2. 哈尔滨医科大学附属第二医院妇产科, 哈尔滨 150001
2. Department of Obstetrics and Gynecology, the 2nd Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang, China
规律性的细胞死亡在发育和维持成体组织的内稳态中具有重要作用。近年研究表明,生理和病理的细胞坏死能以一种有规律的方式发生[1]。这种有规律的坏死被称为程序性坏死[2],它被认为是与经典的Caspase介导的细胞凋亡相互补的程序性细胞死亡方式[3]。细胞坏死也参与一些重要的生理过程,如免疫抵御、排卵、软骨细胞的死亡和肠上皮细胞的死亡等[4]。该过程是由不同细胞水平的生物化学和分子事件共同诱导的,而蛋白激酶受体相互作用蛋白激酶3(receptor interacting protein kinase 3,RIPK3)和它下游的底物混合谱系激酶结构域样蛋白(mixed lineage kinase domain-like protein,MLKL)则是程序性细胞坏死中的关键性细胞成分。
细胞凋亡以及自噬均参与哺乳动物胚胎着床过程,但程序性细胞坏死在胚胎着床过程中的作用尚不清楚。MLKL是程序性细胞坏死的下游重要信号分子,本研究通过观察MLKL在早期妊娠子宫和子宫蜕膜化过程中的表达,探讨程序性细胞坏死是否参与哺乳动物胚胎着床和蜕膜化过程,同时分析雌二醇和孕酮对MLKL的调控作用。
1 材料和方法 1.1 主要试剂TRIzol试剂,Invitrogen公司;SYBR Green Realtime PCR Master Mix-Plus,TaKaRa公司;NBT/BCIP底物显色试剂,Roche公司;左旋咪唑、二甲基亚砜(dimethyl sulfoxide,DMSO)、17β-雌二醇、孕酮、醋酸甲羟孕酮,Sigma公司;MLKL抗体,由厦门大学韩家淮实验室赠送;甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GAPDH)抗体,Cell Signaling Technology公司;辣根过氧化物酶标记山羊抗兔IgG,ABclonal公司;蛋白质相对分子质量标准品(marker),Thermo公司。
1.2 实验动物性成熟的ICR品系小鼠购于黑龙江省中医药大学[许可证号:SYXK(黑)2016-004],饲养于东北林业大学[许可证号:SYXK(黑)2015-002],在人工控制的条件下,室温22 ℃,光照周期为12 h光照、12 h黑暗,自由摄食和饮水。早期妊娠模型建立:雄鼠与雌鼠按1:3合笼交配。次晨检查雌鼠的阴道栓,并把见栓当天记为妊娠第1天。人工诱导蜕膜化模型建立:将健康雄性小鼠的双侧输精管结扎,待其恢复至少2周后,与自然发情的雌鼠按1:3合笼,次日早上检查阴道栓,并把见栓当天记为假孕第1天。假孕第4天上午9:00将小鼠一侧子宫注射芝麻油50 μL,另一侧作为对照。假孕第8天上午9:00取材。激素处理模型:将健康成年雌鼠的双侧卵巢切除,待其恢复至少2周后,随机将这些小鼠分为4组,根据分组分别皮下注射类固醇激素(溶于芝麻油中)或芝麻油:对照组注射芝麻油,每只0.1 mL;孕酮处理组注射孕酮,每只2 mg/0.1 mL;雌二醇处理组注射17β-雌二醇,每只25 ng /0.1 mL;雌二醇+孕酮共处理组注射17β-雌二醇25 ng和孕酮2 mg,每只0.1 mL。激素注射24 h后取材。
1.3 实时荧光定量PCR取小鼠子宫组织50~100 mg,提取组织总RNA,并将总RNA反转录为cDNA。以cDNA为模板进行PCR。MLKL(NCBI序列号:NC_000074.6)引物序列:上游引物5′-CAG AAA CAT CAG CAG CTC CA-3′,下游引物5′-ATG ATT TCC CGC AAC AAC TC-3′,由生工生物工程(上海)股份有限公司合成。反应体系(20 μL)如下:SYBR Premix Ex Taq(Tli RNaseH Plus)(2×)10 μL,上、下游引物(10 μmol/L)各0.4 μL,Rox Reference Dye(50×)0.4 μL,模板cDNA 2 μL,水6.8 μL。反应条件:95 ℃预变性31 s;95 ℃ 5 s、60 ℃ 34 s,40个循环后收集荧光信号。使用Amplied Biosystems® 7500 Real-Time PCR System操作,由仪器自动绘制熔解曲线,采用2—ΔΔCt法计算目的基因相对表达量。
