、发展有关。SLE是一种以大量自身抗体产生为特征的自身免疫性疾病,患者机体内生发中心反应亢进,自身反应性B淋巴细胞显著增加,这种病理改变与Tfh异常密切相关[10]。Odegard等[11]利用狼疮小鼠模型进行研究,发现Tfh增加可以促进小鼠生发中心B淋巴细胞IgG分泌增多,导致狼疮病情进展,而清除体内Tfh病情则改善。Tfh不仅可在生发中心与B淋巴细胞相互作用调节体液免疫,亦可在外周血及SLE患者的肾组织中发挥作用[12-13]。由于人淋巴组织样本采集受限,分析患者外周血Tfh成为一种具有重要临床意义的替代策略。外周血Tfh与生发中心Tfh具有相似的表型和B淋巴细胞辅助功能[14]。利用不同的表面标志,Tfh可被细分为多种亚群,不同亚群的功能存在差异。Choi等[15]发现CXCR5hiICOShiPD-1hi(CXCR5、ICOS、PD-1均高表达)Tfh在SLE患者体内增加,与SLEDAI和抗dsDNA抗体滴度相关。Le Coz等[16]根据CXCR3和CCR6将Tfh分成Tfh1、Tfh2和Tfh3,其中Tfh2扩增与SLE病情相关。但是,既往研究均缺乏对不同自身免疫性疾病中Tfh的对比以及Tfh与B淋巴细胞亚群的相关性分析。
本研究结果示,根据CCR7和PD-1的表达情况,Tfh可被细分为CCR7loPD-1hi Tfh和CCR7hiPD-1loTfh;相比CCR7hiPD-1lo Tfh,CCR7loPD-1hi Tfh表面CXCR3、ICOS和SLAMF5的表达水平较高,说明CCR7loPD-1hi Tfh具有更强的B淋巴细胞辅助能力。CXCR3是一种趋化因子受体,与相应趋化因子结合后可介导Tfh迁移至多种外周炎性组织,特别是肾组织[17]。ICOS和SLAMF5与T淋巴细胞、B淋巴细胞之间相互作用密切相关。ICOS通过与B淋巴细胞表面ICOS配体结合促进B淋巴细胞活化,SLAMF5可增加T淋巴细胞、B淋巴细胞之间的黏附从而产生有效的免疫反应[18]。阻断ICOS和SLAMF5均可抑制B淋巴细胞分化和抗体产生,下调体液免疫的强度[19-20]。Choi等[15]也证实CCR7loPD-1hi Tfh具有更强的分泌IL-21的能力。为了进一步探究CCR7loPD-1hi在自身免疫性疾病中的作用,本研究对不同亚群Tfh进行分析,结果显示CD4+CD45RA-CXCR5+ Tfh的比例在SLE、RA、SS患者和健康对照之间差异均无统计学意义(P均>0.05),但SLE患者外周血中CCR7loPD-1hi Tfh的比例较健康对照增加(P<0.01),CCR7hiPD-1lo Tfh比例降低(P<0.01);RA患者外周血中CCR7loPD-1hi Tfh的比例与健康对照相比亦增加(P<0.01),而CCR7hiPD-1lo Tfh比例差异无统计学意义(P>0.05);SS患者外周血中2个亚群Tfh的比例较健康对照差异均无统计学意义(P>0.05)。研究发现与RA和SS患者相比,SLE患者中自身反应性抗体的种类更加多样[21],Tfh亚群的紊乱可能是SLE自身抗体多样的重要原因。本研究通过相关性分析发现,CCR7loPD-1hi Tfh比例与SLE患者的SLEDAI、抗dsDNA抗体滴度以及CD19+IgD-CD38++成浆细胞比例呈正相关(r=0.447 1、0.517 4、0.466 9,P均<0.05),提示CCR7loPD-1hi Tfh是一种效应型Tfh亚群,可通过辅助B淋巴细胞产生自身抗体促进疾病的发生和发展。