1.4 原位杂交将冻存的正常妊娠第1~8天和人工蜕膜化小鼠子宫组织切片烘片2 min,用4%四氟乙烯-全氟烷氧基乙烯基醚共聚物(polyfluoroalkoxy,PFA)固定1 h,磷酸盐缓冲液(phosphate buffered saline,PBS)洗去固定液,1% Triton X-100透膜20 min,洗涤;预杂交;杂交;洗涤;0.5%封闭液[TSATM PLUS DNP(AP)System试剂盒,PerkinElmer公司]室温封闭;Anti-Digoxigenin-POD(1:100,Roche公司)室温孵育;TNT(0.1 mol/L Tris pH 7.5,0.15 mol/L NaCl,0.05%吐温-20)洗涤;DNP Amplification Reagent(1:50,PerkinElmer公司)避光孵育10 min;洗涤;Anti-DNP-AP(1:200,PerkinElmer公司)4 ℃避光孵育过夜。洗涤,用缓冲液2(0.1 mol/L Tris pH 7.5,0.1 mol/L NaCl,0.05 mol/LMgCl2)平衡5 min,滴加显色试剂NBT/BCIP(1:50)与2 mmol/L左旋咪唑室温显色,甲基绿对染,甘油封片,照相。
1.5 蛋白质印迹分析取冻存的正常妊娠第1~8天、激素处理和人工诱导蜕膜化的小鼠子宫组织匀浆,取上清用BCA蛋白浓度测定试剂盒(Biosharp公司)测定蛋白样品浓度。取等质量的蛋白样品(约20 μL)进行十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(sodium dodecyl sulphate-polyacrylamide gel electrophoresis,SDS-PAGE)。将蛋白转膜后用脱脂奶粉溶液4 ℃封闭过夜,分别与兔源MLKL一抗(1:700)和鼠源GAPDH一抗(1:300,Santa Cruz公司)37 ℃孵育1 h,PBST洗10 min×3次,与5%脱脂奶粉溶液稀释的二抗37 ℃孵育1 h,PBST洗10 min×3次,用电化学超敏发光液(Coolaber公司)显影,曝光。用ImageJ软件对灰度进行相对定量分析,以GAPDH作为内参。
1.6 体外诱导人子宫内膜基质细胞蜕膜化人子宫内膜基质细胞系CRL-4003(美国典型微生物菌种保藏中心)使用不含酚红的DMEM/F-12(1:1)培养基进行传代培养。为了去除血清中本身含有的激素对实验结果的影响,选用活性炭吸附处理后的胎牛血清(fetal bovine serum,FBS),将其以1:10的比例加入培养基中。细胞以1×106/mL的密度接种于35 mm直径培养盘中,在含有5% CO2的37 ℃培养箱中培养。当细胞贴壁生长,长到80%融合时,可以开始诱导蜕膜化。更换新鲜的培养液,在处理组中加入终浓度为36 nmol/L的雌二醇、1 μmol/L的醋酸甲羟孕酮(雌二醇和醋酸甲羟孕酮的溶剂均为无水乙醇),并加入终浓度为0.1 mmol/L的二丁酰环腺苷酸(Sigma公司);在对照组中按体积比1:1 000加入无水乙醇。每隔1 d更换1次新鲜培养液,并按上述浓度重新添加激素和二丁酰环腺苷酸。用TRIzol试剂分别收集处理后第3天、第6天和第9天的细胞,采用实时荧光定量PCR检测催乳素(prolactin,PRL)和MLKL mRNA的表达。
1.7 统计学处理采用GraphPad Prism 5.0软件绘图并分析数据。实验数据用x±s表示,组间比较采用单因素方差分析或Student’s t检验,检验水准(α)为0.05。
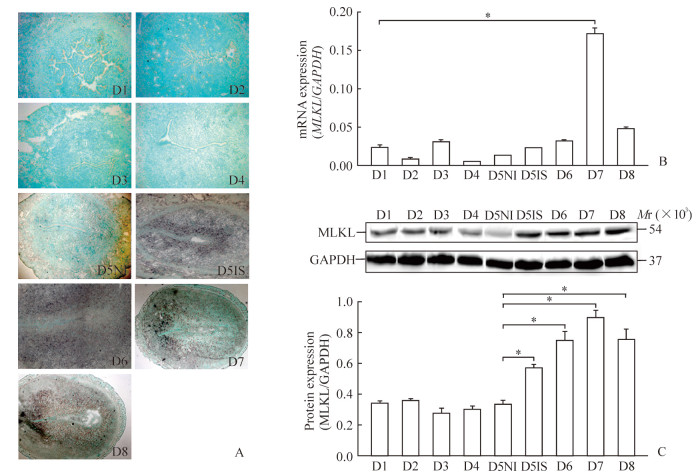
2 结果 2.1 MLKL在小鼠早期妊娠子宫中的表达实时荧光定量PCR、原位杂交和蛋白质印迹分析结果显示,在小鼠早期妊娠子宫中,MLKL mRNA及蛋白在妊娠第1~4天的小鼠子宫腔上皮表达,但表达量较低且不规律;在妊娠第5天的小鼠子宫腔上皮及其周围的蜕膜细胞中表达,与之对照的妊娠第5天非着床位点无明显表达;妊娠第6~8天主要集中在蜕膜组织中表达,着床后表达量逐日增高并在妊娠第7天达到最高峰,第8天表达量稍有下降(图 1)。
|
图 1 MLKL在小鼠早期妊娠第1~8天子宫中的表达 Fig 1 Expression of MLKL in mouse uterus during early pregnancy from day 1 to day 8 A: In situ hybridization (original magnification: ×100 [D1-D4, D5NI, D5IS, D6], ×40 [D7, D8]); B: Real-time PCR; C: Western blotting. MLKL: Mixed lineage kinase domain-like protein; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; D1-D8: Pregnancy day 1 to day 8; NI: Non-implantation site; IS: Implantation site. *P < 0.05. n=3, x±s |
2.2 MLKL在蜕膜化子宫中的表达
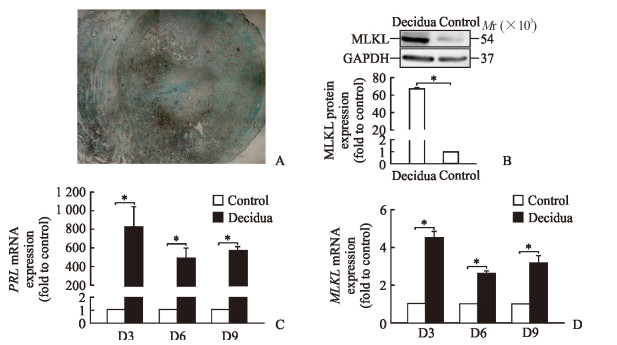
原位杂交结果显示,在人工诱导蜕膜化的小鼠子宫中MLKL mRNA表达量很高,整个蜕膜区域均有表达(图 2A),而在未处理的对照组子宫中则无明显表达。MLKL蛋白的表达与mRNA相一致(图 2B)。为了更精确地研究MLKL在子宫蜕膜化中的作用,将人子宫内膜基质细胞进行传代培养,并对其进行体外蜕膜化诱导。实时荧光定量PCR结果显示,作为蜕膜化标志分子的PRL mRNA在体外诱导蜕膜化的人子宫内膜基质细胞中表达量非常高,证明人子宫内膜基质细胞蜕膜化体外诱导成功且效率较高(图 2C)。MLKL mRNA在体外诱导蜕膜化人子宫内膜基质细胞中表达量高于对照组,差异有统计学意义(P<0.05,图 2D)。
|
图 2 MLKL在小鼠人工蜕膜化子宫组织及体外诱导蜕膜化人子宫内膜基质细胞中的表达 Fig 2 Expression of MLKL in uterus of mice with artificially induced decidualization and human decidual cells induced in vitro A: Expression of MLKL in uterus of mice with artificially induced decidualization by in situ hybridization; B: Expression of MLKL protein in uterus of mice with artificially induced decidualization by Western blotting; C, D: Expression of PRL and MLKL mRNA in human decidual cells induced in vitro by real-time PCR. MLKL: Mixed lineage kinase domain-like protein; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; PRL: Prolactin. D3, D6, D9: Pregnancy day 3, day 6, and day 9. Original magnification: ×100 (A). *P < 0.05. n=3, x±s |
2.3 MLKL在激素处理子宫中的表达
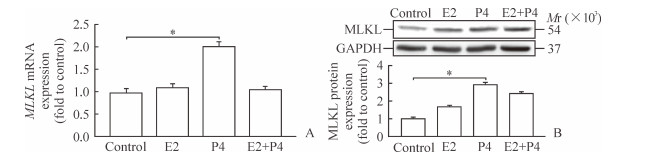
与对照组小鼠子宫相对比,孕酮处理组小鼠子宫中MLKL mRNA和蛋白表达量增高,差异有统计学意义(P<0.05);雌二醇处理组和雌二醇+孕酮共处理组小鼠子宫中,MLKL mRNA的表达量与对照组差异无统计学意义,MLKL蛋白的表达量与对照组和单独孕酮处理组相比虽略有差异但无统计学意义(图 3)。
|