PD-1是含有288个氨基酸的Ⅰ型跨膜蛋白,细胞外含IgV样结构,细胞内含免疫受体酪氨酸依赖抑制基序(immunoreceptor tyrosine-based inhibitory motif,ITIM)和免疫受体酪氨酸依赖转换基序(immunoreceptor tyrosine-based swith motif,ITSM)。PD-1与程序性死亡蛋白配体1(programmed death-ligand 1,PD-L1)结合后,ITIM、ITSM可发生磷酸化,招募蛋白酪氨酸酶,从而使下游分子去磷酸化,转导负向信号,降低T淋巴细胞活化程度及炎症反应[22]。PD-1曾被认为发挥免疫耐受作用,敲除PD-1可以使小鼠产生自身免疫反应,上调PD-1可以改善自身免疫症状[23]。但是多项研究表明,PD-1/PD-L1在自身免疫性疾病患者中表达水平升高,且PD-1hi Tfh具有更强的激活B淋巴细胞能力[15, 24]。这些研究结论并不一致,提示PD-1信号在生发中心对Tfh起着精细的调节作用。上调PD-1虽然可以减少Tfh的数量,但是其具有更强的分泌IL-4和IL-21的能力,从而促进生发中心B淋巴细胞的生长以及高亲和性抗体分泌细胞的形成[4]。
综上所述,本研究表明SLE患者外周血中CCR7loPD-1hi Tfh比例增加与SLEDAI升高、成浆细胞比例的增加相关,检测患者外周血Tfh亚群可间接反映生发中心及B淋巴细胞的功能状态,对于疾病诊断、监测和预后有重要意义,针对该群细胞进行干预可能成为治疗SLE的潜在策略。
| [1] |
LISNEVSKAIA L, MURPHY G, ISENBERG D. Systemic lupus erythematosus[J]. Lancet, 2014, 384: 1878-1888. DOI:10.1016/S0140-6736(14)60128-8 |
| [2] |
BRINK R, PHAN T G. Self-reactive B cells in the germinal center reaction[J]. Annu Rev Immunol, 2018, 36: 339-357. DOI:10.1146/annurev-immunol-051116-052510 |
| [3] |
CRAFT J E. Follicular helper T cells in immunity and systemic autoimmunity[J]. Nat Rev Rheumatol, 2012, 8: 337-347. DOI:10.1038/nrrheum.2012.58 |
| [4] |
GOOD-JACOBSON K L, SZUMILAS C G, CHEN L, SHARPE A H, TOMAYKO M M, SHLOMCHIL M J. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells[J]. Nat Immunol, 2010, 11: 535-542. DOI:10.1038/ni.1877 |
| [5] |
LINTERMAN M A, BEATON L, YU D, RAMISCAL R R, SRIVASTAVA M, HOGAN J J, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses[J]. J Exp Med, 2010, 207: 353-363. DOI:10.1084/jem.20091738 |
| [6] |
HE J, TSAI L M, LEONG Y A, HU X, MA C S, CHEVALIER N, et al. Circulating precursor CCR7loPD-1hi CXCR5+ CD4+ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure[J]. Immunity, 2013, 39: 770-781. DOI:10.1016/j.immuni.2013.09.007 |
| [7] |
HOHBERG M C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus[J/OL]. Arthritis Rheum, 1997, 40: 1725. doi: 10.1002/1529-0131(199709)40:9<1725::AID-ART29>3.0.CO;2-Y.