图 3 MLKL在类固醇激素处理的小鼠子宫中的表达 Fig 3 Expression of MLKL in steroid-treated mouse uterus A: Expression of MLKL mRNA by real-time PCR; B: Expression of MLKL protein by Western blotting. MLKL: Mixed lineage kinase domain-like protein; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; E2: Estrogen; P4: Progesterone. *P < 0.05. n=3, x±s |
3 讨论
细胞凋亡参与哺乳动物胚胎着床过程,在胚胎着床期间大鼠胚胎周围的子宫上皮细胞经历了凋亡性的细胞死亡,最终导致它们被滋养层细胞所吞噬[5]。还有研究发现,自噬与滋养层细胞密切相关,滋养层细胞侵入子宫上皮的过程有可能因为自噬功能的异常而被阻断,滋养层细胞的功能作用也会受到它的影响[6-7]。近年来程序性细胞坏死受到广泛关注,而MLKL在肿瘤坏死因子(tumor necrosis factor,TNF)诱导的程序性坏死通路中具有重要作用[8-9]。本研究结果显示当妊娠进入第5天,胚胎着床发生,MLKL mRNA的表达量开始升高,集中定位在子宫腔上皮,并在早期妊娠过程中持续表达,显示出规律性,而在非着床位点处则观察不到明显的表达;MLKL蛋白同样在妊娠第5天开始表达量显著升高。这表明MLKL与胚胎着床过程关系密切,并且其在子宫腔上皮细胞中的表达可能受着床胚胎的特异性影响。
子宫基质细胞的蜕膜化是胎盘形成以及维持妊娠的必要条件之一。蜕膜化过程包含很多生物学事件,例如子宫内膜基质细胞的生长、细胞形态的显著变化、细胞的凋亡,基质成纤维细胞的再分化、多倍体化,以及细胞外基质的重新建立、细胞间连接的建立等[10-11]。研究显示,完全发育的蜕膜组织能分泌生长因子和细胞因子来支持胚胎发育,并支持母源的血管形成为发育中的胚胎提供营养;同时蜕膜组织还能发挥免疫调节的作用,为胚胎提供阻止其他滋养层细胞侵入的屏障,并且在功能性胎盘形成之前保护胚胎免受母体排斥[5, 12-14]。本研究结果显示MLKL mRNA在妊娠第5天着床位点处表达较明显,并且主要表达于着床位点周围的子宫腔上皮以及蜕膜区域;妊娠第6~8天子宫的蜕膜区域均有较为明显的表达,其表达量在妊娠第7天达到最高峰,之后稍有下降;在人工蜕膜化小鼠模型以及体外诱导人蜕膜化细胞中,MLKL mRNA和蛋白表达量均非常高;而在未发生蜕膜化的对照组中,MLKL mRNA和蛋白的表达基本检测不到。这些结果表明MLKL可能在小鼠子宫蜕膜化过程中起作用。
作为早期妊娠过程中关键的调控因子,雌二醇与孕酮这两种卵巢激素相互作用,调节早期妊娠的正常建立[15]。2014年,Vanden Berghe等[16]发现了孕酮介导的程序性细胞坏死的信号转导途径。随后,Wu等[17]采用孕酮处理,通过激活TNF-α/RIPK1/RIPK3/MLKL途径,诱导小鼠输卵管上皮细胞程序性坏死,再次证实了孕酮对MLKL的激活作用。本研究结果也证实了这一点。与对照组相比,在孕酮处理组小鼠子宫中,MLKL mRNA和蛋白表达量均增高;但在单独雌二醇处理以及雌二醇+孕酮共处理组小鼠子宫中,MLKL mRNA和蛋白表达量并不同步,说明MLKL在转录后的翻译过程中发生了变化,而雌二醇和孕酮的相互作用可能影响了孕酮对MLKL的表达调控。MLKL的表达是否受雌二醇调控还未见研究报道。因此,我们认为MLKL在小鼠子宫中的表达受孕酮的调控。
综上所述,本研究结果表明MLKL在胚胎着床以及蜕膜化过程中发挥作用。今后仍需继续对其上下游的调控因子进行深入研究,以进一步阐明程序性细胞坏死在早期妊娠中的作用及机制。
| [1] |
CHAN F K. Fueling the flames: mammalian programmed necrosis in inflammatory diseases[J/OL]. Cold Spring Harb Perspect Biol, 2012, 4. pii: a008805. doi: 10.1101/cshperspect.a008805.