|
| [8] |
ARNETT F C, EDWORTHY S M, BLOCH D A, MCSHANE D J, FRIES J F, COOPER N S, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis[J]. Arthritis Rheum, 1987, 31: 315-324. |
| [9] |
VITALI C, BOMBARDIERI S, JONSSON R, MOUTSOPOULOS H M, ALEXANDER E L, CARSONS S E, et al. Classification criteria for Sjögren's syndrome:a revised version of the European criteria proposed by the American-European Consensus Group[J]. Ann Rheum Dis, 2002, 61: 554-558. DOI:10.1136/ard.61.6.554 |
| [10] |
BLANCO P, UENO H, SCHMITT N. T follicular helper (Tfh) cells in lupus:activation and involvement in SLE pathogenesis[J]. Eur J Immunol, 2016, 46: 281-290. DOI:10.1002/eji.201545760 |
| [11] |
ODEGARD J M, MARKS B R, DIPLACIDO L D, POHOLEK A C, KONO D H, DONG C, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity[J]. J Exp Med, 2008, 205: 2873-2886. DOI:10.1084/jem.20080840 |
| [12] |
UENO H, BANCHEREAU J, VINUESA C G. Pathophysiology of T follicular helper cells in humans and mice[J]. Nat Immunol, 2015, 16: 142-152. DOI:10.1038/ni.3054 |
| [13] |
CHANG A, HENDERSON S G, BRANDT D, LIU N, GUTTIKONDA R, HSIEH C, et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis[J]. J Immunol, 2011, 186: 1849-1860. DOI:10.4049/jimmunol.1001983 |
| [14] |
ZHANG X, LINDWALL E, GAUTHIER C, LYMAN J, SPENCER N, ALARAKHIA A, et al. Circulating CXCR5+CD4+ helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production[J]. Lupus, 2015, 24: 909-917. DOI:10.1177/0961203314567750 |
| [15] |
CHOI J Y, HO J H, PASOTO S G, BUNIN V, KIM S T, CARRASCO S, et al. Circulating follicular helper-like T cells in systemic lupus erythematosus association with disease activity[J]. Arthritis Rheumatol, 2015, 67: 988-999. DOI:10.1002/art.v67.4 |
| [16] |
LE COZ C, JOUBLIN A, PASQUALI J L, KORGANOW A S, DUMORRTIER H, MONNEAUX F. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease[J/OL]. PLoS One, 2013, 8: e75319. doi: 10.1371/journal.pone.0075319.
|
| [17] |
LACOTTE S, DECOSSAS M, LE COZ C, BRUN S, MULLER S, DUMORTIER H, et al. Early differentiated CD138highMHCⅡ+IgG+ plasma cells express CXCR3 and localize into inflamed kidneys of lupus mice[J/OL]. PLoS One, 2013, 8: e58140. doi: 10.1371/journal.pone.0058140.
|
| [18] |
CANNONS J L, QI H, LU K T, DUTTA M, GOMEZ-RODRIQUEZ J, CHENG J, et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84[J]. Immunity, 2010, 32: 253-265. DOI:10.1016/j.immuni.2010.01.010 |
| [19] |
WEINSTEIN J S, BERTINO S A, HERNANDEZ S G, POHOLEK A C, TEPLITZKY T B, NOWYHED H N, et al. B Cells in T follicular helper cell development and function:separable roles in delivery of ICOS ligand and antigen[J]. J Immunol, 2014, 192: 3166-3179. DOI:10.4049/jimmunol.1302617 |
| [20] |
RAO D A, GURISH M F, MARSHALL J L, SLOWIKOWSKI K, FONSEKA C Y, LIU Y, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis[J]. Nature, 2017, 542: 110-114. DOI:10.1038/nature20810 |
| [21] |
TIPTON C M, HOM J R, FUCILE C F, ROSENBERG A F, SANZ L. Understanding B-cell activation and autoantibody repertoire selection in systemic lupus erythematosus:a B-cell immunomics approach[J]. Immunol Rev, 2018, 284: 120-131. DOI:10.1111/imr.2018.284.issue-1 |
| [22] |
KEIR M E, BUTTE M J, FREEMAN G J, SHARPE A H. PD-1 and its ligands in tolerance and immunity[J]. Annu Rev Immunol, 2008, 26: 677-704. DOI:10.1146/annurev.immunol.26.021607.090331 |
| [23] |
NISHIMURA H, NOSE M, HIAI H, MINATO N, HONJO T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor[J]. Immunity, 1999, 11: 141-151. DOI:10.1016/S1074-7613(00)80089-8 |
| [24] |
LEI H, XUE Y, YIYUN Y, WEIGUO W, LING L, ZOU H. Associations of circulating CXCR3-PD-1+CD4+ T cells with disease activity of systemic lupus erythematosus[J]. Mod Rheumatol, 2018, 25: 1-20. |
 2018, Vol. 39
2018, Vol. 39