|
| [2] |
GALLUZZI L, VITALE I, ABRAMS J M, ALNEMRI E S, BAEHRECKE E H, BLAGOSKLONNY M V, et al. Molecular definitions of cell death subroutines:recommendations of the Nomenclature Committee on Cell Death 2012[J]. Cell Death Differ, 2012, 19: 107-120. DOI:10.1038/cdd.2011.96 |
| [3] |
HILDEBRAND J M, TANZER M C, LUCET I S, YOUNG S N, SPALL S K, SHARMA P, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death[J]. Proc Natl Acad Sci USA, 2014, 111: 15072-15077. DOI:10.1073/pnas.1408987111 |
| [4] |
FESTJENS N, VANDEN BERGHE T, VANDENABEELE P. Necrosis, a well-orchestrated form of cell demise:signalling cascades, important mediators and concomitant immune response[J]. Biochim Biophys Acta, 2006, 1757(9/10): 1371-1387. |
| [5] |
WELSH A O, ENDERS A C. Trophoblast-decidual cell interactions and establishment of maternal blood circulation in the parietal yolk sac placenta of the rat[J]. Anat Rec, 1987, 217: 203-219. DOI:10.1002/(ISSN)1097-0185 |
| [6] |
AKAISHI R, YAMADA T, NAKABAYASHI K, NISHIHARA H, FURUTA I, KOJIMA T, et al. Autophagy in the placenta of women with hypertensive disorders in pregnancy[J]. Placenta, 2014, 35: 974-980. DOI:10.1016/j.placenta.2014.10.009 |
| [7] |
GONG J S, KIM G J. The role of autophagy in the placenta as a regulator of cell death[J]. Clin Exp Reprod Med, 2014, 41: 97-107. DOI:10.5653/cerm.2014.41.3.97 |
| [8] |
ZHAO J, JITKAEW S, CAI Z, CHOKSI S, LI Q, LUO J, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis[J]. Proc Natl Acad Sci USA, 2012, 109: 5322-5327. DOI:10.1073/pnas.1200012109 |
| [9] |
MURPHY J M, CZABOTAR P E, HILDEBRAND J M, LUCET I S, ZHANG J G, ALVAREZ-DIAZ S, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism[J]. Immunity, 2013, 39: 443-453. |
| [10] |
FAZLEABAS A T, BELL S C, FLEMING S, SUN J, LESSEY B A. Distribution of integrins and the extracellular matrix proteins in the baboon endometrium during the menstrual cycle and early pregnancy[J]. Biol Reprod, 1997, 56: 348-356. DOI:10.1095/biolreprod56.2.348 |
| [11] |
TAN J, RAJA S, DAVIS M K, TAWFIK O, DEY S K, DAS S K. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation[J]. Mech Dev, 2002, 111(1/2): 99-113. |
| [12] |
PARIA B C, ZHAO X, DAS S K, DEY S K, YOSHINAGA K. Zonula occludens-1 and E-cadherin are coordinately expressed in the mouse uterus with the initiation of implantation and decidualization[J]. Dev Biol, 1999, 208: 488-501. DOI:10.1006/dbio.1999.9206 |
| [13] |
PENG S, LI J, MIAO C, JIA L, HU Z, ZHAO P, et al. Dickkopf-1 secreted by decidual cells promotes trophoblast cell invasion during murine placentation[J]. Reproduction, 2008, 135: 367-375. DOI:10.1530/REP-07-0191 |
| [14] |
ROGERS P A, MURPHY C R, ROGERS A W, GANNON B J. Capillary patency and permeability in the endometrium surrounding the implanting rat blastocyst[J]. Int J Microcirc Clin Exp, 1983, 2: 241-249. |
| [15] |
KUMAR R, YADAV A, PAKRASI P L. Expression of ER-α and ER-β during peri-implantation period in uterus is essential for implantation and decidualization in golden hamster[J]. Life Sci, 2017, 170: 115-122. DOI:10.1016/j.lfs.2016.12.002 |
| [16] |
VANDEN BERGHE T, LINKERMANN A, JOUAN-LANHOUET S, WALCZAK H, VANDENABEELE P. Regulated necrosis:the expanding network of non-apoptotic cell death pathways[J]. Nat Rev Mol Cell Biol, 2014, 15: 135-147. |
| [17] |
WU N Y, HUANG H S, CHAO T H, CHOU H M, FANG C, QIN C Z, et al. Progesterone prevents high-grade serous ovarian cancer by inducing necroptosis of p53-defective fallopian tube epithelial cells[J]. Cell Rep, 2017, 18: 2557-2565. DOI:10.1016/j.celrep.2017.02.049 |
 2018, Vol. 39
2018, Vol. 39


